How to Cite | Publication History | PlumX Article Matrix
Studies on the Amylase Inhibitors from the Seeds of Adenanthera Pavonina
K. S. Chandrashekharaiah
Department of Biochemistry, Mangalore University, Chikka Aluvara, Kodagu-571232.
Corresponding Author E-mail: kschandraks@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2535
ABSTRACT: An α-Amylase inhibitor was isolated and purified employing ammonium sulphate fractionation, molecular sieve chromatography on sephadex G-10 and G-50 and HPLC from the seeds of Adenanthera pavonina. The molecular weight was found to be 10 - 12 kDa as determined by gel-permeation chromatography on Sephadex G-100. The specific inhibitor activity, fold purity and the yield obtained for Adenanthera pavonina amylase inhibitor was 32.12, 51 and 13.07, respectively. The purified inhibitor was heat stable and retained more than 52% activity at 65°C. The optimum pH obtained for purified inhibitor was 6.3 and 100% Zone of inhibition was observed when it was added on the plated organisms. The Adenanthera pavonina amylase inhibitor inhibited the activity of human salivary α-amylase and inhibitory activity of α-amylase inhibitor against mammalian amylases could suggest its potential in treatment of diabetes and related nutritional problems results in obesity.
KEYWORDS: Amylase Inhibitors; Adenanthera pavonina seeds Characterization; Isolation;
Download this article as:| Copy the following to cite this article: Chandrashekharaiah K. S. Studies on the Amylase Inhibitors from the Seeds of Adenanthera Pavonina. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Chandrashekharaiah K. S. Studies on the Amylase Inhibitors from the Seeds of Adenanthera Pavonina. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27186 |
Introduction
Plants contain proteins with inhibitor activity against animals α-amyalses (Shainkin and Birk, 1970; Silano et al., 1973) occur widely in plants. These amylase inhibitors make plants less palatable by inhibiting α-amyalses of animals thereby preventing the digestion and absorption of dietary starch. This is useful for treating metabolic disorders such as obesity and diabetes mellitus (Ali et al., 2006; Asma Ali et al., 2012). In plants, these enzyme inhibitors are storage or reserve proteins functions as regulators of endogenous enzyme or defensive agents against the attacks of animal predators and insect or microbial pests (Octavio and Rigden, 2002; Octivio and Rigden, 2000; Richardson, 1991). α -amylase inhibitors are attractive candidates in people with diabetes reduce the glucose peaks that can occur after a meal, slowing the speed with which alpha amylase can convert starch to simple sugars until the body can deal with it as well as in plants as a defence mechanism against insect pests highly dependent on starch as energy source (Franco et al., 2000; Nagy-Gasztonyi et al., 2010).
Adenanthera pavonina is a perennial and non-climbing species of leguminous tree. Its uses include food and drink, traditional medicine and timber. It is commonly called Red Lucky Seed and it is cultivated for forage. It is also grown as medicinal plant, an ornamental garden plant or urban tree. The young leaves and seeds can be cooked and eaten. This tree is used for making soap and a red dye can be obtained from the wood. Since the seeds are used as medicine and work on amylase inhibitor is scanty, the present work is undertaken. In the present study, partial purification and characterization of amylase inhibitor is described.
Materials and Methods
Seeds of Adenanthera pavonina were procured from in and around of Puthige Panchayath of Kasaragod District of Kerala, India.
Chemicals
Bovine Serum Albumin, α-amylase, Starch, Sephedex G-10 and G-50 were obtained from Sigma chemical company, USA. All other chemicals used were of technical grade.
Methods
Preparation of Acetone Powder
The Adenanthera pavonina seeds were soaked, dehulled and acetone powder (10 %) was prepared according to the method of Wetter (1957). Soaked and dehulled seeds were blended in a homogenizer for 5 mins with chilled acetone and filtered using suction pump. The cake obtained was dried at 37°C, powdered and stored at 4°C until further use.
Preparation of Crude Extract
A 10 % extracts of acetone powder of Adenanthera pavonina was prepared by stirring on a magnetic stirrer using sodium phosphate buffer pH 7.0 for 1.5 hr followed by centrifugation at 10,000 rpm for 15 mins at 4°C. The supernatant was collected and used for qualitative and quantitative analysis of proteins/peptides for amylase inhibitory activity and antioxidant activity.
Ammonium Sulphate Fractionation
The crude extract was subjected to 0 – 90% ammonium sulphate precipitation. Solid powdered ammonium sulphate was added slowly with constant stirring over magnetic stirrer for 30 min at 4°C. The stirring was continued after the addition of ammonium sulphate salt for one hour. The solution was allowed to stand for 1hr. The precipitated protein was recovered by centrifugation at 10,000 rpm for 30min. The protein pellet was dissolved in small amount of 0.025 M sodium phosphate buffer, pH. 7.0.
Sephadex G -10 and G-50 Gel-Filtration Chromatography
Sephadex G-10 and G-50 were allowed to swell in excess of distilled water on a boiling water bath for 5 hrs, separately. The gels were decanted to remove fines and then equilibrated with 0.05 M sodium phosphate buffer, pH 7.0, separately. The gels were packed into columns of size 1.0 cm X 110.0 cm under gravity. The columns were equilibrated with two bed volumes of 0.05M sodium phosphate buffer, pH 7.0 and fractions were collected at a flow rate of 10 ml/hr. The concentrated Ammonium sulphate fractionation was loaded on to the Sephadex G-10 gel and the proteins were eluted with same buffer with a fraction volume of 2.0 ml. Quantitative analysis of proteins, amylase and protease inhibitor activity was done. The fractions containing amylase inhibitor activity were pooled and subjected to sephadex G-50 chromatography. The elution buffer and fraction volume were similar to sephadex G-10 chromatography. Quantitative analysis of proteins and amylase inhibitor activity was done and the peak fractions containing amylase inhibitor activity were subjected to further purification by RP-HPLC.
RP-HPLC
RP-HPLC is carried out on Reversed-phase octadecylsilica (C18) column using binary solvent system with binary gradient capability and a UV detector. Buffer A is 0.1% (v/v) TFA in water and Buffer B is 100% acetinitrile containing 0.1% (v/v) TFA. Column Equilibration and Blank Run was carried out using Buffer A with a flow rate of 1 mL/min at 220 and 280 nm respectively. Once the stable line is obtained, the sephadex G-50 precipitated sample was injected and eluted the sample with a linear gradient from 0 to 100% buffer B for 30 min.
Amylase Activity
Quantitatively Amylase activity was determined by measuring liberated maltose using method of Bernfeld (1955). A typical Amylase assay mixture consists of 0.1 ml (100µg) of amylase enzyme with 0.9 ml of sodium phosphate buffer (pH 7.0). The reaction was started by the addition of 1.0 ml of 1% soluble starch at 37°C and stopped after 10 minutes by the addition of (DNS) dinitrosalisylic acid reagent (1.0 ml). After incubation in boiling water bath for 15 minutes, the contents of the test tubes were cooled and the volume in each case was made to 6.0 ml by the addition of distilled water. Absorbance of the mixture was measured at 540 nm using UV-Visible spectrophotometer. The amylase activity was defined as liberation of 1mg of maltose from starch at pH 7.0 at 37°C in 10 minutes. The amylase activity was defined as liberation of 1 μmole of maltose formed per min at pH 7.0 at 37°C.
Amylase Inhibitor Activity
Amylase inhibitor activity was determined by measuring reduction in maltose liberated by salivary amylase using (DNS) dinitrosalisylic acid (Fossum et al., 1974; Bernfeld, 1955). A typical Amylase inhibitory assay mixture consists of 0.1 M (100µg) of amylase enzyme with 0.9 ml of sodium phosphate buffer (pH 7.0) with Adenanthera pavonina amylase inhibitor incubated for 10 minutes at room temperature. The reaction was initiated by the addition of 1.0 ml of 1% soluble starch at 37°C. The reaction was stopped after 10 minutes by the addition of DNS (1.0 ml). Absorbance of the mixture was measured at 540 nm as described above. The amylase inhibitory unit was defined as the number of amylase units inhibited under the assay conditions.
Molecular Weight Determination
The apparent molecular mass of the purified amylase inhibitor was determined according to the method of Andrews (1970) using Sephadex G-100 (1.13 x 100 cm) pre-equilibrated with 0.025 M sodium phosphate buffer pH 7.0, at a flow rate of 10 ml/h. The column was calibrated with proteins of different molecular mass such as cytochrome-c (12.3 kDa), carbonic anhydrase (29 kDa), bovine serum albumin (BSA) (66 kDa), alcohol dehydrogenase (150 kDa) and β-amylase (200 kDa). Blue Dextran (2000 kDa) was used to determine the void volume (Vo). The molecular weight of the purified amylase inhibitor was determined from the plot of log molecular weight versus Kav.
Effect of pH and Temperature
The effect of pH on the activity of the purified amylase inhibitor was studied using the buffers of pH 3 – 10. The pH stability was also determined by preincubating the purified amylase inhibitor with different buffers for 30 min. Amylase inhibitor assay was performed as described earlier.The effect of temperature on the activity purified amylase inhibitor was studied at different temperatures ranging between 0 – 90°C. The temperature stability of purified amylase inhibitor was studied by pre-incubating, purified amylase inhibitor at different temperatures (0 – 90°C) for 30 min. The incubated samples were rapidly cooled and assayed at room temperature. Amylase inhibitor assay was performed as described earlier.
Antimicrobial Activity
Antibacterial Activity
Preparation of Inoculums
Stock cultures of three bacterial strains; E.coli, Klebisella sps and Staphylococcus aureus were maintained at 4°C on slopes of nutrient agar. Active cultures for experiment were prepared by transferring a loop full of cells from the stock cultures to the test tubes of sterile water.
Antibacterial Activity by Well Diffusion Method
Antibacterial properties of purified peptides were determined using agar well diffusion method. Approximately 20ml of molten and cooled media (nutrient agar) was poured in sterilized petri dishes. The bacterial test organisms (E.coli, Klebsiella and Staphylococcus aureus) were swabbed uniformly on respected petridishes containing nutrient broth using sterile cotton swabs. Wells were prepared in the agar plates; the wells were labelled and were loaded with 50µl of diluted purified peptides and standard streptomycin. The plates were examined for evidence of zones of inhibition.
Polyacrylamide gel Electrophoresis
An anionic disc gel electrophoresis was carried out essentially according to the method of Davis and Ornstein (1964). A discontinuous gel system consisting of 12% separating gel and 4% spacer gel was used. The electrophoresis was carried out in cold applying a current of 50 – 10 mA for 4 hours using tris–glycine (pH 8.3) as electrode buffer and bromophenol blue as marker dye.
Results and Discussion
Purification
All the purification procedures were performed at 4°C unless otherwise stated. Crude extract of the soaked seeds of Adenanthera pavonina was subjected to ammonium sulphate fractionation (0 – 90%). The precipitated protein obtained was recovered by centrifugation at 10000 rpm at 4°C. The precipitated protein was dissolved in small volume of 0.025M sodium phosphate buffer; pH 7.0 applied on to a Sephadex G-10 chromatography and eluted using same buffer. The fractions were analyzed both for proteins and amylase inhibitor activity. Three fractions were obtained and these fractions were labeled as Fraction –I, II and III. (Fig.1). The fraction – I containing amylase inhibitor activity was pooled and concentrated using ammonium sulphate. The concentrated amylase inhibitor fraction was applied on to a Sephadex G-50 chromatography (Fig.2). Four fractions were obtained and were labeled as Fraction –I, II, III and IV. The fraction – II containing amylase inhibitor activity was further subjected to purification by RP-HPLC (Fig.3). A α-amylase inhibitor was purified employing ammonium sulphate precipitation, ethanol fractionation, chromatographic separation on Sephadex and reversed phase-high profile liquid chromatography (Ho and Whitaker, 1993; Kokiladevi et al., 2005; Hivrale et al., 2011; Mridu Gupta et al., 2014).
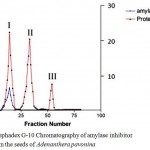 |
Figure 1: Sephadex G-10 Chromatography of amylase inhibitor activity from the seeds of Adenanthera pavonina |
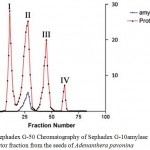 |
Figure 2: Sephadex G-50 Chromatography of Sephadex G-10amylase inhibitor fraction from the seeds of Adenanthera pavonina |
 |
Figure 3: Chromatogram of Elution profile of Sephadex G-50 Amylase inhibitor fraction on RP-HPLC |
The molecular weight of amylase inhibitor from the seeds of Adenanthera pavonina as determined by gel-permeation chromatography on Sephadex G-100 was found to be 13.6 kDa. Alpha amylase inhibitor with a different molecular weight has been reported from bean cultivars, white kidney beans and Phaseolus vulgaris (Mridu Gupta et al., 2014; Yamaguchi, 1993; Wato et al., 2000). In the present studies the maximum activity for the HPLC purified α-amylase inhibitor was observed at 25°C– 45°C. Similar results were observed for bean seed and rye α-amylase inhibitor (Frels and Rupnow, 1985; Power and Whitaker, 1977; Granum, 1978). The purified amylase inhibitor from Adenanthera pavonina was inhibited the human salivary amylase. The α-amylase inhibitor purified from various plant sources was found to be effective on human salivary amylase (Yoshikawa et al., 1999; Iulek et al., 2000; Heidari et al., 2005; Hivrale et al., 2011).Natural antioxidants present in plants are responsible for inhibition or prevention of the injurious effects of oxidative stress caused by free radicals in the body. The HPLC purified fraction also showed the anti-oxidant activity
Anti-bacterial activity of purified inhibitor was tested on three bacterial strains, E.coli, Klebsiella and Staphylococcus aureus using well diffusion method as described earlier. An inhibition zone was observed only for E,coli. The normal growth and development was suppressed by Adenanthera pavonina amylase inhibitor.
Effect of Temperature and pH on Mucuna Amylase Inhibitor
The velocity of the inhibition reaction for amylase inhibitor of Adenanthera pavonina was determined at different temperature ranging between 4°C to 100°C. The optimum temperature obtained from the graph was 45°C. Adenanthera pavonina amylase inhibitor was stable between temperatures 4°C to 65°C.The inhibitor activities of the amylase inhibitor in the different buffers were determined with amylase as enzyme. The optimum pH obtained from the graph was 6.2. The Adenanthera pavonina amylase inhibitor was stable between pH 2.0 to 10.
Inhibition Studies
Adenanthera pavonina amylase inhibitor inhibited both salivary amylase and the growth of streptococcus (Fig.4). The results indicate that amylase inhibitor from the seeds of Adenanthera pavonina is potent antimicrobial agent and inhibition of mammalian amylases suggests its potential in treatment of diabetes.
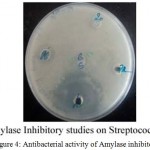 |
Figure 4: Antibacterial activity of Amylase inhibitor |
Conclusion
The α-amylase inhibitor was partially purified from the seeds of Adenanthera pavonina. The partially purified inhibitor showed the molecular weight of 13.6 kDa. Purified amylase inhibitor was found to inhibit the activity of human salivary α-amylase. Inhibitory activity of α-amylase inhibitor against mammalian amylases could suggest its potential in treatment of diabetes.
Acknowledgements
The author would like to thank Prof. K Byrappa, Hon’ble Vice-Chancellor, Mangalore University, Mangalagangothri for research facilities.
Funding source
Nil
Conflict of Interest
I, Dr K S Chandrashekharaiah, corresponding author – representative of submitted research article confirm that I do not have any conflict of interest in the publication of research article.
References
- Ali H., Houghton P. J., Soumyanath A α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. Journal of Ethnopharmacology. 2006;107:449-455.
CrossRef - Andrews P. Estimation of molecular size and molecular weight of biological compounds by gel filtration. Methods in Biochemical Analysis. 1970;18:1-53.
CrossRef - Ali A., Shamkhi A. J., Raghda S. A. Purification and Characterization of Amylase Inhibitor Extracted from White Kidney Bean (Phaseolus vulgaris). Cell & Plant Science. 2012;3(1):17-21.
- Bernfeld P., Amylases A. α and β. Methods in Enzymology. 1955;1(1):149–158.
CrossRef - Brad–Williams W., Cuvelier M. E., Berset C. Use of a radical method to evaluate antioxidant activity. LWT- Food Science and Technology. 1995;28:25-30.
CrossRef - Davis B. J., Ornstein L. Disc Electrophoresis–2, Method and Application to Human Serum Protiens. Annals of the New York Academy of Sciences. 1964;121(2):404-427.
CrossRef - Fossum K., Whitaker J. R. Simple method for detecting amylase inhibitors in biological materials. Journal of Nutrition. 1974;104:930-936.
CrossRef - Franco O. L., Rigden D. J., Melo F. R., Bloch Jr. C., Silva C. P and Grossi-de-Sa M. F. Activity of wheat alpha amylase inhibitors towards bruchid alpha amylases and structural explanation of observed specificities. European Journal of Biochemistry. 2000;267:1466-1473.
CrossRef - Frels J. M., Rupnow J. H. Characterization of two α-amylase inhibitors from black bean (Phaseolus vulgaris). Journal of Food Science. 1985;50:72–78.
CrossRef - Granum P. E. Purification and characterization of α-amylase inhibitor from rye flour. Journal of Food Biochemistry. 1978;2:103–107.
CrossRef - Heidari R., Zareae S., Heidarizadeh M. Extraction, purification and inhibitory effect of α-amylase inhibitor from wheat (Triticum aestivum var. Zarrin). Pakistan Journal of Nutrition. 2005;4:101–105.
CrossRef - Hivrale V. K., Chougule N. P., Giri A. P., Chhabdaa P. J., Kacholea M. S. Biochemical characterisation of α-amylase inhibitors from Achyranthes aspera and their interactions with digestive amylases of coleopteran and lepidopteran insects. Journal of Science of Food and Agriculture. 2011;91:1773–1780.
CrossRef - Ho F.,Whitaker J. F. Purification and partial characterization of white kidney bean α-amylase inhibitor from two experimental cultivars. Journal of Food Biochemistry. 1993;17:15–33.
CrossRef - Iulek J., Franco O. L., Silva M., Slivinski C. T., Bloch J. C., Rigden D. J., Grossi-desa M. F. Purification, biochemical characterization and partial primary structure of a new α-amylase inhibitor from Secale cereale (rye). International Journal of Biochemistry and Cell Biology. 2000;32:1195– 1204.
CrossRef - Kokiladevi E., Manickam A., Thayumanavan B. Characterization of alpha-amylase inhibitor in Vigna sublobata. Botanical Bulletin of Academia Sinica. 2005;46:189–196.
- Lowry O. H., Roseburgh N. J., Farr A. K., Randall R. J. Protein measurements with Folin’s phenol reagent. Journal of Biological Chemistry. 1951;193:265–275.
- Gupta M., Sharma P., Amarjit K. N. Purification of a novel α-amylase inhibitor from local Himalayan bean (Phaseolus vulgaris) seeds with activity towards bruchid pests and human salivary amylase. Journal of Food Science and Technology. 2014;51(7):1286–1293.
CrossRef - Nagi-Gasztonyi M., Nagy A., Nemeth-Szerdahelyi., Pauk J., Gelencser E. The activities of amylases and a-Amylase inhibitor in wide-range herbicide resistant wheat lines. Czech journal of food sciences. 2010;28:217–224.
CrossRef - Octivio L and Rigden D. Activity of wheat amylase inhibitors towards bruchid amylase and structural explanation of observed specificities. European Journal of Biochemistry. 2000;267:2166-2173.
CrossRef - Octavio L and Rigden D. Plant -amylase inhibitors and their interaction with -amylases. Journal of Biochemistry. 2002;269:397-412.
- Power J. R., Whitaker J. R. Purification and some physical and chemical properties of red kidney bean (Phaseolus vulgaris)α-amylase inhibitor. Journal of Food Biochemistry. 1977;1:217–238.
CrossRef - Richardson M. Methods in Biochemistry. 1991;5:259-305.
- Sasikiran K., Rekha M. R., Padmaja G. Protons and alpha amylase inhibitors of sweet potato: Changes during growth phase Sprouting, and wound induced alterations. Botanical Bulletin of Academia Sinica. 2002;43:291-298.
- Shainkin R and Birk Y. D. α-amylase inhibitors from wheat: Isolation and characterization. Biochemica Biophysica Acta. 1970;221:502.
CrossRef - Silano V., Pocchjiari F and Kasarda D. D. Physical characterization of α-amylase inhibitors from wheat. Biochemica Biophysica Acta. 1973;317:139.
CrossRef - Wato S., Kamei K., Arakawa T., Philo J., Wen J., Hara S & Yamaguchi H. A chimera-like a-amylase inhibitor sug- gesting the evolution of Phaseolus vulgaris a-amylase inhibitor. Journal of Biochemistry. 2000;128:139-144.
CrossRef - Wetter L. R. Some properties of lipase present in germinating rapeseeds. Journal of American Oil Chemists Society. 1957;34:66–69.
CrossRef - Yamaguchi H. Isolation and characterization of the subunits of a heat labile a-amylase inhibitor from Phaseolus vulgaris white kidney bean. Bioscience Biotechnology and Biochemistry. 1993;57:297-302.
CrossRef - Yoshikawa H., Kotaru M., Tanaka C., Ikeuchi T., Kwabata M. Characterization of kintoki bean (Phaseolus vulgaris) α-amylase inhibitor: inhibitory activities against human salivary and porcine pancreatic α-amylases and activity changes by proteolytic digestion. Journal of Nutritional Science and Vitaminology. 1999;45:797–802.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





