How to Cite | Publication History | PlumX Article Matrix
Afnan Abdul-Jalil Farhan1, Munazza Gull1 , Sawsan Abdulaziz Rahimuddin1
, Sawsan Abdulaziz Rahimuddin1 , Taha Abdullah Kumosani1, Ahmed Mahmoud Al-Hejin2, Abida Kausar3 and Muhammad Aamer Mehmood4
, Taha Abdullah Kumosani1, Ahmed Mahmoud Al-Hejin2, Abida Kausar3 and Muhammad Aamer Mehmood4
1Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
3Department of Botany, Faculty of Science, Govt: College University (Women) Faisalabad, Pakistan.
4Department of Bioinformatics and Biotechnology, Government College University Faisalabad, 38000, Pakistan.
Corresponding Author E-mail: munagull@hotmail.com
DOI : http://dx.doi.org/10.13005/bbra/2570
ABSTRACT: The need of antibiotics obviate in treated cancer patients when suppression of immune system leads to secondary infections development. The objective of the present study was to evaluate the antibacterial activity and biochemical profiling of various medicinal plants Trigonella foenum-graecum, Ocimum basilicum, Olea europaea, Mentha longifolia and Boswellia sacra against clinical isolates of blood cancer cases. Crude plant extracts in ethanol and methanol were used to test antimicrobial activity through disc diffusion method. Biochemical profiling identified the presence of Gallic acid, parahydroxy benzoic acid, vanillic acid, syringic acid and ferulic acid by high performance liquid chromatography (HPLC). Boswellia sacra showed the maximum antibacterial activity against Streptococcus viridian with 12.4 mm inhibition zone. Trigonella foenum-graecum showed the maximum antibacterial activity against Salmonella Group B 11.8 mm with crude extracts in methanol. The antibacterial activity showed that Streptococcus viridian and Corynebacterium were more inhibited bacteria but Klebsiall pneumonia was found more resistant. Total phenolics analysis by HPLC revealed that parahydroxy benzoic acid was the major phenolic acid found in Olea europaea with 797.8 ng/g. The highest concentration of Gallic acid was found in Ocimum basilicum with 547.02 ng/g. These results indicated that these medicinal plants may serve as antimicrobial agents against clinical bacterial isolates from cancer patient successfully.
KEYWORDS: Antibiotics; HPLC; Leukemia; Plant Extract;Phenolic Acids
Download this article as:| Copy the following to cite this article: Farhan A. A. J, Gull M, Rahimuddin S. A, Kumosani T. A, Al-Hejin A. M, Kausar A, Mehmood M. A. Antimicrobial Activity And Biochemical Profiling Of Selected Medicinal Plants Against Blood Cancer Clinical Isolates. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Farhan A. A. J, Gull M, Rahimuddin S. A, Kumosani T. A, Al-Hejin A. M, Kausar A, Mehmood M. A. Antimicrobial Activity And Biochemical Profiling Of Selected Medicinal Plants Against Blood Cancer Clinical Isolates. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28525 |
Introduction
Cancer is the second leading cause of death worldwide next to cardiovascular diseases. The cancer morbidity and mortality is usually characterized by uncontrolled and abnormal cell growth, invasion of local tissues and distant metastases. Multiple factors such as chemotherapy, radiotherapy, impairment of normal leukocyte function or use of corticosteroids could lead to the immunocompromised conditions in cancer patients.1,2 These patients become highly susceptible to almost any type of infection, especially bacterial and fungal infections. As well as the infections complications remain an important cause of mortality and morbidity between these highly risk and infectious cancer cases.
The heavy antibiotics usage especially in high risk cancer patients cause a selection pressures that result in the emergence of resistant micro-organisms. Currently, many cancer centers have highlighted the fact that an increase in quinolone resistant bacteria (primarily Escherichia coli and Pseudomonas aeruginosa) in patients receiving quinolone prophylaxis. The common use of broad spectrum agents like carbapenems has been linked with the development of multidrug resistant (MDR) in Stenotrophomonas maltophilia and Pseudomonas aeruginosa. Thus, the increase in antibiotic resistance activity is posing an ever-increasing therapeutic problem in cancer patients and the ways by which bacteria overcome drug action are various and multiple, ranging from intrinsic impermeability to acquired resistance.3,4
Rapid spread of resistant clinical isolates in cancer cases indicates the high need to find new antimicrobial agents is of supreme importance for the treatment of infectious diseases. Due to rising of the resistant microorganisms to antibiotics and the price of contemporary allopathic medicines, the scientists are studying various medicinal plants, because of their safety, cost effectiveness and successful therapeutic measure against a wide range of antibiotic resistant microorganisms.5,6,7
A huge section of the world population depends on the traditional systems of medicine to treat a range of diseases. Researchers are increasingly turning their concentration to the medicinal plants. Medicinal plants occupy important position in conventional and modern system of medicines due to their low toxicity for both human and animals. The key benefits of using plant derived medicines are that they are relatively safer than synthetic alternatives, offering valuable therapeutic measures and more affordable treatments.8,9 The scarcity of infective diseases in wild plants is in itself a sign of the successful defense mechanism developed by them.
Plants develop defense mechanisms against diseases due to the presence of a large number of alkaloids, phenols, terpenes derivatives compounds and other antimicrobial compounds makes the essential oils from plants more précised in their mode action against the wide variety of pathogenic microorganisms. Hence, the essential oils could be used as better supplements or alternatives against the pathogenic microorganisms.10 Keeping in view, the present study was designed to assess and compare the in vitro antimicrobial activity of medicinal plants used in Saudi Arabia against clinical bacterial isolates of human blood cancer cases and biochemical profiling through qualitative and quantitative analysis of phenolic acids from these medicinal plants by High performance liquid chromatography (HPLC).
Materials and Methods
Selection of Medicinal Plants for This Study
Five medicinal plants were collected from different regions of Saudi Arabia for this study (Table 1). The collected plants were identified in the laboratory by comparison of flora which made according to different references concerning with the medicinal plants (Royal Botanic Gardens Victoria, Indiana Native Plant and Wildflower Society).
Table 1: Medicinal Plants
| Plant / Part Used | Place | Time of Collection |
| Resin of Boswellia sacra | Jeddah, Yemen origin | 2015 |
| Leaves of Ocimum basilicum | Hail City | 2015 |
| Leaves of Mentha longifolia | Hail City | 2015 |
| Seeds of Trigonella foenum-graecum | Al-Qassim Region | 2015 |
| Leaves of Olea europaea | Jeddah, Syria origin | 2015 |
Microorganisms
Fourty Clinical slants samples were obtained from patients with infection were received from the radiotherapy unit of King Abdul Aziz Medical city, Jeddah, Saudi Arabia. These bacterial cultures were isolated from different blood cancer patients undergoing radiotherapy treatment. All the microorganisms were identified using Analytical profile index (API) system with biomerieux vitek 2. Bacterial strains Salmonella group B, Corynebacterium, Staphylococcus aureus, Klebsiall pneumonia and Streptococcus viridian were chosen for further study on the basis of preliminary analysis.
Preparation of Plants Crude Extract
Dried plants powder (25mg) was taken in 100 ml of 99.8% methanol in a conical flask, plugged with sterile cotton. The samples were placed for 24 hours in shaker with 37oC and for another 24 hours in room temperature.11 The extracts were then filtered using Whatman No. 1 filter paper. The extracts were evaporated using rotary vacuum evaporator to dryness under reduced pressure at 60oC by a rotary evaporator. After vacuum evaporation, the plant extracts were dissolve in 0.25% Dimethyl Sulphoxide (DMSO), which is maximum volume of DMSO that could be used to dissolve solid extracts, and stored at 4oC for further use.12 The solvent DMSO (2.5%) that would not inhibit growth of the microorganisms was used as the negative control for all the experiments.13
Preparation of Inoculum
Stock cultures were maintained at 4oC on nutrient agar slants. Nutrient agar slants that contained peptone (5.0 g), yeast extract (2.0 g), meat extract (1.0 g), NaCl (5.0 g), agar (15 g), pH (7.0), and distilled water (1 liter) were used to culture bacterial strains. The final inoculum of all studied organisms was 104 CFU mL−1 (colony forming units per mL).
Standard Antibiotic Activity Assay
Standard antibiotic discs (Ciprofloxacin) were placed on the surface of a Mueller-Hinton agar that has been inoculated with test microorganisms. During incubation, the antibiotics diffuse outward from the discs creating a concentration gradient which appeared as inhibition zone around these disks. Inhibition zone diameters (mm) were read after 18 h of incubation at 35oC.
Antimicrobial Activity of Plant Extracts by Paper Disk Diffusion Assay
A suspension of testing microorganisms was spread on Muller Hinton Agar (MHA) medium. The sterile filter paper discs (5mm in diameter) was placed on the agar plates which was inoculated with the tested microorganisms and then impregnating with 800-1000μl of plant extract were made in the agar plate with forceps then plates were incubated at 37oC for 18-24 hours. The antibacterial activity of the plant extract was determined by measuring the radius from the middle (center) of the disc to the edge of the zone. The antibacterial assay for each of the extracts against all microorganisms tested was performed in triplicates. The experimental details described earlier were follows to perform antimicrobial activity tests.14
Biochemical profiling of phenolic acids by High Performance Liquid Chromatography (HPLC)
Plant Extraction
The extraction of the phenolic acids from the seeds of Fenugreek, leaves of basil, leaves of olive, leaves of mint and seeds of Frankincense were determined according to earlier described method15,16 with some modification. The fresh leaves (200 g) of plant materials were shade dried and crushed to coarse powder. The powder (20 g) was macerated with 25 ml distilled water of 2 N-HCl and heated in water bath for 1 h at 100oC using air condenser and filtered. The filtrate was extracted with diethyl ether using separating funnel. The diethyl ether layer was washed with distilled water, dried over anhydrous sodium sulphate and evaporated using rotary vacuum evaporator at 25oC to obtain extract. The collected extract was re-dissolved in known amount of (5 ml) HPLC grade methanol. Prior to the injection into HPLC column the sample was filtered through 0.22μm organic filter (Millipore).
Phenolic Acids Analysis
The qualitative and quantitative analysis of phenolic acids were performed by reverse phase high performance liquid chromatography(RP-HPLC) under following conditions: Apparatus: HPLC-Beckman model-322 equipped with 100 A model pump, 420 controllers, mixer, 210 injector and BD-40 recorder, Column: Ultrasphere C18 column 5m, (25 cm x 4.6 mm length), Mobile phase: Methanol: Water (1% acetic acid in 20: 80 v/v); the mobile phase was degassed prior to use in HPLC, Flow rate 1 ml min-1, Chart speed 1 cm min-1, UV Detector, λ max 254 nm, 0.02 aufs (Absorbance Units Full Scale) Attenuation, isocratic mode.
Gallic acid, Parahydroxy benzoic acid, Vanillic acid, Syringic acid and Ferulic acid were analyzed in this study and the detector response for individual phenolic compound was calibrated with authentic standard phenolic acids. All the standard phenolics were procured from Sigma-Aldrich Chemical Company, USA.
The statistical significance of the antibacterial tests was determined. All experiments were conducted in triplicate and the statistical test that generate mean values was used. The resulting data is presented in tabular form. For statistical analysis, OriginLab Origin Pro (version 9.0) was used and the P value < 0 .05 was considered as significant.
Results and Discussion
Evaluation of Antimicrobial Potential of selected Medicinal Plants
Antimicrobial activity of all five medicinal plant extracts in methanol and ethanol are presented in Table 2.
Table 2: Antimicrobial activity of extracts of five medicinal plants against clinical isolates of leukemia cases
| Microbes = zone of Inhibition | Antibiotic | ||||||||||
| Plants crude extracts | Salmonella B | Corynibacterium | Staphylococcus aureus | Klebsiall pneumonia | Streptococcus viridian | Ciprofloxacin (5mg/disc) | |||||
| E | M | E | M | E | M | E | M | E | M | ||
| Boswellia sacra | 4.6 ±1.4 | 9.75 ±1.5 | 4.0 ±1.6 | 9.6 ±2.6 | 7.75 ±0.6 | 6.5 ±1.3 | 4.0 ±2.0 | 4.0 ±1.0 | 4.0 ±0.9 | 12.4 ±2.6 | 9.0 |
| Ocimum basilicum | 1.76 ±0.25 | ND | 1.9 ±0.75 | 3.0 ±0.8 | 1.3 ±0.57 | ND | 2.4 ±0.5 | 1.5 ±0.5 | ND | 1.0 ±0.2 | 9.0 |
| Mentha longifolia | 2.0 ±1.0 | 2.5 ±1.0 | 4.0 ±1.0 | 3.3 ±1.2 | 7.0 ±1.6 | ND | 3.6 ±0.5 | 1.0 ±0.2 | 3.25 ±1.2 | 3.6 ±0.5 | 10.0 |
| Trigonella foenum-graecum | 2.3 ±0.5 | 11.8 ±2.0 | 4.0 ±1.6 | 11.5 ±1.2 | 5.8 ±1.6 | 6.0 ±0.2 | 5.3 ±1.4 | 1.0 ±0 | 6.4 ±1.2 | 10.0 ±1.6 | 6.0 |
| Olea europaea | 2.0 ±0.7 | 1.0 ±0.9 | 4.0 ±0.9 | 3.0 ±0.4 | 3.0 ±0.9 | 1.5 ±0.5 | 1.75 ±0.4 | 1.0 ±0 | 9.0 ±1.0 | 4 ±0.5 | 7.0 |
The value represent zone of inhibition (mm) Mean ± Standard Error. 800mg of extract for all discs. (E) Ethanol, (M) Methanol, ND: No inhibition zone, Ciprofloxacin was used as positive control.
The methanol extracts of Boswellia sacra, Trigonella foenum-graecum and Ocimum basilicum presented higher activities than ethanol extracts i. e., Boswellia sacra showed the maximum antibacterial activity against Streptococcus viridian with 12.4 mm inhibition zone. Trigonella foenum-graecum showed the maximum antibacterial activity against Salmonella Group B 11.8 mm with crude extracts in methanol. The highest effect of ethanol extract was found against Staphylococcus aureus with 7.75 mm inhibition zone. The largest inhibition zones of Trigonella foenum-graecum were observed for methanol extract against Corynibacteria 11.5 mm (Figure 1) and Salmonella Group B 11.8 mm (Figure 2).
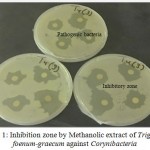 |
Figure 1: Inhibition zone by Methanolic extract of Trigonella foenum-graecum against Corynibacteria
|
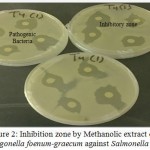 |
Figure 2: Inhibition zone by Methanolic extract of Trigonella foenum-graecum against Salmonella B
|
The largest inhibition zones (3mm) of Ocimum basilicum were observed for methanol extract against Corynibacteria. On the other hand, the ethanolic extract of Mentha longifolia and Olea europaea showed higher activities than methanolic extract. The largest inhibition zones of Mentha longifolia were observed for ethanol extract against Staphylococcus aureus 7mm and Corynibacteria 4mm. The highest effect of Methanol extract against Streptococcus viridian 3.6mm. While, the largest inhibition zones of Olea europaea were observed for ethanol extract against Corynibacteria 4 mm and Staphylococcus aureus 9mm. The highest effect of methanol extract against Streptococcus viridian 4mm and Corynibacteria 3 mm.
The antimicrobial effect of the medicinal plants is well documented. The results of different studies provide evidence that some medicinal plants might indeed be potential sources of new antibacterial agents even against some antibiotic-resistant strains.17,18 The antimicrobial effect of three species of Boswellia against eleven different bacterial strains including Corynibacterium, Staphylococcus aureus, Salmonella typhi and Klebsiella pneumoniae were studied previously and they found that the antibacterial activity mainly against the Gram-positive bacteria.19 They observed the same interesting fact that the multi-resistant staphylococcus strains showed great sensitivity to investigated plant extract supporting the results of this study. Highest antimicrobial activity against Salmonella, Klebsiall pneumonia and Staphylococcus aureus with essential oil of Mentha longifoli was recorded supporting the data of this study as described earlier.20 Trigonella foenum-graecum was giving inhibition zone in the range of 2.3 – 6.4 mm for ethanol and 1.0 – 11.8 mm for methanol crude extracts. Trigonella foenum-graecum crude extract of methanol was found very effective compared to the antimicrobial effect of Ciprofloxacin (6 mm) against Gram negative (Salmonella B) and Gram positive (Corynibacterium and Streptococcus viridian) studied the antibacterial effect of Olea europaea with number of solvent include water, ethanol, methanol, Ethyl acetate, n-Hexane, n-Hexane and Diethyl ether and it recorded antibacterial effect of Olea europaea leaves against Klebsiella pneumonia, Salmonella and Staphylococcus aureus while, no effect on Salmonella was recorded.21 All plants in this study (Boswellia sacra, Ocimum basilicum L, Mentha longifolia L., Trigonella foenum-graecum L and Olea europaea L) showed antimicrobial activity against different types of bacteria but the differences may be probably due to different environmental and genetic factors, different chemotypes and the nutritional status of the plants as well as other factors that can influence the oil composition.22
Qualitative and Quantitative Analysis of Phenolic Acids by HPLC
Qualitative and quantitative analysis of phenolic acids from five medicinal plants by HPLC are presented in Table 3 accordingly. Gallic acid and Ferulic acid were detected in all tested plants with different concentration. The highest concentration of gallic acid and ferulic acid were found in Ocimum basilicum 547.02 ng/g and 236.75 ng/g, respectively. Parahydroxy benzoic acid was found in all tested plant except Boswellia sacra and the highest concentration was detected in Olea europaea 797.8 ng/g. While, the highest concentration of vanillic acid and syringic acid were found in Mentha longifolia174.02 ng/g and 21.76 ng/g, respectively.
Table 3: Quantitative analysis of phenolic acids by HPLC found in various medicinal plants
| Plants crude extracts | Gallic acid (ng/g) | Parahydroxy benzoic acid (ng/g) | Vanillic acid (ng/g) | Syringic acid (ng/g) | Ferulic acid (ng/g) |
| Boswellia sacra | 138.82 | ND | ND | ND | 10.14 |
| Ocimum basilicum | 547.02 | 24.27 | ND | ND | 236.75 |
| Mentha longifolia | 311.72 | 367.98 | 174.02 | 21.76 | 48.92 |
| Trigonella foenum-graecum | 244.87 | 65.73 | ND | 21.52 | 129.57 |
| Olea europaea | 397.91 | 797.8 | 140.42 | ND | 30.45 |
ND: Not detected
Phenolic acids have important biological and pharmacological properties and may be benefit for human health. From ages, these biochemical compounds are important components of the human food, due to their antioxidant activity, their potential to reduce oxidative stress, preventing tissue damage and resultant chronic diseases particularly for anticancer activities.23,24 Gallic acid and Ferulic acid were also identified and quantified in ethanol extract previously.25 The crude extracts of Mentha longifolia and Mentha spicata contained gallic acid compounds while those extracts differed in their content of other studied phenolic compounds.26 In the ethanol extract of Mentha longifolia, Ferulic acids were identified in the aerial parts extract, but they were in very low concentration to be quantified.27,28 Analysis of data by HPLC recorded that pararhydroxy benzoic acid was the phenolic acid with concentration 797.8 ng/g followed by gallic acid 397.91 ng/g in Olea europaea crude extract. The presence of phenolic compounds in olive leaves including phenols (tyrosol, hydroxytyrosol, vanillin, vanillic acid, and caffeic acid) and oleuropein were reported.29 In olive fruit, Vanillic acid, Syringic acid, Gallic acid and Ferulic acid were reported earlier.30,31 Phenolic acids are quite susceptible to degradation under environmental stress (pH, temperature, light and oxygen) that explain the differences between the studies.32,33,34
Conclusion
The antibacterial activity showed that Streptococcus viridian and Corynebacterium were more inhibited bacteria but Klebsiall pneumonia was found more resistant.Boswellia sacra and Trigonella foenum-graecum showed great antimicrobial potential against clinical bacterial isolates from cancer patients. Total phenolic analysis by HPLC revealed that Gallic acid and Parahydroxy benzoic acid were the major phenolic acid found among all the tested medicinal plants and may serve as antimicrobial agent against clinical bacterial isolates from cancer patients successfully. Based on the findings of this study it could be recommended that the extracts of these plants should be further analyzed to isolate the specific antibacterial compounds and defense mechanisms in them and speedy clinical trials should be carried out to explore the pharmaceutical potential of medicinal plants in the treatment of bacterial and fungal infectious diseases.
Acknowledgements
The authors acknowledge, Microbial biotechnology Lab, King Fahd center for Medical Research, King Abdulaziz University, Jeddah, Saudi Arabia for providing all facilities/equipments required.
Competing Interest
It is stated that there is no conflict of interests regarding the publication of this paper among authors and there is no conflict of interest on the side of the reviewers also.
Funding sources
Financial support of this research work was partly provided by King Abdulaziz University, Jeddah, Saudi Arabia, Project No. (54-37-TA) R&D.
References
- Klastersky J, Paesmans M, Rubenstein E. The MASCC Risk Index: a multinational scoring system to predict low-risk febrile neutropenic cancer patients. Clin. Onco. 2000;18:3038-3051.
CrossRef - Panghal M, Kaushal V, Yadav J.P. In vitro antimicrobial activity of ten medicinal plants against clinical isolates of oral cancer cases. Clin. Micro. Antimicrob. 2011;10:1-11.
CrossRef - Sher H, Hussain F. Ethnobotanical evaluation of some plant resources in Northern part of Pakistan. Afri J. Biotech. 2009;8:4066-4076.
- Akthar M.S, Degaga B, Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms. Sci. Pharma. Res. 2014;2:001-007.
- Al-Ali K, Abdelrazik M, Alghaithy A, Diab A, El-Beshbishy H, Baghdadi H. Antimutagenic and anticancer activity of Al Madinah Alhasawy mint (Mentha longifolia) leaves extract. J. Biol. Sci. 2014;17:1231-1236.
CrossRef - Amzazi S, Ghoulami S, Bakri Y, Idrissi A. I, Fkih-Tétouani S and Benjouad A. Human immunodeficiency virus type 1 inhibitory activity of Mentha longifolia. Therapie. 2003;58:531-534.
CrossRef - Akthar M. S, Degaga B and Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms. Sci. Pharma. Res. 2014;2:001-007.
- Adams-Haduch J.M, Potoski B.A, Sidjabat H.E, Paterson D.L, Doi Y. Activity of Temocillin against KPC-producing Klebsiella pneumoniae and Escherichia coli. Agents. Chemo. 2009;53:2700-2701.
- Arun P, Purushotham K.G, Jayarani J.J, Kumari V. In vitro antibacterial activity and flavonoid contents of Lawsonia inermis (Henna). J. Pharm Tech. Res. 2010;2:1178-1181.
- Hussain A.I, Anwar F, Sherazi HS.T. Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Chem. 2008; 108:986-985.
- Doss A, Pugalenthi M. Evalution of antioxidant activity and phythochemical screening of Malus domestica borkh (apple) and Phaseolus vulgaris (green beans). J. Pharma.scie. inno. 2012;1:1-4.
- Dineshbabu J, Srinivasan P, Manimekalai K, Guna G.D, Darsini T.P. Uses of traditional medicinal plants against the biofilm forming Streptococcus pyogenes isolated from upper respiratory tract. J. Pharma. Bioscie. 2015;6:464-479.
- Zgoda J.R, Porter J.A. Convenient microdilution methods for screening natural products against bacteria and fungi. Biol. 2001;39:221-225.
CrossRef - Kumara M, Agarwala R, Deyb K, Raib V, Johnsonc B. Antimicrobial activity of aqueous extract of Terminalia chebula on Gram positive and Gram negative microorganisms. Int. J. Curr. Pharma. Res. 2009;1:56-60.
- Tandon S, Sand N.K, Pant A.K, Ram B. Evaluation of phenolic acids from some plants of family Asteraceae. 2001;25:30-31.
- Joshi R.K. Qualitative analysis of phenolic constituents from leaves of Anaphalis contorta. J. Nat. Prod. Res. 2011;1:23-25.
- Dubey N.K, Kumar R, Tripathi P. Global promotion of herbal medicines. India’s opportunity. Scie. 2004;86:37-41.
- Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of turkish Artemisia absinthium, Artemisia dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. Agri. Food chem. 2005;53:9452-9458.
CrossRef - Hasson S.S, Al-Balushi M.S, Sallam T.A, Idris M.A, Habbal O, Al-Jabri A.A. In vitro antibacterial activity of three medicinal plants-Boswellia (Luban) Species. Paci. J.Tropi. Biomed. 2011;1:178-182.
CrossRef - Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Ozkan H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia ssp. Longifolia. Food chem. 2007;103:1449-1456.
CrossRef - Hussain A, Qarshi I. A, Liaqat R, Akhtar S, Aziz I. R. U. M, Ullah I, Shinwari Z. K. Antimicrobial potential of leaf and fruit extracts and oils of wild and cultivated edible olive. J. Bot. 2014;46:1463-1468.
- Özcan M, Chalchat J.C. Essential oil composition of Ocimum basilicum and Ocimum minimum L. in turkey. Czech. J. Food Scie. 2002:20:223-228.
- Harri C. S, Mo F, Migahed L, Chepelev L, Haddad P. S, Wright J. S, Willmore W.G, Arnason J.T, Bennett S. A. Plant phenolics regulate neoplastic cell growth and survival: a quantitative structure-activity and biochemical analysis. J. Physio. Pharmaco. 2007;85:1124-1138.
CrossRef - Laura A, Alvarez-Parrilla E, Gonzalez-Aguilar G. A. (Eds.), Fruit and vegetable phytochemicals: Chemistry, nutritional value and stability, John Wiley & Sons, ISBN: 978-0-8138-0320-3,DOI: 1002/9780813809397. 2009;384.
- Juliani H. R, Simon J.E. Antioxidant activity of basil. new crops. new use. 2002;5:575-579.
- Vlase L, Benedec D, Hanganu D, Damian G, Csillag I, Sevastre B, Tilea I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for. Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. 2014;19:5490-5507.
- Al-Mandeel F. A. Investigation and chromatographic separation of some phenolic compounds from flowers of Mentha longifolia and Mentha spicata L. growing in Iraq. Int. Ayurved. Med. 2013;4:352-360.
- Benedec D, Vlase L, Oniga I, Mot A.C, Silaghi-Dumitrescu R, Hanganu D, Tiperciuc B, Crişan G. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of. Mentha. 2013;61: 262-267.
- Rababah T.M, Ereifej K.I, Esoh R.B, Al-u’datt M.H, Alrababah M.A, Yang W. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Prod. Res. 2011;25:596-605.
CrossRef - Sedef N.E.l, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Rev. 2009;67:632-638.
- Kountouri A.M, Mylona A, Kaliora A.C, Andrikopoulos N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. 2007;14:659-667.
- Viola P, Viola M. Virgin olive oil as a fundamental nutritional component and skin protector. Dermatol. 2009;27:159-165.
CrossRef - Bianco A, Buiarelli F, Cartoni G, Coccioli F, Muzzalupo I, Polidori A, Uccella N. Anlaysis by HPLC-MS/MS of biophenolic components in olives and oils. Analytical letters. 2001;34:1033-1051.
CrossRef - Friedman M, Jürgens H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agri. Food Chem. 2000;48:2101-2110.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





