How to Cite | Publication History | PlumX Article Matrix
Characterization of Antibacterial Lactic Acid Bacteria Isolated from Moroccan Fermented Olives.
Kaoutar El-Issaoui1 , Sanae Zinebi1, Jamal Abrini1, Rajae Zahli1, Nadia Amajoud1,2, Nadia Skali Senhaji1 and El-Ouardy Khay1
, Sanae Zinebi1, Jamal Abrini1, Rajae Zahli1, Nadia Amajoud1,2, Nadia Skali Senhaji1 and El-Ouardy Khay1
1Laboratory of Biology and Health. Department of Biology. Faculty of Sciences. BP: 2121. Abdelmalek Essaadi University, Tetouan (93002). Morocco.
1,2Laboratory of bacteriological analysis of water and foodstuffs of the urban commune of Tetouan, Faculty of Sciences M'hannech 2 (93002) Tetouan. Morocco.
Corresponding Author E-mail: issaoui.kaoutar@hotmail.fr
DOI : http://dx.doi.org/10.13005/bbra/2575
ABSTRACT: The research for antibacterial activities of lactic acid bacteria isolated from Moroccan table olives, revealed 15 bacterial strains having an inhibitory activity against pathogenic germs: Listeria monocytogenes, Listeria innocua and Staphylococcus aureus; as well as Gram-negative germs, such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and others. From 127 samples of table olives, 143 bacterial strains with antibacterial effect were isolated by the double layer method, 15 strains were retained. All are Gram positive, catalase negative and non-sporulating. Cocci constitute 66.33% of the total effective. The remaining 33.33% are represented by bacilli/Ovoid. The lactic acid bacteria retained have a greater inhibitory effect against Gram positive and Gram negative bacteria. Listeria monocytogenes CECT 4032 and Staphylococcus aureus MBLA were inhibited by all the lactic strains studied. The smallest inhibition zones were recorded for the two isolates LB15 and LB96 against E. coli 87739, with diameter less than 10 mm.
KEYWORDS: Antibacterial Activity Bacteriocins; Lactic Acid Bacteria;Tables Olives;
Download this article as:| Copy the following to cite this article: El-Issaoui K, Zinebi S, Abrini J, Zahli R, Amajoud N, Senhaji N. S, Khay E. Characterization of Antibacterial Lactic Acid Bacteria Isolated from Moroccan Fermented Olives. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: El-Issaoui K, Zinebi S, Abrini J, Zahli R, Amajoud N, Senhaji N. S, Khay E. Characterization of Antibacterial Lactic Acid Bacteria Isolated from Moroccan Fermented Olives. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28461 |
Introduction
Table olive is obtained by lactic fermentation, which consists in promoting the development of lactic acid bacteria (LAB). These bacteria acidify gradually the medium culture by producing lactic acid, which prevents the proliferation of other microorganisms, in particular pathogenic or undesirable microorganisms.
Lactic acid bacteria as probiotics, witch when administered in adequate amounts, confer a health benefit on the host (FAO/WHO., 2006). They are present in many fermented and unfermented foods, and they produce a great diversity of bacteriocins whose nisin is the most widely used as a food preservative.
The bacteriocins produced by LAB have attracted increasing attention because they are active in a nanomolar range and have no toxicity. They are defined as protein-like molecules produced by lactic acid bacteria, which inhibit the growth of other bacteria, especially pathogenic bacteria such as Listeria monocytogenes (Klaenhammer, 1988).
In addition to the synthetic pathway and the concentration required for inhibitory activity, bacteriocins differ from antibiotics in that they have a relatively narrow spectrum of action and bactericidal activity is directed against taxonomically close strains of the producing bacterium (Dobson et al., 2012).
Bacterial fermentation of perishable raw materials has been used for centuries to preserve the nutritional value of foods and beverages, a long period of time (Deegan et al., 2006). In recent years, bio-preservation systems such as bacteriocinogenic cultures and/or their bacteriocins have attracted increasing attention, and new approaches to the control of pathogenic microorganisms have been developed (Ross et al., 1999).
Bacteriocins produced by LAB are of particular interest to the food industry since these bacteria have generally been considered safe.The interest in LAB occurring in foods is primarily due to the biotechnological potential of new bacterial species and strains. In the present study we selected table olives as a potential source of new species or strains of LAB because table olives represent a fermented product that supports the dominance of LAB.
This study aimed to isolate and characterize LAB from fermented olives of Morocco and to evaluate their biotechnological properties.
Material and Methods
Isolation of LAB Strains for Their Antibacterial Activity
143 bacterial strains with antibacterial effects were isolated from 127 samples of Moroccan table olives by the double layer method.
The isolation was carried out on De Man Rogosa and Sharpe (MRS) agar (Biokar Diagnostics, Beauvais, France) (De Man et al., 1960). Temperature incubation was 30°C for 24h. The well isolated colonies were picked up and transferred to two plates of MRS agar. After incubation for 24h at 30°C, one plate is stored at + 4°C and the other is used to detect the antibacterial activity of the isolates. Thus, the latter was overlaid with 5 ml of soft Brain Heart Infusion (HiMedia Laboratories, Mumbai, India) inoculated with the target strain (Listeria monocytogenesCECT 4032). After 18 to 24 hours of incubation at 30°C, the antibacterial activity was detected by the appearance of a clear halo around the colonies.
Physiological and Biochemical Characterization
Microscopic examination (form, grouping and mobility of cells), Gram staining, catalase test and sporulation were performed. The metabolism of gas, acetoin, gelatin and sugars was also carried out (Okada et al., 1992; Abriouel et al., 2012).
Only the Gram positive, catalase negative non-motile and non-sporulating strains were retained.
Preparation of Cell free Culture Supernatants
LAB isolates were grown in MRS broth at 30oC for 24h. The cultures broth obtained were used for the preparation of culture free supernatants (CFS) for assays. The 24h old cultures were centrifuged at 8 000g for 20 min by using a centrifuge (Model Hettich universal 230) and then the supernatants were adjusted to pH 6.5 with 1N NaOH in order to rule out possible inhibition effects due to organic acids.
Antibacterial Activity Spectrum of LAB Isolates
Indicator strains used for assessment of antimicrobial activity of the selected LAB isolates were; six Gram positive (Listeria monocytogenes CECT 4032, Staphylococcus aureus 25983 ATCC, Staphylococcus aureus 43300 ATCC, Staphylococcus aureus 3920 ATCC, Staphylococcus aureus MBLA and Staphylococcus epidermidis ATCC 12228), and seven Gram negative (Escherichia coli 87739 ATTC, Escherichia coli 25922 ATTC, Escherichia coli MBLA K12, Klebsiella pneumonia ATCC 13883, Proteus mirabilis (Institut d’hygiène Rabat, Maroc), Salmonella typhimirium ATCC 14028 and Enterococcus feacium CECT 410).
Several methods for detecting the antibacterial activity of bacteriocin-producing strains have been described. However, the agar well diffusion assay (AWDA) remains the most common methods. It based on diffusion of the antibacterial substance through solid or semi-solid culture media previously inoculated with an indicator strain.
In the present investigation, the antibacterial activity of LAB isolates was performed by AWDA on Mueller Hinton Agar (MHA, Biokar Diagnostics, Beauvais, France) plates overlaid with soft BHI agar inoculated with overnight cultures of target strains. A blunt end of a sterile Pasteur pipette was used to create 4 wells with diameters of 6 mm into the MHA plate. 3 wells were filled with 100 μl of CFS sample adjusted to pH 6.5 with 1N NaOH as mentioned above. The fourth well was filled with 100 μl sterile distilled water to serve as a negative control. The plates were pre-incubated at 4°C for 4 h to allow uniform diffusion into the agar and then incubated at 30°C for 24h. The antibacterial activity was expressed in millimeters by measuring the diameter of clear zone around the wells.
Confirmation of Bacteriocins
Cell free culture supernatant from LAB strains was adjusted to pH 6.5 with 1N NaOH. To eliminate the effect of hydrogen peroxide, catalase enzyme was added to the supernatant at a final concentration of 1 mg/mL. The mixture was filtered through a 0.22 μm pore size filter followed by incubation at 25°C for 2 h and the residual activity was examined by the AWDA as described above.
Effect of Temperature on Activity of Antibacterial Substances
The CFS samples adjusted at pH 6.5 and treated by catalase as described above were heated at different range of 60, 80 and 100°C each for 30 min and autoclaved at 121°C for 15 min. The heat treated CFS samples were then assayed for antimicrobial activity by AWDA against L. monocytogenes. pH adjusted and H2O2 eliminated CFSs without any heat treatments served as a controls. Residual antimicrobial activity of heat-treated CFS was expressed on percent of inhibition zone size (mm) compared to the control (100% at 30°C for 30 min) using L. innocua CECT 4030 as the indicator bacteria.
Location of the Inhibitory Substances
By the AWDA described above, the intra and extracellular fraction represented respectively by the pellet and the culture supernatant of each retained LAB strain were tested against L. innocua CECT 4030.
Measurement of Arbitrary unit of Antimicrobial Activity
Antimicrobial activity was determined essentially by the AWDA. On the surface of the MHA medium, 5 ml of semi-solid BHI medium inoculated with 100 μl of an overnight culture of L. monocytogenes CECT 4032, were poured. After solidification of the surface layer, wells were created into the agar plate as mentioned above. A serial two-fold dilution of neutralized supernatant of selected LAB isolate was carried out. 100μl of each dilution were used to fill the wells. The plate was pre-incubated at 4°C for 2h and then incubated at 37°C for 24h. Antibacterial activity was defined as the reciprocal of the dilution that resulted in an inhibition zone and expressed as arbitrary units (AU) per milliliter.
The Growth Kinetics of Selected LAB Strains
Bacterial growth was monitored by optical density (OD) measurements as a function of time. In parallel, the pH of the culture was determined.
Overnight culture of each selected LAB isolates was inoculated (2%, v/v) into100 ml of MRS broth. Each lactic strain was grown for three days at 25°C, 30°C and 37°C. For each bacterial culture, sterile samples were taken at regular time intervals and the pH was determined by using a pH meter (Model PHSJ-3F). In parallel, 1ml of each culture was centrifuged at 8000 g for 20 min at 4oC, the pellet was watched twice, re-suspended in 1ml of physiological water and the OD of the suspension was measured at 620 nm using a spectrophotometer (Model Rayleigh UV-1800). All samples were in duplicates.
Results
Lactic Acid Bacteria Isolation
143 LAB strains were isolated from 127 samples of fermented olives collected from diverse geographic regions in Morocco. LAB isolates were selected based on their ability to produce antibacterial substances, against L. innocua, on MRS-agar plates using the double-layer method. Only 54 strains exhibited antibacterial activity against indicator bacteria. However, 15 isolates maintained their inhibitory substance production using agar well diffusion assay. These isolates were retained for further tests.
The morphological, physiological and biochemical identification revealed that all retained strains are Gram positive, catalase negative and not producing gas (Table 1). Several remarks can be made as soon as these results are read:
All LAB isolates are immobile, produced no acetoin except LB6, LB8, LB21, LB48 and LB97. They are all the homo-fermentation type, due to the absence of CO2 production.
All the isolates are also able to growing in 6.5% NaCl and are all thermophilic.
Coccoid-shape lactic acid bacteria able to growing in 6.5% NaCl are identified as Enterococci.
LB96 unable to grow at 50°C are identified as Enterococcus faecalis.
Table 1: Morphological, physiological and biochemical characteristics of isolated genera of LAB
| LAB strain | ||||||||||||||||
| LB6 | LB97 | LB98 | LB8 | LB48 | LB62 | LB78 | LB93 | LB21 | LB91 | LB15 | LB96 | LB104 | LB105 | LB114 | ||
| Gram | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Form | bacilli | ovoid | Ovoid | ovoid | ovoid | Cocci | Cocci | Cocci | Cocci | Cocci | Cocci | Cocci | Cocci | Cocci | Cocci | |
| Catalase | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Production | CO2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| VP | + | + | – | – | + | – | – | – | + | – | – | – | – | – | – | |
| Mobility | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Gelatinase | – | – | – | – | – | + | – | – | – | – | – | + | + | + | – | |
| Growth in 6,5% NaCl | – | +/- | +/- | +/- | +/- | + | + | + | + | + | + | + | – | + | + | |
|
Growth at |
45C | + | +/- | +/- | +/- | +/- | + | + | + | + | + | + | + | + | + | + |
| 50C | – | + | + | + | – | + | + | + | + | + | + | – | + | + | + | |
| 7°C | + | – | – | – | – | + | + | + | + | + | + | + | + | + | + | |
| Fermentation of carbohydrate | ||||||||||||||||
| Glucose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Lactose | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Mannose | – | + | + | + | – | – | + | – | + | + | – | + | – | + | + | |
| Dextrose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Sucrose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Arabinose | + | – | + | + | – | + | + | + | – | – | – | – | – | – | + | |
| Fructose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Saccharose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Galactose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Lactobacillus | Leuconostoc | Enterococcus | ||||||||||||||
Antibacterial Activity Spectrum of Selected LAB
The antibacterial activity exhibited by the majority of strains especially toward Gram-negative bacteria may be due to the organic acid effect. For this purpose, the inhibitory effect was checked after supernatant neutralization. By this way, the selected LAB strains retained their antibacterial effect against different Gram-positive and Gram-negative pathogenic bacteria (Table 2). Some isolated strains inhibited almost all pathogenic bacteria tested; but the most susceptibility of them was against Gram-positive pathogens.
Table 2: Antibacterial activity of CFS derived from LAB isolates using Agar Well Diffusion Assay
| LAB strain | |||||||||||||||
| Targeted strain | LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 |
| Listeria monocytogenes CECT 4032 | +++ | +++ | +++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| Staphylococcus epidermidis ATCC 12228 | – | – | ++ | – | ++ | ++ | – | – | – | ++ | – | + | – | ++ | – |
| Staphylococcus aureus 25983 ATCC | ++ | – | ++ | – | ++ | ++ | – | + | – | ++ | – | – | – | ++ | – |
| Staphylococcus aureus 43300 ATCC | – | – | ++ | – | ++ | ++ | – | – | – | ++ | – | – | – | + | – |
| Staphylococcus aureus 3920 ATCC | – | ++ | + | – | ++ | ++ | – | – | – | + | – | – | – | ++ | – |
| Staphylococcus aureus MBLA | + | + | ++ | + | +++ | ++ | + | + | + | + | ++ | + | ++ | + | + |
| Escherichia coli MBLA K12 | – | ++ | + | – | – | + | ++ | ++ | + | + | + | + | + | + | + |
| Proteus mirabilis (Institut d’hygiène Rabat, Maroc) | + | + | + | + | + | + | + | + | ++ | ++ | ++ | ++ | + | + | + |
| EnterococcusfeaciumCECT 410 | – | + | + | – | ++ | + | + | ++ | + | + | + | + | – | + | + |
| Salmonella typhimirium ATCC 14028 | – | – | + | – | ++ | ++ | – | – | – | + | – | – | – | ++ | – |
| Escherichia coli 87739 ATTC | – | – | + | – | – | – | – | – | – | + | – | – | – | – | – |
| Escherichia coli 25922 ATTC | + | ++ | ++ | + | ++ | ++ | – | + | – | ++ | + | – | – | ++ | + |
| Klebsiella pneumoniae ATCC 13883 | + | + | + | + | + | + | + | ++ | ++ | ++ | + | ++ | + | + | + |
+++: ; ++: ; +: ; -:
The results of antagonistic activity of LAB isolates showed that the inhibitory effect differs depending on the targeted bacterium. The isolated strains have a greater inhibitory effect against the growth of three Gram positive indicator bacteria, whose inhibition zones is often exceed 20 mm (Table 2). L. monocytogenes CECT 4032 and S. aureus MBLA were inhibited by all the lactic strains studied. S. aureus ATCC 3920, S. aureus ATCC 3300, S. aureus ATCC 25983 and S. epidermidis ATCC 12228 showed significant sensitivity to LB15, LB48, LB62, LB96 and LB105.
Klebsiella pneumonia ATCC 13883 and Proteus mirabilis showed a sensitive profile in the presence of the 15 lactic strains. E. coli MBLA K12, Enterobacter cloaceae, Salmonella typhimirium ATCC 14028, and E. coli 25922 ATTC were all inhibited by LB15, LB62 and LB96.
The lowest zones of inhibition were shown against E. coli 87739, with a diameter less than 10 mm for both LAB strains LB15 and LB96.
Effect of Incubation Period on the Production of Antibacterial Substances
The production of antibacterial substances at different days was determined by AWDA measuring the zone of inhibition against L. monocytogenes. Antimicrobial metabolites production by the strains was detected at all days of incubation (Table 3).The highest level was obtained after 24h of incubation and then production was declined very slowly over time.
Table 3: Effect of incubation time on the antibacterial substances production expressed by the inhibition diameters (mm).
| Incubation time
at 30 °C |
LAB strain | ||||||||||||||
| LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 | |
| 24h | 21 | 26 | 19 | 25 | 21 | 27 | 24 | 22 | 20 | 19 | 24 | 18 | 23 | 18 | 20 |
| 48h | 20 | 21 | 19 | 21 | 21 | 20 | 24 | 21 | 20 | 17 | 23 | 18 | 19 | 18 | 20 |
| 72h | 20 | 19 | 19 | 21 | 21 | 20 | 24 | 18 | 20 | 17 | 22 | 18 | 12 | 18 | 20 |
| 96h | 15 | 17 | 19 | 21 | 21 | 17 | 24 | 12 | 17 | 11 | 22 | 18 | 12 | 18 | 16 |
The results group the LAB isolates into two categories: the first is that of the strains LB15, LB48, LB78, LB98 and LB105 having a constant inhibition zones over incubation time. The diameters measured are of the order of 19mm, 21mm, 24mm and 18mm respectively. The second category consists of LB6, LB8, LB21, LB62, LB91, LB93, LB96, LB97, LB104 and LB105 having downward inhibitory potency over incubation time. The best inhibition zones were recorded for LB8, LB21 and LB62 with diameters of 26mm, 25mm and 27mm, respectively. After incubation for 96 h, the inhibition zone diameter of LB8 and LB62 was reduced by 10 mm, while that of LB21 was reduced by 4mm (25mm to 21mm).
Confirmation of Bacteriocins
The inhibiting substances produced by isolated LAB strains have undergone several treatments: neutralization of pH, elimination of the effect of hydrogen peroxide, temperature and pH…
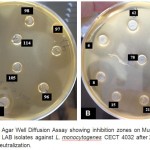 |
Figure 1: A and B: Agar Well Diffusion Assay showing inhibition zones on Mueller Hinton Agar medium (MHA) by LAB isolates against L. monocytogenes CECT 4032 after 24 h of incubation at 30°C after pH neutralization.
|
The antimicrobial activity of the CFSs was determined thrice (i.e before and after neutralization to pH 6.5 and treatment by catalase) using AWDA. The measure of inhibition zone diameters showed that all of the isolates have varying antibacterial activity against L. monocytogenes (Table 4).
It can be seen that pH neutralization doesn’t eliminate the effect of substances produced against Listeria monocytogenes CECT 4032. On the contrary, the inhibition diameters are greater after pH neutralization for the majority of the strains. This suggests that the substances produced are highly active at neutral pH.
Treatment of the supernatant by catalase showed that the antibacterial effect is not due to the production of hydrogen peroxide. Indeed, neutralization of supernatant and addition of catalase tend to increase the inhibition degree (Labioui et al., 2005).
Table 4: Antibacterial activity of treated supernatants against L, monocytogenes CECT 4032 (inhibition zone diameter measured in mm)
| LAB strain | |||||||||||||||
| LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 | |
| Untreated supernatant | 15 | 19 | 19 | 21 | 21 | 19 | 24 | 21 | 20 | 17 | 23 | 17 | 19 | 17 | 20 |
| Supernatant
at pH 6.5 |
21 | 20 | 23 | 22 | 25 | 27 | 24 | 28 | 20 | 24 | 27 | 24 | 26 | 28 | 25 |
| Supernatant treated
with catalase |
21 | 20 | 22 | 21 | 22 | 25 | 24 | 28 | 20 | 23 | 25 | 23 | 25 | 29 | 23 |
Effect of Temperature on Activity of Antibacterial Substances
The residual antibacterial activity of cell free supernatants from LAB isolates after thermal treatments at 60°C, 80°C, 100°C for 30 min and in an autoclave at 121°C for 15 min is presented in Table 5.
Inhibitory substances were found to be thermo-stable and retained their activity up to 121 °C for 15 min while partial loss of activity was noticed with continuous increase in temperature.
Table 5: Effect of temperature on antibacterial substances.
| LAB strain | ||||||||||||||||
| LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 | ||
| 30°C/
30min |
100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| 60°C/
30min |
100% | 92% | 100% | 100% | 93,33% | 100% | 96,15% | 95,65% | 100% | 95,83% | 100% | 100% | 100% | 95,45% | 89,47% | |
| 80°C/
30min |
95,83% | 92% | 95,65% | 95,45% | 93,33% | 95% | 96,15% | 95,65% | 96% | 83,33% | 100% | 100% | 95,65% | 95,45% | 89,47% | |
| 100°C/
30min |
91,66% | 88% | 95,65% | 90,90% | 86,66% | 95% | 96,15% | 91,30% | 96% | 83,33% | 95,83% | 96,29% | 95,65% | 90,90% | 84,21% | |
| 121°C/
15min |
91,66% | 84% | 91,30% | 90,47% | 86,66% | 95% | 96,15% | 86,95% | 88% | 83,33% | 95,83% | 96,29% | 91,30% | 90,90% | 78,94% | |
The antibacterial activity of the isolated strains is more or less stable at 60°C and 80°C.The residual activity was 100% for certain strains and more than 92% for others. Above 100°C, the antibacterial substances are less thermostable and the residual antimicrobial activity decreases to 78%. The substances produced by LB97, LB98, LB58 and LB78are considered to be the most thermostable, they retained more than 95% activity at 121°C for 15 min. However, LB114 produces a less thermostable substance; its residual activity was78.94%.
Location of the Inhibitory Activity
The cell fraction (pellet) had no effect on the growth of L. innocua CECT 4030 while the extracellular fraction corresponding to the supernatant exhibits a strong antibacterial power (Table 6). The result demonstrate that the inhibitory substance is present in the extracellular fraction for all LAB isolates
Table 6: Antibacterial activity of cellular and neutralized extracellular fraction of LAB strains
| LAB strain | |||||||||||||||
| LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 | |
| Supernatant | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Pellet | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
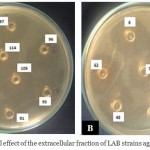 |
Figure 2: Antibacterial effect of the extracellular fraction of LAB strains against Listeria innocua
|
Antibacterial Compounds Production
Table 7 shows the titers of the different substances produced. The most important sub-inhibitory concentration recorded is 640 AU/ml for LB8, LB15 and LB104 while LB91 showed a smaller inhibitory concentration of 80 AU/ml.
Table 7: Arbitrary unit of antimicrobial activity
| LAB strain | |||||||||||||||
| LB6 | LB8 | LB15 | LB21 | LB48 | LB62 | LB78 | LB91 | LB93 | LB96 | LB97 | LB98 | LB104 | LB105 | LB114 | |
| Activity (AU/ml) | 320 | 640 | 640 | 640 | 320 | 160 | 160 | 80 | 160 | 320 | 320 | 160 | 640 | 160 | 320 |
Growth Kinetics
The behavior of the LAB isolates was determined by the kinetics of cell growth and acidification of medium in MRS broth at 25°C, 30°C and 37°C. the result is show in Tables 8, 9 and 10.
At 25°C, the initial pH of the LB6, LB62 and LB21 cultures decreases by approximately 2.4 units after 3 days of incubation (Table 8), followed by LB98, LB15 and LB93 with 2 pH units. Based on the criteria set by Cogan et al. (1997), a fast acidifying strain is able to decrease pH by 1.3 units after the first 6 hours of incubation. At 25°C, LB6 and LB21 are considered to be fast acidifying LAB strain.
Table 8: Growth of isolates at 25°C.
| LAB strain | ||||||||||||||||
| LB 21 | LB 78 | LB 98 | LB 48 | LB 6 | LB 91 | LB 104 | LB 15 | LB 96 | LB 97 | LB 105 | LB 8 | LB 93 | LB 114 | LB 62 | ||
| initial pH | 5.7 | 5.7 | 5.7 | 5.7 | 5.8 | 5.8 | 5.8 | 5.9 | 6 | 6 | 6 | 6.1 | 6.1 | 6.1 | 6.4 | |
| final pH | 3.4 | 4.09 | 3.6 | 4 | 3.4 | 4.12 | 4 | 3.79 | 4.14 | 4.73 | 4.3 | 4.32 | 4.11 | 4.4 | 3.98 | |
|
Optimal values |
O.D | 0.713 | 0.714 | 0.901 | 0.736 | 0.475 | 0.72 | 0.516 | 0.872 | 0.809 | 0.88 | 0.732 | 0.89 | 0.79 | 0.78 | 0.836 |
| pH | 3.4 | 4.12 | 3.69 | 4.07 | 3.58 | 4.12 | 4.1 | 4.05 | 4.25 | 4.29 | 4.34 | 4.45 | 4.24 | 4.4 | 4.06 | |
| incubation time (h) | 72 | 72 | 72 | 48 | 48 | 72 | 48 | 48 | 48 | 72 | 72 | 72 | 48 | 72 | 48 | |
The optical density recorded during the three days differs from the pH of the medium. When the culture pH decreases, the OD increases. Bacterial biomass often reaches 0.7 to 0.8 OD values. The highest OD 0.9 was obtained for LB98. However, the smallest OD values (0.475 and 0.516) were recorded for LB6 and LB104, respectively.
At 30°C, the growth kinetic of LAB strains evaluated by OD and pH measurement is presented in table 9. The data show a gradual decrease in pH for all bacterial cultures studied. LB21 and LB96 showed a fast acidifying activity (6 to 3.14) after 48 h of incubation. However, LB78 was as low acidifying strain with an initial and final pH 5.7 and 4.14, respectively. The final OD values ranged from 0.6 to 0.9. LB96, LB105, LB8 and LB62 showed maximum growth of 0.9. However, low optical densities between 0.5 and 0.6 were recorded for LB6, LB48, LB98 and LB104 after 48 h and 72 h of incubation.
Table 9: Growth of LAB isolates at 30°C.
| LAB strain | ||||||||||||||||
| LB 21 | LB 78 | LB 98 | LB 48 | LB 6 | LB 91 | LB 104 | LB 15 | LB 96 | LB 97 | LB 105 | LB 8 | LB 93 | LB 114 | LB 62 | ||
| initial pH | 5.7 | 5.7 | 5.7 | 5.7 | 5.8 | 5.8 | 5.8 | 5.9 | 6 | 6 | 6 | 6.1 | 6.1 | 6.1 | 6.4 | |
| final pH | 3.8 | 4.14 | 3.56 | 4 | 3.4 | 4.08 | 3.9 | 4.05 | 3.14 | 3.82 | 4.19 | 4.27 | 4.19 | 4.11 | 4 | |
|
Optimal values |
O.D | 0.836 | 0.782 | 0.658 | 0.667 | 0.683 | 0.789 | 0.524 | 0.851 | 0.902 | 0.75 | 0.941 | 0.948 | 0.838 | 0.77 | 0.96 |
| pH | 3.66 | 4.13 | 3.56 | 4.04 | 3.58 | 4.11 | 4.09 | 4.05 | 4.22 | 4.38 | 4.29 | 4.4 | 4.28 | 4.5 | 4.19 | |
| incubation time (h) | 48 | 72 | 72 | 48 | 48 | 48 | 48 | 72 | 48 | 48 | 72 | 72 | 48 | 72 | 48 | |
At 37°C, the evolution of pH and OD of LAB cultures is presented in table 10. It is similar to that observed at 30°C. PH of LB21 culture decreased by 1.4 units after 6 h, LB21 is considered as fast acidifying strain. The biomass production is greater at 37°C than 25°C and 30°C. The OD value varies between 0.8 and 0.9 for all strains studied. However, LB105 reached a maximum biomass of 0.96 after 72 h of incubation.
Table 10: Growth of LAB isolates at 37°C.
| LAB strain | ||||||||||||||||
| LB21 | LB 78 | LB 98 | LB 48 | LB 6 | LB 91 | LB 104 | LB 15 | LB 96 | LB 97 | LB 105 | LB 8 | LB 93 | LB 114 | LB 62 | ||
| initial pH | 5.7 | 5.7 | 5.7 | 5.7 | 5.8 | 5.8 | 5.8 | 5.9 | 6 | 6 | 6 | 6.1 | 6.1 | 6.1 | 6.4 | |
| final pH | 3.36 | 4.04 | 3.6 | 4.1 | 3.39 | 4.05 | 4.05 | 3.6 | 4.13 | 3.51 | 3.89 | 4.16 | 4.04 | 4.14 | 3.86 | |
| Optimal values | O.D | 0.805 | 0.826 | 0.801 | 0.892 | 0.825 | 0.869 | 0.849 | 0.94 | 0.867 | 0.852 | 0.968 | 0.852 | 0.892 | 0.757 | 0.891 |
| pH | 3.41 | 4.11 | 3.6 | 4.11 | 3.52 | 4.19 | 4.21 | 3.62 | 4.15 | 4.16 | 4.12 | 4.21 | 4.14 | 4.25 | 4.31 | |
| incubation time (h) | 72 | 72 | 72 | 48 | 48 | 48 | 48 | 72 | 48 | 48 | 72 | 72 | 48 | 72 | 48 | |
The growth of isolates at the three temperatures studied 25°C, 30°C and 37°C clearly showed that some isolates grow much better at 30°C and/or 37°C. Other isolates have almost the similar profile for the three incubation temperatures. Final pH is always between 3 and 4. However, the time required for maximal growth sometimes exceeds 48 hours and may reaches 72 h.
Depending on their behavior, LAB isolates are classified into two categories, the first grouping those with a high acidification rate during the first 6 hours of incubation time, and the second comprising those with low acidification rate.
For the three incubation temperatures 25°C, 30°C and 37°C, the pH of LB8, LB62, LB105 and LB114 decreased by 0.4 pH units after 5 h of incubation. They are therefore considered as strains with a low rate of acidification (Lairini et al., 2014).
The growth of LB21 as an example of fast acidifying strain and LB8 as a low acidifying strain was studied in MRS broth at 25°C, 30 °C and 37°C. Growth was followed by optical density (OD620) and the production of antibacterial substance was determined against L. innocua by the AWDA and expressed in arbitrary units (AU ml-1) (Figure 3).
 |
Figure 3: Time course of growth and inhibitory substances production by LB8 (a) and LB21 (b) at 25°C, 30°C and 37°C.
|
According to the graphs above, the optimal growth temperature varies between 30°C and 37°C. For the two strains with different acidification rates, maximum growth ranged from 48 h to 72 h. The antibacterial activity is the same for the three temperatures studied, and it’s reduced when the medium pH is less than 3.5.
The study of different characteristics of LAB isolates, mentioned in Table 11, show that LB15, with a high acidifying capacity, and LB8, LB105, LB62, with low acidifying power, produce heat-resistant inhibiting substances. The inhibitory substance titer is important for LB15, LB8. However, it is less important for LB105 and LB62. The strains LB104, LB6, LB21 and LB96 have a high rate of acidification, heat-resistant inhibiting substances and a high arbitrary unit, with the exception of LB96 which shows a small inhibitory substance titer and a high bactericidal power. The strains LB48, LB114 and LB97 with medium acidification rate have a high inhibitory substance titer; Hence, LB48, and LB114 are considered as the best strains inhibiting pathogenic bacteria.
Table 11: Important characteristics of LAB isolates
| LAB strain | |||||||
| LB15 | LB8 | LB104, LB6, LB21 | LB96, LB98 | LB105, LB62, LB78, LB91, LB93 | LB48, LB114 | LB97 | |
| Acidification rate | ++++ | – | ++++ | ++++ | – | + | + |
| Thermoresistance | ++++ | ++++ | ++++ | ++++ | ++++ | + | ++++ |
| Antibacterial activity | ++++ | ++++ | + | ++++ | ++++ | ++++ | + |
| Arbitrary unit of
antimicrobial activity |
++++ | ++++ | ++++ | + | + | ++++ | ++++ |
Discussion
Analysis of 127 samples of Moroccan fermented table olives resulted in the isolation of 143 bacterial strains by the double layer method. 54 strains were gram positive, catalase negative, immobile and non-sporulating. After confirmation of their belonging to lactic acid bacteria, the 54 isolates were purified and then tested for their antibacterial effect against pathogenic bacteria. Only, 15 isolates retained their antibacterial activity using agar well diffusion assay
Based on their biochemical characteristics, the retained strains are homo-fermented because they don’t produce CO2. The genus-specific identification of strains allowed us to classify strains LB8, LB15, LB62, LB104, LB105, LB96 and LB114 as Enterococcus due to their ability to grow in a medium containing 6.5% NaCl. The inability of LB48 and LB96 to grow at 50°C, suggests that they are Enterococcus faecalis. The antibacterial effect of the LAB strains was tested against a range of 13 Gram positive and Gram negative bacteria. The results revealed an important inhibitory effect of isolated bacteria, especially against Gram-positive bacteria.
Indeed, the study showed a potential antibacterial activity, revealed by large inhibition zones against Gram-positive bacteria, notably L. monocytogenes CECT 4032 and S. aureus EMBL. On the other hand, Proteus mirabilis and Klebsiella pneumonia ATCC 13883 were more sensitive than the other Gram-negative bacteria used.
LB15 and LB96 are considered to be the most effective, and have been retained for their bactericidal effect against all the target pathogenic bacteria. LB62, LB105 and LB48 inhibited all indicator strains tested with the exception of E. coli 25922 and E. coli K12 not inhibited by LB48.
Similar results have been reported by Gaamouche et al. (2014) who have isolated, from the brine of traditional table olives, 40 lactic strains with antibacterial activity against L. monocytogenes, from which 8 strains were also active against E. coli O157. Also, Labioui et al. (2005) demonstrated that the isolated lactic strains were all active against Gram-positive, but only one strain was effective against Proteus mirabilis a Gram-negative bacteria.
The study of antimicrobial activity of thermophilic lactobacilli, carried out by Allouche et al. (2010), revealed a narrow spectrum of inhibition, directed in particular towards the target germs: Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. Also, Todorov (2008) found a Lactobacillus plantarum strain that inhibits the growth of Listeria spp., Escherichia coli and Klebsiella pneumoniae.
The antibacterial activity of lactic acid bacteria may be due to various compounds such as hydrogen peroxide, organic acids or bacteriocins (Luo et al., 2011). The results obtained after addition of catalase, neutralization of the pH and incubation of the CFS at high temperature, showed that the molecules produced are thermostable and exerted an antibacterial activity with respect to pathogenic bacteria. These molecules meet the criteria retained by Klaenhammer (1988) and can therefore be considered as bacteriocins.
The bactericidal activity of the all LAB isolates is detected in extracellular fraction. Similar result was obtained by Labioui et al. (2005) reported that the bactericidal activity of bacteriocin BLh5 is found exclusively in the culture medium.
The effect of incubation period on antibacterial activity of LAB isolates at 30°C showed that the inhibition zones of LB15, LB48, LB78, LB98 and LB105 incubated for 24h, remained constant after 96h of incubation under the same conditions. However, LB6, LB8, LB21, LB62, LB91, LB93, LB96, LB97, LB104 and LB105 showed a reduction in their antibacterial effect after 24h of incubation at 30°C; as shown in Table 7. The pH measured after 48h of incubation is between 3 and 4. These results are similar to those obtained during the study of pH effect on the production of inhibiting substances, the antimicrobial activity is reduced when the pH is below 4.5 (Kebede et al., 2007; Johanningsmeier and McFeeters, 2011;Medeiros etal., 2015). The work of Corsetti et al. (2004) showed that Lactobacillus strains were fully active at pH values ranging from 3 to 8, but Lb. plantarum 4DE and Lb. plantarum 3DM showed a reduction in antibacterial activity also at pH values of 3-4 and 8-9. In the work of Batdorj et al. (2006), the two bacteriocins studied were active over a wide pH range (2-10). Other studies realized by Bhattacharya and Das (2010) and Patil (2013) on the stability of bacteriocins at different pH levels, showed that these compounds are more stable in acidic than basic pH. Karaoglu et al. (2003) showed that the six bacteriocins tested were stable between pH 4.5 and 7 but sensitive outside this interval.
On the other hand, several studies of the influence of incubation time on the bactericidal activity of bacteriocins have been carried out. Tufail et al. (2011) have demonstrated that bacteriocin production by Lactobacillus bulgaricus is maximal after 48h of incubation. The work of Noordiana et al. (2013) reported that the antibacterial activity of the isolates is optimal between 48 and 72h of incubation, and is reduced after 96h of incubation. Recently, Kp et al. (2016) have found that the best incubation time for maximum bacteriocin activity was 48 h.
Our study also showed that the optimal incubation temperature varies between 25°C and 30°C for some lactic strains, but all isolated strains showed an optimal growth at 30°C and 37°C. Similar results have been reported by Chramostova et al. (2014) who showed that 37°C is the optimum growth temperature of Lactobacillus acidophilus, Bifidobacterium sp. and Streptococcus thermophilus. For their part, Panesar et al. (2010) showed a maximum production of lactic acid bacteria at 37°C. Recently, Patel and Parikh (2016) showed that 37°C is the optimum growth temperature of Lactobacillus sp.
Conclusion
In this study, morphological, physiological and biochemical criteria of the identified isolates allowed to link them to three lactic genera, the most dominant being the genus of Enterococcus (66.6%) followed by Leuconostoc (26.6%) and Lactobacillus (6.8%).
In view of the results, the bacterial species isolated from fermented table olives of Morocco, have interesting technological characteristics. Six isolates are considered as good acidifiers, they are the main agents of acidification and preservation of table olives. However, nine isolates are less acidifying, and they can be used for the production of flavors and for post-acidification operations.
Acknowledgements
This work was supported by the Regional Environmental Laboratory of the Urban Community of Tetouan.
Conflict of Interest
We declare no conflict of interest in this work
Funding Source
The research was supported by Abdelmalek Essaâdi University.
References
- A. O., W. H. O. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation.Rome: Food and Agriculture Organization of the United Nations . World Health Organization. 2006.
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988;70:337-349.
CrossRef - Dobson A., Cotter P. D., Ross R. P., Hill C. Bacteriocin production a probiotic trait. Environ. Microbiol. 2012;78:1-6.
CrossRef - Deegan L. H., Cotter P. D., Hill C., Ross P. Bacteriocins biological tools for bio-preservation and shelf-life extension. Dairy J. 2006;16:1058-1071.
CrossRef - Ross R. P., Galvin M., McAuliffe O., Morgan S. M., Ryan M. P., Twomey D. P., Meaney W. J., Hill C. (ed) Developing applications for lactococcal bacteriocins in Lactic Acid Bacteria Genetics Metabolism and Applications. Antonie Leeuwenhoek. 1999;337–346. pays
- Man D. J. C., Rogosa M., Sharpe M. E. A medium for cultuvation of lactobacilli. Journal of Applied Bacteriology. 1960;23:130-135.
CrossRef - Okada S., Ishikawa M., Yoshida I., Uchimura T., Ohara N., Kozaki M. Identification and characteristics of lactic acid bacteria isolated from sour dough sponges. Biotechnol. Biochem. 1992;56:572-575.
CrossRef - Abriouel H., Benomar N., Cobo A., Caballero N., Fuentes M. Á. F., Pérez-Pulido R., Gálvez A. Characterization of lactic acid bacteria from naturally-fermented Manzanilla Aloreña green table olives. J. Food Microbiol. 2012;32(2):308-316.
CrossRef - Labioui H., Elmoualdi L., Yachioui E. M., Ouhssine M. Sélection de souches de bactéries lactiques antibactériennes. Soc. Pharm. Bordx. 2005;144:237.
- Cogan T. M., Barbosa M., Beuvier E., Bianchi-Salvadori B., Cocconcelli P. S., Fernandes I., Gomez J., Gomez R., Kalantzopoulos G., Ledda A. Characterization of the lactic acid bacteria in artisanal dairy products. Dairy Res. 1997;64:409-421.
CrossRef - Lairini S., Beqqali N., Bouslamti R., Belkhou R., Zerrouq F. Isolement des bactéries lactiques à partir des produits laitiers traditionnels Marocains et formulation d’un lait fermenté proche du Kéfir. Sci. Rev. Int. Sci. Technol. 2014;10:267-277.
- Gaamouche S., Arakrak A., Bakkali M., Laglaoui A. Antimicrobial activity of lactic acid bacteria and bacteriocins isolated from a traditional brine table olives against pathogenic bacteria. J. Curr. Microbiol. App. Sci. 2014;3:657-666.
- Allouche F. N., Hellal A., Laraba A. Etude de l’activité antimicrobienne des souches de lactobacilles thermophiles utilisées dans l’industrie laitière. Rev Nat Technol. 2010;3:13-20.
- Todorov S. D. Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of the adsorption of bacteriocin AMA-K to Listeria sp. J. Microbiol. 2008;39:178-187.
CrossRef - Luo F., Feng S., Sun Q., Xiang W., Zhao J., Zhang J., Yang Z. Screening for bacteriocin-producing lactic acid bacteria from kurut, a traditional naturally-fermented yak milk from Qinghai–Tibet plateau. Food Control. 2011;22:50-53.
CrossRef - Kebede A., Viljoen B. C., Gadaga H., Narvhus J. A., Lourens-Hattingh A. The effect of incubation temperature on the survival and growth of yeasts in sethemi, South African naturally fermented milk. Food Technol. Biotechnol. 2007;45:21-26.
- Johanningsmeier S. D., McFeeters R. F. Detection of Volatile Spoilage Metabolites in Fermented Cucumbers Using Nontargeted, Comprehensive 2 Dimensional Gas Chromatography Time of Flight Mass Spectrometry (GC× GC TOFMS). Food Sci. 2011;76.
CrossRef - Medeiros A. C., Souza D. F., Correia R. T .P. Effect of incubation temperature heat treatment and milk source on the yoghurt kinetic acidification. Food Res. J. 2015;22:1030-1036.
- Corsetti A., Settanni L., Sinderen V. D. Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity. Appl. Microbiol. 2004;96:521-534.
CrossRef - Batdorj B., Dalgalarrondo M., Choiset Y., Pedroche J., Metro F., Prévost H., Chobert J. M., Haertlé T. Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. Appl. Microbiol. 2006;101:837-848.
CrossRef - Bhattacharya S., Das A. Study of physical and cultural parameters on the bacteriocins produced by lactic acid bacteria isolated from traditional Indian fermented foods. Am J Food Technol. 2010;5:111-20.
CrossRef - Patil S. R. Extraction partial purification and characterization of bacteriocin from Lactobacillus casei NCIM NO. 2732. Recent Adv. Appl. Sci. 2013;28:90-95.
- Karaoğlu Ş. A., Aydin F., Kiliç S. S., Kilic A. O. Antimicrobial activity and characteristics of bacteriocins produced by vaginal lactobacilli. J. Med. Sci. 2003;33:7-13.
- Tufail M., Hussain S., Malik F., Mirza T., Parveen G., Shafaat S., Wajid A., Mahmood R., Channa R. A., Sadiq A. Isolation and evaluation of antibacterial activity of bacteriocin produced by Lactobacillus bulgaricus from yogurt. J. Microbiol. Res. 2011;5:3842-3847.
- Noordiana N., Fatimah A. B., Mun A. S. Antibacterial agents produced by lactic acid bacteria isolated from Threadfin Salmon and Grass Shrimp. Food Res. J. 2013;20:17-124.
- Sure K .P., Kotnis P. V., Bhagwat P. K., Ranveer R. C., Dandge P. B & Sahoo A. K. Production and Characterization of Bacteriocin Produced by Lactobacillus Viridescence (NICM 2167). Brazilian Archives of Biology and Technology. 2016;59.
- Chramostova J., Mošnovà R., Lisova I., Pešek E., Drbohlav J., Nemeckova I. Influence of Cultivation Conditions on the Growth of Lactobacillus acidophilus, Bifidobacterium sp., and Streptococcus thermophilus and on the Production of Organic Acids in Fermented Milks. Czech J. of Food Sci. 2014;32(5).
- Panesar P. S., Kennedy J. F., Knill C. J., Kosseva M. Production of Llactic acid using Lactobacillus casei from whey. Arch. Biol. Technol. 2010;53:219-226.
CrossRef - Patel S. A., Parikh S. C. Production of lactic acid from whey by Lactobacillus sp. isolated from local dairy products. Int. J. Curr. Microbiol. Appl. Sci. 2016;5:734-741.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





