How to Cite | Publication History | PlumX Article Matrix
College of Science and Humanitarian Studies, Shaqra University, Qwaieah 11971, Saudi Arabia.
Corresponding Author E-mail: dr.alsaggaf10@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2613
ABSTRACT: Green biosynthesis of nano-metals is an important research demand to have these minute active particles. The biosynthesis of zinc oxide nanoparticles (ZnO-NPs) was conducted using the marine macro algae Sargassum muticum, as an eco-friendly approach for NPs synthesis. The biosynthesized ZnO-NPs was characterized and evaluated for their antimicrobial potentiality against skin pathogens, Candida albicans and Staphylococcus aureus, concerning antibiotic sensitive and resistant strains. The ZnO-NPs were applied for fabrication of bioactive cotton textiles, which were also evaluated as antimicrobial coatings. ZnO-NPs was successfully synthesized using S. muticum extract, with uniform distribution, spherical shapes, and particle size range of 4 to 23 nm. The antimicrobial potentiality of biosynthesized ZnO-NPs was evidenced against the entire examined skin pathogens, which included antibiotic resistant strains. The treatment of cotton textiles with ZnO-NPs resulted in bioactive fabrics with comparable shape and surface. The treated textiles had a remarkable microbicidal activity toward examined skin pathogens and maintain their potentiality even after tow laundering cycles. Algal biosynthesized ZnO-NPs is, however, advised for the fabrication of antimicrobial textiles to protect skin from antibiotic resistant pathogens.
KEYWORDS: Antimicrobial Textiles; Biosynthesis; Characterization; Nano-metals; Skin Protection
Download this article as:| Copy the following to cite this article: Alsaggaf M. S. Applicable Control of Antimicrobial Resistant Skin Pathogens Using Algal-Synthesized Zinc Oxide Nanoparticles. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Alsaggaf M. S. Applicable Control of Antimicrobial Resistant Skin Pathogens Using Algal-Synthesized Zinc Oxide Nanoparticles. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29523 |
Introduction
The infectious pathogens emergence and their unceasing development into antibiotic resistance, e.g skin pathogens, is possessing a serious public health threat worldwide.1
Among these resistance-acquiring pathogenic microbes, Staphylococcus aureus is from the most aggressive organisms that cause numerous variety of diseases and infections.2
The resistant S. aureus strains to methicillin (MRSA) are well documented and could be spread via skin contact or personal items sharing with low health care precautions;3 they are very challenging to be prevented or control. The main suggested keys for MRSA control were the raised standards of hygiene, skin trauma prevention and the usage of surface-coating or ointments having powerful and innovative antibacterial properties.4
Candida albicans is from the aggressive opportunistic human pathogens that could survive in the different internal body organs/systems and superficially and threat the lives of immune-deficient patients.5 Invasive candidiasis could be transmitted through surgery, burns and skin wounds, especially in IC unit and immunosuppressive patients.6
Although the presence of numerous and diverse antifungal drugs, only limited classes are applicable to treat systemic or mucosal Candida spp. infections, particularly the resistant strains.7 The emergence of resistant C. albicans strains to antifungal agents, e.g. azole compounds, is documented to elucidate its dangerousness and mechanisms.8,9
The promising advances in nanotechnology characteristics and applications led to their potentiality and powers for development novel biocidal agents /formulations; numerous investigations recommended the application of nano-metals as powerful antimicrobial factors for application in pathogens control and fabrication of biocidal materials.10-12
Zinc oxide nanoparticles (ZnO-NPs) is from the well-studied NPs for their characteristics and bioactivity. While ZnO powder was repeatedly applied in dermatological formulations, e.g. ointments, creams and lotions, as the active antimicrobial and sunblock ingredient,13 ZnO-NP was confirmed as highly effective agents for inhibiting numerous microbial pathogens from dermatophytes and foodborne pathogens.4,14
The traditional methods for NPs synthesis are chemical-based methods, however, interests are increased to develop eco-friendly and biosafe methods, i.e. biosynthesis using plants, microorganisms, enzymes and algae, which exclude toxic materials from NPs synthesis procedures.15 Many attempts were conducted and succeeded to produce Zn-NPs using marine algae.16,17
The fabrication of functional “smart” textiles aroused to respond to the increasing demand for safety and high- technology fabrics. Antimicrobial textiles are highly demanded, in this context, to provide the hygienic conditions and to prevent the contamination with pandemic diseases.18 Many fabricated antibacterial textiles were based on traditional bioactive agents, e.g. disinfectants, antibiotics … etc., whereas others, recently, applied metals-NPs for production of more active antimicrobial textiles.19
The metal oxides NPs, ex. TiO2, CaO, MgO and ZnO, have additional interests due to their increasing biosafety for human and environment, and their high stability under various process conditions.11 Specifically, ZnO-NPs was characterized as generally nontoxic, chemically stable at high temperature, and active photo oxidation catalyst.20 Besides, compared to other NPs like nano Ag, ZnO-NPs have many advantages such as their cost-efficacy, UV-blocking and white color.21
Therefore, the biosynthesis of ZnO-NPs using the marine macroalgae S. muticum and their application for fabrication of antimicrobial textiles against resistant skin pathogens, were all investigated.
Materials And Methods
Algal Culture and Extracts
Dried identified samples from Sargassum muticum (brown macroalgae from marine source) were generously supplied from Kafrelsheikh university- Faculty of Aquatic & fisheries Science, Egypt. Algal extraction was performed by grinding dried biomass to ~ 60 mesh size, immersion of powder (20 g) in 1 L from distilled water (DW) then boiling the mixture for 120 min. The extract was then filtered through filter paper (Whatman 41) to eliminate algal residues. Algal extract was kept in sterile dark bottles at 4 ºC until use.
Biosynthesis and Characterization of ZnO-NPs
0.1 M zinc nitrate [Zn (NO3)2-6H2O] solution was prepared and react 75 ml from this solution with 25 ml from algae extract for 220 min at 75 ºC in a shaking water bath. The white semi-solid product was attained via centrifugation at 3600 xg for 12 min, then carefully washed with deionized water and dried for 6 h at 105 ºC. For more purification of NPs, the attained dried ZnO product was resuspended in 20 ml from algae extract and subjected to 150 ºC for 250 min, then purified NPs were suspended in deionized water with the addition of 5 mM from sodium dodecyl sulfate (SDS) as stabilizer. The NPs size, shape and distribution were characterized using transmitted microscopy (TEM, GERMANY -ZEISS-EM10).
Antimicrobial Evaluation
Different microbial skin pathogens were challenged in this study; Candida albicans R (ATCC MYA574, Fluconazole-resistant) and Staphylococcus aureus R (ATCC 43300, Methicillin resistant) were used as models for resistant pathogens, whereas C. albicans I and S. aureus I (isolated from hospitalized skin infected patients) were used as normal sensitive pathogens. The C. albicans stains were propagated/screened using yeast malt extract medium (YM browth/agar at 25 °C), whereas the used growth parameters for S. aureus strains were the trypticase soy medium (TS broth/agar at 37 °C), respectively.
In Vitro Evaluation
The inhibition zones method (ZOI) was applied for evaluating the antimicrobial activity of produced ZnO-NPs. Microbial cultures were streaked onto suitable media the pores of 6 mm diameter were aseptically made in agar. Different concentrations from ZnO-NPs, i.e. 25, 50 and 100 µg/ml, were prepared using sterile citrate buffer and 50 µl form each concentration were pipetted into wells and incubated for 24 h at suitable temperature for each microbe. The appeared ZOI were precisely measured for each treatment.
Application for Antimicrobial Textiles Production
Standardized bleached cotton fabrics (100%, 110 g/ m2) were purchased from Misr Co. for Weaving and Spinning, Egypt. The fabric were washed four times with distilled water then dried by air and cut into ~1 cm2 pieces. Textile pieces were immersed into the biosynthesized ZnO-NPs solution and left for 90 min at 45°C with stirring, then air dried. The influence of cotton textile treatment with NPs on the topography of fibers compared with untreated textiles was evaluated using scanning microscope imaging.
The antimicrobial activity of NPs-treated textiles were determined toward examined skin pathogens using the inhibition zone assay; as mentioned before, microbial cultures were spread onto appropriate media then NPs-treated textiles were placed in the surface of inoculated agar and incubated for 24 h. The appeared inhibition zones surrounding the textile pieces were measured and their mean diameters were calculated.
The durability of treated textiles, after washing cycles, were evaluated repetitive laundry treatment,22 using neutral water at 40 ±3°C in a home laundry machine. NPs-treated fabrics were subjected to 2 successive laundering cycles then squeezed and air-dried, after each of them. The textiles activity, after laundering cycles, was measured toward examined strains as mentioned above.
Results
ZnO-NPs was successfully synthesized using the reducing powers of algal extract of S. muticum; the SDS was used to maintain the well-distribution of NPs in the solution and prevent their agglomeration. The micrograph of biosynthesized ZnO-NPs with S. muticum extract elucidated that the NPs had spherical shapes, uniformly distribution and average particle size in the range of 4 to 23 nm (Fig 1).
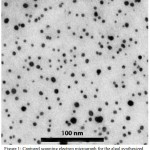 |
Figure 1: Captured scanning electron micrograph for the algal synthesized nano-ZnO to elucidate their shape, size and distribution
|
The antimicrobial potentiality of ZnO-NPs against examined skin pathogens, i.e. C. albicans (I), C. albicans (R), S. aureus (I) and S. aureus (R), was confirmed via ZOI assay (Fig. 2).
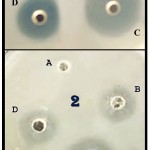 |
Figure 2: Antimicrobial potentiality of ZnO-NPs against resistant strains of Candida albicans (1) and Staphylococcus aureus (2), after challenging with 50 µL from nanoparticles concentrations of 25 µg/ml (B), 50 µg/ml (D) and 100 µg/ml (C), comparing with control (A).
|
The antimicrobial activity was more evidenced in C. albicans strains than in S. aureus strains, especially in the higher concentrations of ZnO-NPs. The results for inhibition zones in sensitive and resistant strains to antibiotics were comparable with no significant difference between trials in each species. The recorded ZOIs, including wells diameters, were 12.4, 19.7 and 25.5 mm against C. albicans (R), and 13.8, 16.3 and 22.6 mm against S. aureus (R), for the ZnO-NPs concentrations of 25, 50 and 100 µg/ml, respectively.
The influence of cotton textile treatment with ZnO nanoparticles on the topography and shape of fibers is illustrated in Fig (3). The micrographs indicated that the textile fibers’ topography was not undesirably affected by the treatment of NPs; the shape and surface of control and treated fibers was comparable, although the treated textiles fibers became more expanded and enlarged than control fibers, with the appearance of some ZnO-NPs on their surface. The NPs did not form aggregates during or after textile treatment.
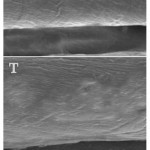 |
Figure 3: Influence of cotton textile treatment with ZnO nanoparticles (T) on the topography of fibers compared with untreated textiles (C), using scanning microscope imaging.
|
The durability of treated textiles ZnO-NPs was evaluated after 2 laundering cycles; fabrics could maintain most of their antimicrobial action of laundering (Table 1). The laundering cycles reduced the antimicrobial activity of treated textiles, against antibiotic resistant strains, by 7.35 and 19.62% for C. albicans R, and by 13.55 and 17.92% for S. aureus R, after the first and second laundering cycle, respectively. The antimicrobial potentiality of washed textiles was comparable, with no different significance, between the sensitive and resistant strains, among the same species, after textiles’ repeated washing.
Table 1: Antimicrobial potentiality of nano ZnO – treated textiles against skin pathogens after laundering cycles*
| Laundering cycles | Antimicrobial activity (zones of inhibition, mm) | |||
| Candida albicans I | Candida albicans R | Staphylococcus aureus I | Staphylococcus aureus R | |
| 0 | 28.3±0.5 | 27.5±0.4 | 25.7±0.4 | 25.1±0.3 |
| 1st cycle | 25.8±0.2 | 25.2±0.5 | 21.6±0.3 | 21.7±0.4 |
| 2nd cycle | 22.5±0.4 | 22.1±0.3 | 19.8±0.4 | 20.6±0.2 |
* Inhibition zones are triplicates means (including textile width of 10 mm) ± standard deviation
Discussion
The green synesthesia of NPs was successfully achieved in this study, SDS was added to biosynthesized ZnO-NPs to prevent their agglomeration because of the high polarity when using water as the intermediate solvent.20
While the toxicity of heavy metals and their oxides are recognized upon human cells exposure, at high concentrations, this toxicity were not predicted at low concentrations; ZnO was established to protect intestinal cells from many microbial infections via inhibiting bacterial internalization and adhesion.23 Consequently, the bacterial ability to grow at low ZnO concentrations advocates that ZnO-NPs could be non-toxic for every microbial cultures and recommended their potential biosafety for human usage.
Microbial cell walls contain many charged components; the surface proteins are from the main component of microbial pathogens’ cell wall that responsible for colonization and adhesion, the other components contain teichoic acid and polysaccharides.24 Therefore, these components could exposed to specific interactions with NPs that lead to disrupt their organization and function. Long-chain polycations was reported to kill bacterial strains when coated onto their surfaces.10
The biocidal potentiality from ZnO-NPs is much powerful than from bulk ZnO; this is simply because of the larger surface/volume ratio in smaller particles which gives them efficacy as antimicrobial agent.25 ZnO-NPs could generate hydrogen peroxide (H2O2) from their surfaces, which presents other elucidation for their microbicidal activity.26 The generated H2O2 concentration, from NPs surfaces, increases with NPs size decrements, which could explain the activity of current biosynthesized ZnO-NPs, as they have a low particle sizes range, as detected with TEM imaging.
Numerous studies proposed the production of reactive species from oxygen (ROS), that relate to the photosensitivity of ZnO-NPs, as the direct mechanism to damage microbial cell membranes; that the mechanism of NP toxicity may relate to their and to under specific wavelength high-intensity light, therefore nanomaterials that The architecture of these membranes are impaired by ROS through lipid peroxidation.27
The treatment of cotton fabrics with ZnO-NPs evidenced its efficacy to transfer the NPs antimicrobial activity to treated textiles. Textile treatments with nano-metals was recurrently recommended to provide them with powerful bioactivity; chemically synthesized ZnO-NPs were used for coating cotton textiles and this treatment led to significant antibacterial activity from treated textiles against wide variety of bacterial pathogens.28,29 The shape of prepared ZnO-NPs was reported to have little impact on their antibacterial activity when applying for textile coating.30 However, current study presented novel application of algal synthesized ZnO-NPs for control of skin resistant pathogens.
The high durability of nano ZnO-treated textiles was reported by many investigators; their bactericidal activity was reduced by 2 – 25 % from the unwashed treated textiles.29,31
The TEM micrographs of ZnO-NPs treated cotton fibers indicated that the NPs could efficiently interact with cotton fibers, as evidenced from the absence of NPs aggregates. The minute traces of NPs on the surface of fibers suggested that they could penetrate inside the textile fibers and stabilize within their cellulose network.32
Accordingly, the produced antimicrobial textiles with ZnO-NPs could be advised for the fabrication of skin protection fabrics for usage in hospitals, laboratories and personal care.
Conclusions
ZnO-NPs could be effectually biosynthesized using marine algae S. muticum, the antimicrobial activity of NPs was proved against many microbial pathogens, e.g. C. albicans and S. aureus, including antibiotic resistant strains. Treated cotton textiles with ZnO-NPs had powerful activity to inhibit microbial pathogens even after repeated washing cycles.
Acknowledgement
Thanks are indebted forever for our God (ALLAH) for the mercy helping and guiding.
Conflict of interest
There is no conflict of interest.
References
- Desselberger U. Emerging and re-emerging infectious diseases. J. Infect. 2000;40:3–15.
CrossRef - Lowy F. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–32.
CrossRef - Zaoutis T.E, Toltzis P, Chu J, Abrams T, Dul M, Kim J, McGowan K.L and Coffin S.F. Clinical and molecular epidemiology of community-acquired methicillin-resistant Staphylococcus aureus infections among children with risk factors for health care-associated infection: 2001–2003. Pediatr. Infect. Dis. J. 2006;25:343–8.
CrossRef - Jones N, Ray B, Ranjit K.T and Manna A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008;279:71–6.
CrossRef - Lopez-Martinez R. Candidosis, a new challenge. Clin. Dermatol. 2010;28:178–84.
CrossRef - Pfaller M.A and Diekema D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–63.
CrossRef - Denning D.W and Hope W.W. Therapy for fungal diseases: opportunities and priorities. Trends. Microbiol. 2010;18:195–204.
CrossRef - Akins R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005;43:285–318.
CrossRef - Cannon R. D, Lamping E, Holmes A. R, Niimi K, Tanabe K, Niimi M and Monk B.C. Candida albicans drug resistance – another way to cope with stress. Microbiology. 2007;153:3211–7
CrossRef - Tiller J.C, Liao C.J, Lewis K and Klibanov A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. 2001;98:5981–5.
CrossRef - Stoimenov P.K, Klinger R.L, Marchin G.L and Klabunde K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–86.
CrossRef - Ma D, Guan J, Normandin F, Denommee S, Enright G, Veres T and Simard B. Multifunctional nano-architecture for biomedical applications. Chem. Mater. 2006;18:1920–7.
CrossRef - Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods. 2003;54:177–82.
CrossRef - Tayel A.A, El-Tras W.F, Moussa S, Mahrous H, El-Baz A.F, Salem,M.F and Brimer L. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J. Food Saf. 2011;31:211–8.
CrossRef - Sinha S, Pan I, Chanda P and Sen S.K. Nanoparticles fabrication using ambient biological resources. J. Appl. Biosci. 2009;19:1113–30.
- Jain N, Bhargava A, Tarafdar J.C, Singh S.K and Panwar J. A biomimetic approach towards synthesis of zinc oxide nanoparticles. Appl. Microbiol. Biotechnol. 2013;97:859–69.
CrossRef - Azizi S, Ahmad M.B, Namvar F and Mohamad R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014;116: 275–7.
CrossRef - Windler L, Height M and Nowack B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013;53:62-73.
CrossRef - Dastjerdi R and Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids. Surf. B. Biointerfaces. 2010;79:5-18.
CrossRef - Yadav A, Prasad V, Kathe A.A, Raj S and Yadav D. Sundaramoorthy C, Vigneshwaran N. Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull. Mater. Sci. 2006;29:641-45.
CrossRef - Becheri A, Durr M, Lo Nostro P and Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J. Nanopart. Res. 2007;10:679-89.
CrossRef - American Association of Textile Chemists and Colorists (ed). AATCC Technical Manual, test method 61(2A)-1996, NC. Research Triangle Park. 2000;75.
- Roselli M, Finamore A, Garaguso I, Britti M.S and Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by E. coli. J. Nutr. 2003;133:4077–82.
CrossRef - Navarre W.W and Schneewind O. Surface proteins of Gram positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999;63:174–229.
- Baker C, Pradhan A, Pakstis L, Pochan D. J and Ismat S. S. Synthesis and Antibacterial Properties of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005;5(2):244-9.
CrossRef - Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001;3: 643-6.
CrossRef - Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti M.F and Fievet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6:866–70.
- CrossRef
- Rajendran R, Balakumar C, Mohammed H.A, Jayakumar S, Vaideki K and Rajesh E.M. Use of zinc oxide nano particles for production of antimicrobial textiles. Int. J. Eng. Sci. 2010;2:202-8.
- Subash A.A, Chandramouli K.V, Ramachandran T, Rajendran R and Muthusamy M. Preparation characterization, and functional analysis of zinc oxide nanoparticle-coated cotton fabric for antibacterial efficacy. J. Text. Instit. 2012;103:298-303.
- Sricharussin W, Threepopnatkul P and Neamjam N. Effect of various shake of zinc oxide nanoparticles on cotton fabric for UV-blocking and anti-bacterial properties. Fiber. Polym. 2011;12:1037–41.
CrossRef - Sivakumar P.M, Balaji S, Prabhawathi V, Neelakandan R, Manoharan P.T and Doble M. Effective antibacterial adhesive coating on cotton fabric using ZnO nanorods and chalcone. Carbohydr. Polym. 2010;79:717-23.
CrossRef - Gouda M. Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J. Ind. Text. 2011;41:222–40.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






