How to Cite | Publication History | PlumX Article Matrix
Priyanka C. Nandanpawar1, Mohd Ashraf Rather2, Mohan Ramesh Badhe1 and Rupam Sharma1
1Fish Genetics and Biotechnology Central Institute of Fisheries Education Panch Marg, Versova Mumbai – 400061, India.
2College of Fisheries shirgaon, Ratnagiri-India.
Corresponding Author E-mail: mashraf38@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2606
ABSTRACT: The increasing application of nanoparticles both in industries and in agricultural fields has led to its accumulation in the aquatic ecosystem through water run-off. Insights into the validity of safer nanoparticles such as gold and chitosan are fairly established. However, its effect on aquatic invertebrates has been less studied. The present study was aimed to study effects of chitosan reduced gold nanoparticles (CRGNPs) during green fluorescent protein (GFP) encoding plasmid delivery in giant freshwater prawn, macrobrachium rosenbergii. The mean particle size and zeta potential CRGNPs was 33.7 nm and 24.79 mV respectively. Prawn juveniles were exposed to nanoparticles concentrations (10 µg/L, 20 µg/L) of CRGNPs by immersion treatment for a period of 36 hours. GFP was ubiquitously expressed in muscle tissues of prawns. The comet assay indicated dose dependent genotoxicity of CRGNPs in gill, pleopod and muscle tissues which was in conformity with its bioaccumulation pattern in vivo. The highest bioaccumulation of CRGNPs was found in Gills, followed by pleopods and least in muscles. Hence, the toxicological potential of CRGNPs to the environment cannot be denied and demands more research on the particular aspect. The doses standardized in the present study would be helpful in safer nano-gene delivery in aquatic invertebrates and development of transgenics employing less cost.
KEYWORDS: Bioaccumulation; Comet Assay Gold Genotoxicity; GFP; Nanoparticles;
Download this article as:| Copy the following to cite this article: Nandanpawar P. C, Rather M. A, Badhe M. R, Sharma R. Assessment of DNA Damage During Gene Delivery in Freshwater Prawn by Chitosan Reduced Gold Nanoparticles. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Nandanpawar P. C, Rather M. A, Badhe M. R, Sharma R. Assessment of DNA Damage During Gene Delivery in Freshwater Prawn by Chitosan Reduced Gold Nanoparticles. Orient J Chem 2018;34(2). Available from: https://www.biotech-asia.org/?p=29484 |
Introducation
In this era of modern biomedical sciences, nanoparticles are being extensively used for applications such as delivery of genes, hormones, vaccines, peptides or proteins intended for therapeutic purpose. Moreover, cell labeling (Bhirde et al., 2011), drug targeting ( Hans and Lowman 2002), biosensors, and hyperthermia therapy are only a portion of the wide nanoparticle application spectrum (Jeng and Swanson.,2006). Various metal and polymeric nanoparticles (1-100 Nanometers) like gold, Titanium oxide (Ghosh et al. 2008; Rather et al., 2013)) and Chitosan (Rather et al.,2016; Duceppe and Tabrizian 2010)) (Kashyap et al.,2015)), poly Lactic-co-Glycolic Acid (PLGA)(Li Y-P et al., 2001), polyLactic Acid (PLA)(Kumari et al.,2010), poly-ε-caprolactone (PCL)(Tang et al., 2014)) and gelatin etc. are used for the above purposes. (Soppimath et al., 2001; Mahapatro A and Singh DK. 2011). These nanoparticles are being used mainly because of their unique properties such as electrostatic binding, higher intracellular uptake, convenient release profiles and better encapsulation efficiency (Shan et al.,2012). In mammalian systems such as humans, rats and mouse models, nanoparticles are being used for cancer treatments, targeted gene integration and generation of immunogenic potential(Roy et al. 1999). Various non-mammalian and vertebrate models such as zebrafish, black seabream, are also being studied for efficient gene/drug delivery and payload efficiency using nanoparticles (Li et al., 2013; Sharif et al.,2012)Despite the enormous research done in nanoparticle applicability, little is known about its ill effects to aquatic animal models. The nanoparticles are reported to be genotoxic to host DNA , alters nucleic acids, causes mutations and random inactivation of genes (Shinde et al., 2012). Commonly used nanoparticles such as Titanium oxide, silver nanoparticles, carbon nanotubes, fullerenes are responsible to produce chronic cellular damage, apoptosis, genotoxicity and bioaccumulation ( Lai et al.,2008 ; Chang et al.,2012 ;Zhu et al.,2016;Cheng SH.,2012) has been recently documented. A number of non-mammalian animal models such as zebrafish have been used to assess the risk associated with nanoparticles on human health (Asharani et al; 2008)). Even the widely used gold nanoparticles are biocompatible and believed to be non-toxic, but, but several studies have indicated its semi lethal effects such as lipid peroxidation, cytotoxicity and oxidative stress. (Tedesco et al.,2010; Pan et al., 2007; Freese et al., 2012 Sharma et al.,2016).
In the present aquaculture scenario, the emerging use of nanoparticles for maintaining water quality (Pradeep T and Anshup.,2009), sedimentation of algal biomass (Xu et al ., 2011), growth promoting and gene delivery for production of transgenic is taking a step forward. Nanotechnology based DNA vaccines are being developed against White Tail disease in shrimps (Ramya et al., 2014), but such treatments may leave behind traces of nanoparticle residues which may find its way to the biological systems. When released as effluents into the aquatic environment, the metal nanoparticles may accumulate in invertebrates and can enter the human food chain. (Baun et al.,2008 ;(Judy et al.,2011).Since this nanotoxicity is size and dose dependent, it becomes mandatory to formulate the safe dose of nanoparticles before delivering it in farmed conditions (Rather et al., 2013).
The giant freshwater prawn (Macrobrachium rosenbergii) is a commercially important species for aquaculture and is recently being used in various genetic improvement programs. Very scarce literature is available regarding gene delivery in this species (Ramya et al.,2014) and hence, this species was considered as an aquatic invertebrate model to test the possibility of standardization of safer nano-gene delivery doses for transgenic production.
The CRGNPs are reported to be nontoxic in the case of mammalian systems ( Pokharkar et al., 2009; Bhumkar et al.,2007; Stefan et al.,2013) but the toxicity potential of these is yet to be established in non-mammalian aquatic invertebrates. Another reason for considering CRGNPs includes the fact that chitosan acts as a penetration enhancer (Bhumkar et al.,2007) and hence is helpful in gene delivery practices. As chitosan imparts positive charge to the gold nanoparticles, it has better electrostatic affinity towards the negatively charged DNA/Plasmid. The present study aims to demonstrate Chitosan reduced gold nanoparticles as gene delivery vehicles in M. Rosenbergii and its possible effects on cells as well as genetic gradients.
Materials and Methods
Chemicals
For the synthesis of CRGNPs, Gold chloride, Chitosan (degree of deacetylation 90%) were procured from Sigma Aldrich (St. Louis, MO) company. For comet assay, Dimethyl Sulphoxide (DMSO) and Low Melting Point Agarose (LMPA) from SRL (Mumbai, India) company were used. Triton X-100 (Sigma) for comet assay and other laboratory wares were obtained from Tarsons (GIBCO, BRL, UK). All other chemicals were of analytical and molecular biology grade. Plasmid PDB402 having 367 bp GFP (Green Fluorescent Protein) gene with CMV (Cytomegalovirus) promoter was procured from Central Institute of Fisheries Education (CIFE), Mumbai,India.
Procurement of Animals
Juvenile prawns were collected from a local prawn hatchery at Bharuch, Gujarat, India and kept in FRP (fiber reinforced Plastic) tanks at the density of 10 prawns/tank of 50 litre capacity in the wet lab of Central Institute of Fisheries Education, Mumbai, India. They were acclimatized to hatchery conditions for two weeks before commencement of the experiment. The water quality parameters were checked fortnightly following APHA guidelines (APHA,1999). The photoperiod maintained in the tank was 12h light/ 12 h darkness. The research undertaken complies with the current animal welfare laws in India. The care and treatment of animals used in this study were in accordance with the ethical guidelines of ICAR-Central Institutute of Fisheries Education, Mumbai,India. As the experimental animal Macrobrachium rosenbergii is not an endangered shellfish, the provisions of the Govt of India’s Wildlife Protection Act of 1972 are not applicable for experiments on this shellfish.
Preparation and Confirmation of CRGNP- plasmid Conjugates
CRGNPs were prepared by Turkevich et al. method (Turkevich et al.,1951) with slight modification. 100 ml of aqueous solution of chloroauric acid (HAuCl 4 ) of 8mM (4ml) concentration was added to 10 ml of chitosan solution (6.92mg/ml) prepared in 1% acetic acid and heated for 15 min to reduce the chloroauric acid which yields a ruby-red solution. The ruby red colored solution formed is the indication of formation of gold nanoparticles.
For preparing CRGNP- plasmid conjugates, 1 ml of CRGNP solution was dissolved in 9 ml triple distilled water and topped the volume up to 10ml ( Rajeshkumar et al.,2009). Different concentrations of plasmid solutions (50ng/μl, 100ng/μl) were prepared by dissolving in 50mM sodium sulfate buffer separately followed by heating at 550C. Both plasmid and nanoparticles of variable concentrations were mixed proportions and vortexed on magnetic stirrer at 700 g for 30 seconds. Lastly,the solutions were stored at room temperature. The characterization of resultant nano-plasmid conjugates was done with Beckman Coulter Delsa Nano C- Nano Particle Size Analyzer.for the mean particle size and size distribution. For Scanning electron microscopy, drops of the nanoparticle dispersions were placed on carbon-coated grids. The solution was allowed to air-dry for 1 min. After drying of the samples, they were treated with a high energy electron beam and analyzed using Quanta 200 Environmental Scanning Electron Microscopy (ESEM) system at Icon analytics, Mumbai.
Gel Retardation Assay
Conjugation of DNA with the CRGNPs was confirmed by centrifuging the conjugate solution at 13,000 g at 480 C for 20 min ( Wu et al.,2010);(Shan et al., 2012).After discarding the supernatant, 20 ml autoclaved triple distilled water was added to the pellets. The solution was vortexed for 10 min and then loaded onto 1% agarose gel. The agarose gel was stained with 1μg/mL ethidium bromide and visualized under UV (Ultraviolet) transilluminator (Syngene, UK).
Dose Administration and Sampling
The CRGNP doses selected for the study were selected by performing a preliminary test to confirm the rate of mortality in prawns.The CRGNP test concentrations were 10,20,30,40 ug/L of tank water. An immersion treatment to 10 prawn juveniles/ tank was given for 24 hrs. Accordingly, doses which caused least mortality(10ug/L,20ug/L) were selected for final treatement.
The treatment of CRGNP-plasmid conjugates were given (viz. 10ug/L,20ug/L)to animals by immersion treatment in Fibre Reinforced Plastic(FRP) tanks for a period of 36 hours. The animals were stocked at the rate of 6 individuals/Litre in every tank. The animals were not fed 24 hours prior to the treatment. Regular aeration was maintained during the treatment. After 36 hours of treatment, the tissue samples were collected from animals by anaesthetizing them with MS222.
Fluorescence Microscopy and Comet Assasy
The freshly sampled tissue sections were washed in triple distilled water and the exoskeleton was removed with the help of forceps. The muscle tissues of midbody and near tail end of prawns were then analysed under TCS-SP Leica microscope (Germany) for fluroscence detection. GFP fluorescence absorption peak on 488nm and an emission peak on 509nm was observed.
The comet assay was performed on gills, pleopods and muscle tissues following the method of singh et al. [Pavlica et al.,2001] with slight modifications. As a positive control, silver nanoparticles (2μg/L) were administered to prawns. For comet assay, the tissues were placed in 1ml of cold Hanks Balanced Salt Solution (HBSS) containing 20mM EDTA in 10% DMSO. The tissues were minced into fine pieces and a suspension was prepared. Slides were prepared in triplicates per concentration and were immersed in cold lysis solution (4oC) at pH 10 for 60 minutes.
After unwinding in the electrophoresis buffer (300 mM NaOH: 1 mM Na2EDTA) at pH13. for 20 min, electrophoresis was conducted at a constant voltage of 25 V and 300 mA at 4oC.Slides were neutralized in 0.4 M Tris having a pH of 7.5 for 5 min and then stained with 75μL Ethidium bromide, cover slipped and immediately analyzed in Zeiss axiophore image analyser. Twenty cells per slide ( Pavlica et al., 2001) were examined using the software from metasystems (Germany) at the National Center for Preclinical Reproductive and Genetic Toxicology Genetic Toxicology in National Institute for Research in Reproductive Health (NIRRH), Mumbai.
Bioaccumulation studies by Graphite Furnace- Atomic Absorption Spectroscopy (GF-AAS)
GF-AAS was performed on Gill, muscle and pleopod tissues following the method of kehoe ( Kehoeet al (1988)) with some modifications. The tissues were wet digested using concentrated supra-pure trace-metal free HNO3 (Nitric acid) followed by Conc HClO4 (Perchloric acid). The dried residue was dissolved in 5ml 0.1M HNO3. GF-AAS was performed using a Perkin–Elmer Model-800 atomic absorption spectrophotometer with a Perkin–Elmer HGA-600 graphite furnace. For the analysis graphite tubes were used. After digestion, the sample was diluted with 0.25N supra-pure nitric acid @ 1:2 v/v, prior to analysis. For calibration of the equipment, elemental gold standard (Merck) was used(Lasagna et al., 2010).
Data Analysis
The results were analyzed by one-way ANOVA and the comparisons of mean values were carried out by Duncan’s multiple range test (DMRT). Statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL). All the data analyses were expressed as a mean ± standard error.
Results
Different concentrations of CRGNPs showed color variations ranging from bright red to magenta color (Fig 1). The mean particle size ,polydispersity index and zeta potential of CRGNPs was found to be 33.7nm,0.292 and 24.6mV respectively. The results of SEM confirmed that nanoparticles were compact, spherical in structure and well dispersed. (Fig 1 and 2).
 |
Figure 1: Chitosan Reduced Gold Nanoparticles of variable concentrations
|
 |
Figure 2: Particle size distribution and Scanning Electron Microscopy of chitosan reduced gold nanoparticles
|
The conjugation of plasmid with CRGNPs was confirmed by gel retardation assay. Blank CRGNPs and naked plasmid DNA (pDB 402) was used as controls. The CRGNP- plasmid nanoconjugate did not migrate in the gel. On the other hand, control DNA showed migration from the well (Fig 3). CRGNPs devoid of plasmid did not show any fluorescence in the gel. Further, the bands developed by the CRGNP- plasmid nanoconjugate were less bright than the naked DNA indicating encapsulation of plasmid by CRGNPs.
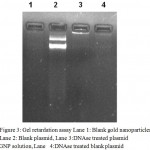 |
Figure 3: Gel retardation assay Lane 1: Blank gold nanoparticles, Lane 2: Blank plasmid, Lane 3:DNAse treated plasmid GNP solution, Lane 4:DNAse treated blank plasmid
|
The intracellular distribution of CRGNP- plasmid nanoconjugates after 36 hours of exposure was detected mostly in gill lamellae (Fig 4a), Pleopods and uropod muscles (Fig 4b, 4c) suggesting the protein expression of the transfected GFP gene. There was no significant difference between GFP expression intensities of samples administered with variable CRGNP- plasmid nanoconjugates concentrations.
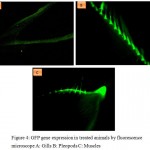 |
Figure 4: GFP gene expression in treated animals by fluorescence microscope A: Gills B: Pleopods C: Muscles
|
Results of cell viability assay showed no significant change in viability compared to Control cells. The % tail DNA content (±S.D) at 10μg/L was 34.7±16.3% for gills, 41.37±17.7% for pleopods and 14.39 ±11.5% for muscles (Fig 5). At concentration of20 ug/L, it was 48.536±36.03% for gills, 37.6±21.3% for pleopods and 10.9±7.2% for muscles was detected. The negative control group showed very less damaged DNA content in tail DNA(9.75±6.1%) while the positive control (2ug/L silver nanoparticles) it was highest (75.12± 13.8) confirming heavy DNA damage (Fig 6).
 |
Figure 5: Percentage of tail DNA in various tissues.
|
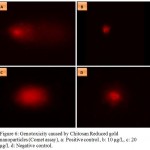 |
Figure 6: Genotoxicity caused by Chitosan Reduced gold nanoparticles (Comet assay), a: Positive control , b: 10 μg/L, c: 20 μg/L d: Negative control.
|
DNA damage is visible in positive control(Silver nanoparticles) while tail length shows concentration dependant damage. No DNA damage is present in negative control shown by absence of tail.
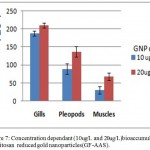 |
Figure 7: Concentration dependant (10ug/L and 20ug/L)bioaccumulation of chitosan reduced gold nanoparticles(GF-AAS).
|
Least bioaccumulation is evident in muscle tissues and highest in gills
GF-AAS study showed maximum accumulation of CRGNPs in gill tissues of the treated due to maximum exposure to tank water containing nanoparticles.
Discussion
The polymer coated metal nanoparticles are becoming popular as they are known to have less toxicity (Castillo et al., 2008 ;Singh et al., 2009). The unique features of CRGNPs are tunable core size, large surface to volume ratio, monodispersity and easy functionalization with many biomolecules ( Shan et al., 2012; Zhang et al., 2010). The mean particle size of gold nanoparticles was found to be in the size range as previously reported in studies. Many authors have reported size of the gold nanoparticles ranging from 20 nm to 450nm ( Gan et al .,200; Li et al.,2010). Analysis of gold nanoparticles by polydipersity index suggested narrow range of particle size distribution. Nanoparticles had higher value of zeta potential which showed moderate stability as zeta potential above 30 mV are considered to be more stable ( Torchilin VP.,2007) (Fig 1). CRGNPs were synthesized using Chitosan which acted as a reducing agent and provided the positive charge to nanoparticles. The overall positive charge on CRGNPs was evident in Zeta potential measurement and also confirmed the presence of a Chitosan coating over gold nanoparticles. GNP concentrations (10µg/L, 20µg/L) were administered to the animals to ascertain toxic effect on various tissues. Concentration above 80µg/L was found to be toxic by initial trials carried out in our lab. (Li et al.,2010) found gold nanoparticles toxicity at the dose of 15µg/L during immersion treatment in Daphnia magna. Immersion treatment facilitated even distribution of GNPs in the solution.
Conjugation of plasmid DNA to CRGNPs was the deciding factor during the study. Since the conjugation could be detected as soon as the buffer containing DNA was added to the nanoparticle solution as evidenced by the change in coloration. The conjugated solution looked purplish in color suggesting increased particle size and increased light scattering resulting in red-shift effect (Huang X and El-Sayed M 2010). When the gel retardation assay for conjugated nanoparticles was carried out, the DNA-nanoconjugates could not migrate in the gel as the neutralization of the negatively charged phosphate group in DNA with the positive charge of nanoparticle obstructed its migration (Wu et al.,2010).This confirmed the conjugation of DNA with nanoparticles. Lesser fluorescence in DNA-nanoconjugates was mainly due to fluorescence quenching by gold nanoparticle and increase in publication in the nano-conjugated sample (Kainthan et al.,2006).
Consequently, the nanoconjugates after administration to experimental animals via immersion treatment, may possibly escape the endo-lysosomal sections and entered the cytoplasm. Upon entering in endosomes, the proton sponge effect of nanoparticles caused osmotic swelling and subsequent endosomal rupture. This facilitated the escape of DNA-gold nanoconjugate into the cytoplasm and subsequent integration in the nuclear DNA (Guo et al.,2010 ;Benjaminsen et al.,2012).
Successful delivery of nanoparticles also brings with it the chances of toxic effects.( Li et al., 2010; Nandanpawar et al.,2013) has reported that the toxicity of silver nanoparticles ranged from 3-4 µg/L in Daphnia magna. (Bar-Ilan et al., 2009) exposed transparent zebrafish embryos to colloidal silver nanoparticles at various sizes (3, 10, 50, and 100 NM), at a concentration of 100 or 250 μM, from 4 to 120 hours post fertilization (hpf). Silver nanoparticles produced almost 100% mortality at 120 hpf. In our experiment, when the prawns were exposed to silver nanoparticles (2µl/L) as a positive control, significant mortality was observed and necrosis was visible. Comet assay for CRGPs showed significant DNA damage at higher dose, i.e. 20 µg/L was found to be significant. Higher percentage of tail DNA was indicative of more DNA damage as a consequence of single and double strand breaks (AshaRani et al., 2009). On the contrary (Schulz et al., 2012) found no genotoxicity in the trachea of rats at 18 μg concentrations of GNPs. However, in the present study, dose dependant toxicity of GNPs was evident since the exposure time of animals to the variable concentrations was constant. The similar result was also reported by various researchers (Wiwanitkit et al., 2009; Hirn et al., 2011). The concentration of gold nanoparticles@10µg/L was less toxic with wide tissue distribution and hence could be considered as a safe dose for using CRGNP as delivery vehicles in biomedical applications like gene and vaccine delivery. Low toxicity may be attributed to the non-toxic polymer Chitosan coating over CRGNPs. (Das et al.,2012) established a correlation between the capping material and genotoxicity of Gold Nanoparticles. The highest toxicity was reported in gold nanoparticles capped with aspartic acid treatment, while those coated with bovine serum albumin (GNPB) were most biocompatible.
Our study showed a direct correlation between CRGNP dose and bioaccumulation in the organs like gill, pleopods and muscle (Fig 7). The extent of DNA damage and bioaccumulation of gold nanoparticles in pleopods on an average were lesser than gills (Fig 5,7) which may be due to the hardened cell structure of pleopods. The least bioaccumulation was found in muscle tissues. (Lasagna et al.,2010) reported reduced bioaccumulation of GNPs as the dose increased. Our study supported this finding as concentration dependant bioaccumulation was observed (Fig 7). The systemic toxicity of gold nanoparticles in the intermediate size range (18–37 nm) was linked to major organ damage in the liver, spleen, and lungs in mice. The muscles being less exposed to aquatic environment had less chances of damage as the treatment was given by immersing the animals in the nanoparticles suspended water. In vivo studies of gold nanoparticles have reported bioaccumulation in important body organs, acute inflammation and apoptosis in the liver ( Wiwanitkit et al.,2009; Xia et al ., 2006). On the other hand, repeated administration of higher dose caused no toxicity of gold nanoparticles in mice ( Lasagna et al.,2010). The absence of mortality at lower doses may be because of the hardy nature of prawn juveniles as compared to the zebrafish embryos.
Conclusion
The present study concludes that gold nanoparticles do not cause significant genotoxicity but is responsible for bioaccumulation at higher concentrations. The toxicologically safe doses identified in this study can be used for various applications like gene and drug delivery. The immersion treatment can be considered as an efficient and convenient method of nanoparticles administration when large numbers of animals have to be treated simultaneously with minimum stress. Though Chitosan nanoparticles and gold nanoparticles are considered as safe in nature, their combined effects may indicate some chemical or biological interactions that are still uncharacterized. Further studies on the interaction of these nanoparticles to different biological systems also needs to be advocated for the development of a better delivery system in terms of safety and efficiency.
Acknowledgement
The authors are thankful to ICAR, New Delhi and Director, ICAR-CIFE for providing funds and necessary equipments for this research.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater 20th edition. Public Health. 1999.
- Asharani P .V, Wu L.Y, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19(25):255102.
Crossref - AshaRani P V, Kah L.M.G, Hande M.P, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells – SOM. ACS Nano. 2009;3(2):279–90.
- Bar-Ilan O, Albrecht R.M, Fako V.E, Furgeson D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5(16):1897–910.
CrossRef - Baun , Hartmann N.B, Grieger K, Kusk K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17(5):387–95.
- Benjaminsen R.V, Mattebjerg M.a, Henriksen J.R, Moghimi S.M, Andresen T.L .The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Molecular Therarpy. 2012; 21(1):149–57.
- Bhirde A, Xie J, Swierczewska M, Chen X. Nanoparticles for cell labeling. Nanoscale. 2011;3(1):142–53. Available from: http://www.mendeley.com/catalog/nanoparticles-cell-labeling/
CrossRef - Bhumkar D.R, Joshi H.M, Sastry M, Pokharkar V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharmaceutical Research. 2007;24(8):1415–26.
CrossRef - Castillo P.M, Herrera J.L, Fernandez-Montesinos R, Caro C, Zaderenko A.P, Mejías J.A. Tiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by Toll-like receptor ligands. Nanomedicine. 2008;3(5):627–35.
CrossRef - Chang Y-N, Zhang M, Xia L, Zhang J, Xing G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials (Basel). 2012;5(12):2850–71.
CrossRef - Chen Y.S, Hung Y.C, Liau I, Huang G.S. 2009 Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4(8):858–64.
CrossRef - Cheng J, Cheng S.H. Influence of carbon nanotube length on toxicity to zebrafish embryos. International Journal of Nanomedicine. 2012;7:3731–9.
CrossRef - Das S, Debnath N, Mitra S, Datta A, Goswami A. Comparative analysis of stability and toxicity profile of three differently capped gold nanoparticles for biomedical usage. Biometals. 2012;25(5):1009–22.
CrossRef - Duceppe N, Tabrizian M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opinion in Drug Delivery. 2010;7(10):1191–207.
CrossRef - Freese C, Uboldi C, Gibson M.I, Unger R.E, Weksler B.B, Romero I.A. Uptake and cytotoxicity of citrate-coated gold nanospheres : comparative studies on human endothelial and epithelial cells.Particle and Fibre Toxicology. 2012
CrossRef - Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surfaces B; Biointerfaces. 2005; 44(2–3):65–73.
CrossRef - Ghosh P, Han G, De M, Kim C.K, Rotello V.M. Gold nanoparticles in delivery applications. Advances in Drug Delivery Reviwes. 2008;60(11):1307–15.
CrossRef - Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z. Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte. ACS Nano. 2010;4(9):5505–11.
CrossRef - Hans M, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State Material Science. 2002;6(4):319–27.
CrossRef - Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. European Journal of Pharmeutics and Biopharmaceutics. 2011;77(3):407–16.
CrossRef - Huang X, El-Sayed M. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of Advanced Research. 2010;(1):13–28.
CrossRef - Jeng H.A, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances and Environmental engineering. 2006;41(12):2699–711.
CrossRef - Judy J.D, Unrine J.M, Bertsch P.M. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environmental Science Technology. 2011;45(2):776–81.
CrossRef - Kainthan R.K, Gnanamani M, Ganguli M, Ghosh T, Brooks D.E, Maiti S. Blood compatibility of novel water soluble hyperbranched polyglycerol-based multivalent cationic polymers and their interaction with DNA. Biomaterials. 2006;27(31):5377–90.
CrossRef - Kashyap P.L, Xiang X, Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Intermational Journal of Biological Macromolecules. 2015;77:36–51.
CrossRef - Kehoe D.F, Sullivan D.M, Smith R.L. Determination of gold in animal tissue by graphite furnace atomic absorption spectrophotometry Journal-Association of official Analytical Chemists. 1988;71(6):1153–5.
- Kumari A, Yadav S.K, Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B Biointerfaces. 2010;75(1):1–18.
CrossRef - Lai J.C.K, Lai M.B, Jandhyam S, Dukhande V.V, Bhushan A, Daniels C.K. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. International Journal Nanomedicine. 2008;3(4):533–45.
- Lasagna-Reeves C, Gonzalez-Romero D, Barria M.a, Olmedo I, Clos , Sadagopa Ramanujam V.M. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochemical Biophysical Research Communications. 2010;393(4):649–55.
CrossRef - Li L, Lin S.L, Deng L, Liu Z.G. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black seabream Acanthopagrus schlegelii Bleeker to protect from Vibrio parahaemolyticus. Journal of Fish Diseases. 2013;36(12):987–95.
- Li T, Albee B, Alemayehu M, Diaz R, Ingham L, Kamal S. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anaytica and Bioanaytical Chemistry. 2010;398:689–700.
- Li Y-P, Pei Y-Y, Zhang X-Y, Gu Z-H, Zhou Z-H, Yuan W.F. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. Journal of Controlled Release. 2001;71(2):203–11.
CrossRef - Mahapatro A, Singh D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. Journal of Nanobiotechnology. 2011;9(1):55.
CrossRef - Nandanpawar P, Badhe M, Rather M.A and Sharma R. Chitosan nanoparticles for gene delivery in Macrobrachium rosenbergii (DE MAN 1879) Journal of Cell and Tissue Research. 2013;13(2):3619-3624.
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–9.
CrossRef - Pavlica M, Klobučar G.I .V, Mojaš N, Erben R, Papeš D. Detection of DNA damage in haemocytes of zebra mussel using comet assay. Mutatation Research – Genetic Toxicology Environmental Mutagens. 2001;490:209–14.
- Pokharkar V, Dhar S, Bhumkar D, Mali V, Bodhankar S, Prasad B.L.V. Acute and Subacute Toxicity Studies of Chitosan Reduced Gold Nanoparticles: A Novel Carrier for Therapeutic Agents. Journal of Biomedical Nanotechnology. 2009;5(3):233–9.
CrossRef - Pradeep T, Anshup . Noble metal nanoparticles for water purification: A critical review. Thin Solid Films. 2009;517(24):6441–78.
- Rajeshkumar S, Venkatesan C, Sarathi M, Sarathbabu V, Thomas J, Anver Basha K. Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish and Shellfish Immunology. 2009;26(3):429–37.
CrossRef - Ramya V.L, Sharma R, Gireesh-Babu P, Patchala S.R, Rather A, Nandanpawar P.C. Development of chitosan conjugated DNA vaccine against nodavirus in Macrobrachium rosenbergii (De Man, 1879). Journal of Fish Diseases. 2014;37(9):815–24.
CrossRef - Rather M.A, Bhat I.A, Gireesh-Babu P, Chaudhari A, Sundaray J.K and Sharma R. Molecular characterization of kisspeptin gene and effect of nanoencapsulted kisspeptin-10 on reproductive maturation in Catla catla. Domestic Animal Endocrinology. 2016;56:36-47.
CrossRef - Rather M.A, Sharma R, Gupta S, Ferosekhan S, Ramya V.L, Jadhao S.B. Chitosan-Nanoconjugated Hormone Nanoparticles for Sustained Surge of Gonadotropins and Enhanced Reproductive Output in Female Fish. PLoS One. 2013;8(2):1–10.
CrossRef - Roy K, Mao H.Q, Huang S.K, Leong K.W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nature Medicine. 19995(4):387–91.
CrossRef - Schulz M, Ma-hock L, Brill S, Strauss V, Treumann S, Gröters S. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. Mutation Research Toxicology Environmental Mutagens. 2012;745(1–2):51–7.
CrossRef - Shan Y, Luo T, Peng C, Sheng R, Cao A, Cao X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials. 2012;33(10):3025–35.
CrossRef - Sharif F, Porta F, Meijer A.H, Kros A, Richardson M.K. Mesoporous silica nanoparticles as a compound delivery system in zebrafish embryos. International Journal of Nanomedicine. 2012;7:1875–90.
CrossRef - Sharma N, Rather M.A, Gireesh-Babu P, Kundan K, Malachy N.O.A and Sharma R. Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nanoparticles. Food and chemical toxicology. 2016;96 96-122.
- Shinde S.K, Grampurohit N.D, Gaikwad D.D, Jadhav S.L, Gadhave M .V, Shelke P.K. Toxicity induced by nanoparticles. Asian Pacific Jouranl of Tropical Diseases. 2012;2(4):331–4.
CrossRef - Singh A.V, Patil R, Kasture M.B, Gade W.N, Prasad B.L.V. Synthesis of Ag-Pt alloy nanoparticles in aqueous bovine serum albumin foam and their cytocompatibility against human gingival fibroblasts. Colloids Surfaces B; Biointerfaces. 2009;69(2):239–45.
CrossRef - Singh N.P, McCoy M.T, Tice R.R, Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175(1):184–91.
CrossRef - Soppimath K.S, Aminabhavi T.M, Kulkarni a R, Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release. 2001;70(1–2):1–20.
- Stefan M, Melnig V, Pricop D, Neagu A, Mihasan M, Tartau L.Attenuated effects of chitosan-capped gold nanoparticles on LPS-induced toxicity in laboratory rats. Material Science Engineering Part C. 2013;33(1):550–6.
CrossRef - Tang S, Huang Z, Zhang H, Wang Y, Hu Q, Jiang H. Design and formulation of trimethylated chitosan-graft-poly(ε-caprolactone) nanoparticles used for gene delivery. Carbohydrate Polymers. 2014;101(1):104–12.
CrossRef - Tedesco S, Doyle H, Blasco J, Redmond G, Sheehan D. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquatic Toxicology. 2010;100(2):178–86.
CrossRef - Torchilin V.P. Nanoparticulates as Drug Carriers. Nano Today. 2007;2(2):54.
- Turkevich J, Stevenson P.C, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussion Faraday Society. 1951;11:55.
CrossRef - Wiwanitkit V, Sereemaspun A, Rojanathanes R. Effect of gold nanoparticles on spermatozoa: the first world report. Fertility Sterility. 2009;91(1):e7–8.
CrossRef - Wu Y, Wang W, Chen Y, Huang K, Shuai X, Chen Q. The investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitro. International Journal of Nanomedicine. 2010;5(1):129–36.
CrossRef - Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Letters. 2006;6(8):1794–807.
CrossRef - Xu L, Guo C, Wang F, Zheng S, Liu C.Z. A simple and rapid harvesting method for microalgae by in situ magnetic separation. Bioresources Technology. 2011;102(21):10047–51.
CrossRef - Zhang X.D, Wu H.Y, Wu D, Wang Y.Y, Chang J.H, Bin Z.Z. Toxicologic effects of gold nanoparticles in vivo by different administration routes. International Journal of Nanomedicine. 2010;5(1):771–81.
CrossRef - Zhu S, Oberdörster E, Haasch M.L. Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Marine Environmental Research. 2016;62:5-9.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





