Manuscript accepted on : 20 March 2018
Published online on: --
Cold Active Amylases Producing Psychrotolerants Isolated from Nella Lake, Antarctica
Abhas Kumar Maharana and Shiv Mohan Singh
and Shiv Mohan Singh
Polar Biology Laboratory, National Centre for Antarctic and Ocean Research, Vasco-da-Gama, Goa-403804, India.
Corresponding Author E-mail: drshivmohansinghncaor@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2603
ABSTRACT: Cold active amylase was investigated by bacteria and yeast isolates from the sediment core samples of Nella Lake,Larsemann Hills region, East Antarctica. Between potential yeast and bacteria isolates screened for amylases, best isolates were identified asRhodotorula sp. Y-37 and ArthrobacteralpinusN16 by molecular technique.Amylase production capabilities of both the isolate subjected for optimization processes by using submerged fermentation technique with soluble starch as substrate.The results indicate that a supplement of 1% w/v glucose, 1% w/v yeast extract and 0.1% w/v KCl at pH 7.0with 5% v/v inoculum enhances the amylase production by 5.72-fold using Rhodotorula sp. Y-37. In other hands, the activators are 1% w/v of galactose and peptone, 0.1% w/v KCl and 2.5% v/v inoculum at pH 7.0 enhances the amylase production by 3.74-fold using ArthrobacteralpinusN16. Cold-active amylasecan be used in detergent, textile, food and beverage industries. Bio-degradation of starchy materials by cold active amylases can contribute in cleaning of environment at cold regions without harming the climate.
KEYWORDS: Amylase; Arthrobacteralpines; Cold Active; East Antarctica; Larsemann Hills; Rhodotorula sp.
Download this article as:| Copy the following to cite this article: Maharana A. K, Singh S. M. Cold Active Amylases Producing Psychrotolerants Isolated from Nella Lake, Antarctica. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Maharana A. K, Singh S. M. Cold Active Amylases Producing Psychrotolerants Isolated from Nella Lake, Antarctica. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29380 |
Introduction
An amylase is an enzyme that catalyses the hydrolysis of starch into sugars, are also regarded as glycoside hydrolases and act on α-1, 4-glycosidic bonds. It is of three types, i.e. α-amylases (EC 3.2.1.1), β-amylase (EC 3.2.1.2) and γ-amylase (EC 3.2.1.3).α-amylase acts up on starchy materials, and yields maltotriose, maltose and glucose as end product.Because it can act anywhere on the substrate, α-amylase tends to be faster-acting than β-amylase having optimum pH is 6.7-7.0.While optimum pH for β-amylase is 4.0-5.0 and γ-amylase has the most acidic optimum pH 3.0.
Many researchers focused on thermophilic amylase, and cold-active amylase very is less studied.1,2,3 Cold active amylase from bacteria likeAlteromonas sp,4 Nocardiopsis sp,5 Janthinobacterium sp,6 Arthrobacter sp7; yeasts likeCandida antarctica,8 Cryptococcus flavus,9 Rhodotorulaglacialis and Mrakiagelida10 and moldslike Aspergillus sp,11 Penicillium sp, Alternaria sp.,Absidia sp. and Fusarium sp.12,13 were studied from various sources.
Cold-active amylases have immense biotechnological applications like additives in processed food industries and detergents, textile industries, waste-water treatment, bio-pulping, bio-remediations in cold climates and molecular biology applications.14,15,16 Thus, cold-active amylases are becoming promising enzymes and a world-wide choice for biotechnologists, microbiologists, biochemists and pharmacists. Cold activeamylases function effectively at cold temperatures with high rates of catalysis in comparison to the amylases from their mesophilic or thermophilic counterparts, which shows little or almost no activity at a low temperature.17 Cold active α-amylasessave energy by overcoming the heating requirements, and minimize undesirable chemical reactions that could occur at high temperatures by removing contaminants.18
As cold-active enzymes have high catalytic activity at low temperatures with low thermo-stability at high temperatures,it is worthwhile to search for new cold activeamylases with novel properties from different sources. There is no information available on enzymes from microorganisms isolated from Nella Lake, Antarctica, which may be an ideal habitat for cold adapted micro-organisms. Besides, primary screening optimization is necessary in the enzymatic study to make the yield commercially accepted.19
Therefore, in the present study psychrotolerant yeasts and bacterial strains have been isolated from the sediment core samples of Nella Lake and production of α-amylases are optimized through submerged fermentation technology. The investigation led to the identification of a high α-amylase producing isolates asRhodotorula sp. Y-37 and ArthrobacteralpinusN16.
Materials and Methods
Sample Collection and Isolation of Bacteria and Yeasts
Sedimentcoresamples were collected from Lake Nella (76°22′ S, 69°24′ E), an ultra-oligotrophic lake located at an altitude of 15 m a.s.l. in the Broknes peninsula, Larsemann Hills region, East Antarctica. Core samples were cut off into small pieces from 0.5 cm to 90 cm, which were brought back to Polar Biology Lab, National Centre for Antarctic & Ocean Research with ice packs and kept at -20°C till investigation. Core samples were serially diluted by 10 fold dilution technique and spread over various media viz. Antarctic Biological Medium (ABM), Nutrient Agar (NA), Zobell Marine Agar (ZMA), Potato Dextrose Agar (PDA) and their 1/10th strengths also used for isolating the oligotrophic bacteria and yeasts. Plates were incubated for 1-2 weeks at various temperatures viz. 1, 5, 15 and 22°C and cultures after proper visibility were streaked on the same agar plate for further purification using microscopic technique (Epi-fluorescence research microscope, BX51 Olympus, Japan). Purified isolates were stored at 1°C (Refrigerated incubator, MIR-554-PE, Panasonic) and glycerol stocks were also prepared in their respective broth with 20% glycerol (v/v) and kept at -20°C (Biomedical freezer, MDF-U537D, Sanyo).
Primary Screening for Cold Active Amylase
Amylase assay was done by taking soluble starch agar plates (g/L: beef extract, 3.0; peptone, 5.0; soluble starch, 2.0; agar, 20.0; pH 7.0) and incubating at 4, 15 and 22°C by spot inoculation method for 10 days.12 After incubation, Gram’s iodine (Hi-media) was used for the zone of clearance.
Production and extraction of cold active amylase by submerged fermentation
Isolates were inoculated separately in Potato Dextrose Broth (PDB) and incubated at 15°C for 48 h at 150 rpm (Refrigerated incubator shaker, IS-971RF, Jeio tech, Korea). For production of amylase, freshly prepared inoculum was used. The production was done by using the modified mineral salt medium (%w/v: peptone, 0.6; KCl, 0.05; MgSO4, 0.05; K2HPO4, 0.03; KH2PO4, 0.03; CaCl2. 2H2O, 0.01; soluble starch, 0.1; pH 7.0) as recorded by Abe and co-workerswith some modifications.20 The production medium was incubated at 15°C and 150 rpm after addition of 5% (v/v) inoculum to autoclaved medium for 120 h. Each day about 5 ml medium was pulling out aseptically and extraction was done by centrifugation at 10,000 rpm for 20 minat 4°C. The supernatant was regarded as crude amylase enzyme and subjected for assay.
Amylase Assay
Amylase activity was measured spectro-photometrically(UV-Vis spectrophotometer, Specord S 205, Analytik Jena, AG Germany) using soluble starch (Hi-media) as a substrate.21 Modifications were done in the reaction mixture incubated at 15°C for 20 min along with respective sample blanks. One unit of amylase activity is defined as the quantity of enzyme that caused 0.01% reduction of blue color intensity of starch-iodine solution at 15°C in one min per ml at 690 nm.
Identification of Potential Isolates
Molecular identification of the potential isolates producing cold active amylase was done by using ITS-D1/D2 gene and 16s RNA gene sequencing based molecular technique for yeasts and bacteria, respectively. The isolates were outsourced to MTCC, Chandigarh, India for molecular identification. The gene sequences were used to carry out BLAST with the nr-database of GenBank database. The consensus sequence of sample was submitted at BankIt, GenBank, and NCBI for the accession numbers.
Partial Characterization of Potential Isolates
The potential isolates were subjected for different biochemical tests (catalase, oxidase, MRVP, indole, citrate utilization, esculinase and phosphatase tests).22 Carbon utilization testswere carried out using Hi Carbo™ kits (KB009, HiMedia,India) incubated for 2-7 days at 15°C. Besides, for physiological characterization, a loopful of culture was inoculated in flask containing 50 ml of broth and the flask were incubated at 5, 15, 22 and 35°C at 150 rpm in a shaking incubator. Growth was analyzed by spectrophotometer at 600 nm in the interval of 24 h. About 50 ml of broth with varied pH values (3, 5, 7, 9 and 11) and NaCl concentrations (0, 1, 5, 10 and 20% w/v) were tested for the growth of isolates at 15°C and 150 rpm in a shaking incubator. Furthermore, potential isolates were screened for other hydrolytic enzymes like protease, lipase and cellulase.
Production Optimization
Production optimization for cold active amylase was done by “one factor at-a-time” (OFAT) method. Factors investigated were incubation period (day 1-5), temperature (15 and 35°C), inoculum size (0.1-20% v/v), initial pH (5-12), nitrogen sources (1% w/v: yeast extract, beef extract, peptone, NaNO3, KNO3 and Ca(NO3)2.4H2O) and carbon sources (1% w/v: glucose, maltose, fructose, lactose, galactose, sucrose and xylose), and minerals (NaCl, KCl, MgCl2, MnCl2, BaCl2, HgCl2 and CaCl2). In each experiment, the optimized factor is replaced with the old one and a control devoid of additional factors was also incubated collaterally. All experiments were conducted in triplicates.
Statistical Analysis
The data recorded during the investigation were subjected to significance testing by analyses of variance (ANOVA) using Microsoft excel 2007. Statistical significance was set at P<0.05, and for high significance set at P<0.001. Results were denoted as mean ± standard error (SE) of triplicate experiments.
Results
Screening and Selection of Potential Amylase Producers
All the bacterial and yeast isolates were investigated for amylaseproduction at various temperatures (4, 15 and 22°C) by plate assay method using soluble starch. Only 11.24% isolate were positive for amylase (data not shown). Then best seven from both yeasts (Y-23, Y32, Y-33, Y-34, Y-35, Y-36 and Y37) and bacteria (N16, N21, N29, N33, N39, N59 and N60) were selected for further quantification by submerged fermentation technique using soluble starch at 15°C for 5 days. Day-wise quantification was done and Fig. 1 revealed the maximum amylase produced by Y-35 and N39 among yeasts and bacteria, respectively.ANOVA revealed that there is a significant difference between the yeastand bacterial isolates producing cold active amylase at P<0.01 while a high significant is seen i.e. P<0.001 between the incubation periodand amylase activity.
 |
Figure 1: Production of amylase by Nella Lake isolates using soluble starch at 15°C.
|
The results are the means of 3 independent experiments, and the bars correspond to standard errors.
Effects of Temperature on production of Amylase
The best 3 isolates from yeasts(Y-32, Y-35 and Y-37) and 5 from bacteria (N16, N21, N29, N39 and N60) were investigated for amylase production at both 15°C and 35°C which revealed maximal production by the yeast isolate Y-37 i.e. 358.75 IU/ml/min at 35°C where asbacteria N16 showed maximum amylase 666.51 IU/ml/min at 35°C on 4th day of incubation (Fig. 2A, B). But as the amylase assay was conducted at 15°C the amylase can be regarded as cold active amylase. Further optimization was conducted by using Y-37 and N16 using various parameters. ANOVA reveals significant differences in amylase activities among the yeast isolates day wise at P< 0.05 while same significant is found in case of amylase activities among all the bacteria at different temperatures.
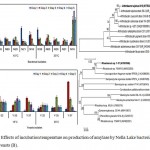 |
Figure 2: Effects of incubation temperature on production of amylase by Nella Lake bacteria (A) and yeasts (B).
|
Molecular phylogenetic analysis of N16 (C) and Y-37 (D), showing the position of strain N16 and Y-37 in relation to the other genera,GenBank accession numbers for the sequences are shown in parentheses;numbers at nodes denote bootstrap values based on 1000 replicates andevolutionary analyses were conducted in MEGA5.
Identification and Partial Characterization of Y-37 and N16
From molecular identification, it was found that N16 is identified as Arthrobacteralpinus N16 (GenBank accession no. KY783361) and Y-37 as Rhodotorula sp. Y-37 (GenBank accession no. KY887686) and phylogenetic tree is represented in Fig. 2C, D. Macro- and micro- morphology of isolatesN16 and Y-37with amylase assay plates are depicted in Fig. 3A-F.
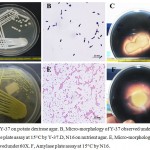 |
Figure 3: A, Y-37 on potato dextrose agar. B, Micro-morphology of Y-37 observed under 60X. C, Amylase plate assay at 15°C by Y-37.D, N16 on nutrient agar. E, Micro-morphology of N16 observed under 60X. F, Amylase plate assay at 15°C by N16.
|
Rhodotorula sp. Y-37 can utilize various sugars as carbon sources viz. D-glucose, raffinose, sucrose, L-sorbose, D-gluconate, D-ribose, D-arabinose, lactose, glycerol, DL-lactate, myoinositol, D-xylose, maltose, cellobiose, melezitose, D-glucoronate, mannitol, D-galactose, rhamnose and L-arabinose. Between nitrogen sources, Y-37 utilizes nitrate, nitrite, lysine, tryptophan and creatinine. It is negative for growth at 1% acetic acid and 50% glucose. Arthrobacteralpinus N16 is an aerobic, Gram-positive rod actino-bacterium and was tested for many biochemical analyses. It is positive for catalase and negative for oxidase tests, MRVP, indole, citrate utilization, esculinase and phosphatase tests. Besides, it can utilize carbon sources viz. glycerol and inositol. Optimum temperature for the growth of both the isolate is 15°C, followed by 5°C. It was found that there is little growth at 35°C and almost no growth in case of 40°C. It denotes psychrotolerant nature of Rhodotorula sp. Y-37 and ArthrobacteralpinusN16. Both the isolate have a wide range of pH tolerance from pH 3-11 while optimum is pH 9 and 11 by N16 and Y-37, respectively (Fig. 4). Salt tolerance tests revealed no growth found at 5%w/v by N16 while Y-37 can tolerate higher salt concentrations of 10% w/v. Y-37 is negative for cellulasewhile positive for protease and lipase whileN16 can degrade substrate apart from starch, i.e. tributyrin (cold active lipase) and negative for protease and cellulase.
 |
Figure 4: Physiological analysisofArthrobacteralpinusN16 and Rhodotorulasp. Y-37.
|
The results are the means of 3 independent experiments. NB: Nutrient Broth; PDB: Potato Dextrose Broth.
Effects of Inoculum Size on Production of Amylase
Various inoculum sizes ranging from 0.1-20 v/v were taken for optimal production of amylase by Rhodotorulasp. Y-37 and ArthrobacteralpinusN16 at 35°C and results are depicted in Fig. 5A, B. Maximum production was found by inoculum 5% v/v on 3rd day of incubation by Y-37 (204.35 IU/ml/min) while inoculum 2.5% v/v on 4th day of incubation was optimum for N16 (184.74 IU/ml/min). ANOVA reveals that there is a significant difference between the inoculum sizes and cold active amylase activities at P<0.01 along with the incubation periodby both the isolate.
Effects of Carbon Sources on Production of Amylase
Among1% w/v carbon sources were taken and added additionally into the production medium with a control having no additional carbon sources. Fig. 5C, D reveals all the carbon sources have induced effect on amylase production while glucose showed the maximum, i.e. 472.42 IU/ml/min by Y-37and1% sucrose was regarded as an optimum carbon source for N16. ANOVA reveals a high significant difference between the carbon sources investigated and cold active amylase activities at P<0.001 along with the incubation period by both the isolate.
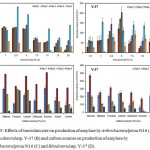 |
Figure 5: Effects of inoculum size on production of amylase by ArthrobacteralpinusN16 (A) and Rhodotorulasp. Y-37 (B) and carbon sources on production of amylase by ArthrobacteralpinusN16 (C) and Rhodotorulasp. Y-37 (D).
|
The results are the means of 3 independent experiments, and the bars correspond to standard errors.
Effects of Nitrogen Sources on Production of Amylase
Various types of organic and in-organic nitrogen sources as mentioned earlier were taken and additionally put into the production medium with a control having no additional nitrogen sources (Fig. 6A, B).All the organic nitrogen sources have induced effect, and yeast extract showed the maximum, i.e. 342.33 IU/ml/min on 2nd day of incubation by Y-37. Same pattern was seen in case of N16 in less production due to inorganic nitrogen sources and optimum was found due to 1% peptone on 3rd day of incubation. ANOVA reveals a high significant difference between the nitrogen sources investigated and cold active amylase activity at P<0.001 along with the incubation period by both Y-37 and N16.
Effects of Minerals on Production of Amylase
Various types of mineral sources (0.1% w/v) were taken as mentioned earlier and added additionally into the production medium with a control having no additional mineral sources (Fig. 6C, D). All the minerals except HgCl2, and CaCl2 has induced effect on amylase production while KCl showed the maximum, i.e. 418.3 IU/ml/min on 4th day of incubation by Rhodotorula sp. Y-37. ArthrobacteralpinusN-16 showed maximum amylase production by 0.1% KCl, i.e. 615.51 IU/ml/min on 4th day of incubation. ANOVA reveals that there is a high significant difference between the minerals and cold active amylase activities along with the incubation period at P<0.001 by Y-37 while at P< 0.05 by N16.
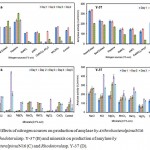 |
Figure 6: Effects of nitrogen sources on production of amylase by ArthrobacteralpinusN16 (A) and Rhodotorulasp. Y-37 (B) and minerals on production of amylase by Arthrobacteralpinus N16 (C) and Rhodotorulasp. Y-37 (D).
|
The results are the means of 3 independent experiments, and the bars correspond to standard errors.
Effects of Initial pH on Production of Amylase
Medium pH ranging from 5.0-12.0 was investigated, which revealed pH 7.0 as optimal for amylase production (936.74 IU/ml/min) on 4th day of incubation by Rhodotorula sp. Y-37 while Arthrobacteralpinus N16 produced 1147.51 IU/ml/min amylase on 3rd day of incubation (Fig. 7A, B). ANOVA reveals that there is a significant difference between the initial pH of the medium with that of cold active amylase at P<0.001 along the incubation period by both Y-37 and N16.
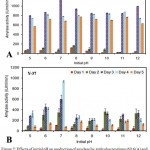 |
Figure 7: Effects of initial pH on production of amylase by ArthrobacteralpinusN16 (A) and Rhodotorulasp. Y-37 (B).
|
The results are the means of 3 independent experiments, and the bars correspond to standard errors.
Discussion
Sediment cores from Nella Lake showed heavy loads of both bacterial and yeast strains at lower temperatures. Besides, only fewer strains were positive for amylase production at a cold temperature. Among them, two isolate one each from bacteria and yeasts was selected assuming both qualitative and quantitative methods. By molecular technique, isolates were identified as Rhodotorula sp. Y-37 and ArthrobacteralpinusN16. The isolate Y-37 showed closest similarity (98%) with Rhodotorula sp. YSAR15(GenBank accession no. AM922292). YSAR15 was isolated from sediments of melt water stream of Arctic MidtreLovenbreen glacier. This strain was positive for amylase more at 22°C as compared to 8°C.23 Isolate N-16 showed closest similarity (99%) with ArthrobacteralpinusstrainS6-3 having GenBank accession no. NR_117254, a psychrophilic bacterium, which was isolated from alpine soil and cells exhibited a rod-coccus growth cycle and produced a yellow pigment and was also positive for starch degradation.24 There was a report of cold lipase produced by Antarcticstrain Rhodotorula sp. Y-23 isolated from Nella Lake which also have ability to produce cold active amylase25
There are many reports on cold adapted amylase from bacteria17,22 and fungi26,12,13,27 from various sources. Carrasco and co-workers reported about yeasts isolated from King George Island, the sub Antarctic region in which Cryptococcus and Rhodotorulaspp. showed amylase activity at a low temperature.28 Buzzini and Martini reported about amylase production by Cryptococcus and Rhodotorulaspp. at 25°C isolated from Brazilian rain forest.29 Rhodotorulamucilaginosa PT1, psychrotolerant yeast exhibited optimal amylase production at 25°C26. In contrast, Hatha and co-workersreported about amylase produced neither by Cryptococcus nor Rhodotorulaat 4°C or 20°C.30 The same pattern was also seen.10 Present study contradicts with the results of Sahay (2015)who reported about a psychrotolerant Rhodotorulamucilaginosa BPT1 which showed maximal production (2.16 U/ml) of cold active amylase after 96 hwith 1% (v/v) inoculum at 40°C incubation using 2% (w/v) soluble starch.27 However, there are many reports on cold active amylase production more at neutral pH by psychrotolerant Rhodotorula mucilaginosa.26,27
Temperature is the most vital factor that not only affects the growth of microorganism but also meant for the thermo stability nature of enzymes. Present study reported maximum production was found at 35°C as compared with 15°C which denotes the psychrotolerant nature of both the isolates and also denotes the stability of amylases produced at that condition. As the assay was done at 15°C with 1% soluble starch, therefore it can be regarded as cold tolerant amylase. Enzymes from psychrotolerants are much important than the enzymes from psychrophilic and mesophilic counterparts, which may be due to a wider range of temperature tolerance. Besides, there are many reports of getting amylase from psychrotolerant isolates at a higher temperature than colder.26-27 Cold tolerant amylase can be used in textile industries for desizing of grey fabrics, and low temperature saves energy consumptions effectively. Another application is fermentation of starch to ethanol, for which in general much higher temperature is needed with heavy applications of amylase. However, by introduction of cold active amylase, it minimizes energy consumptions with minimal enzyme concentrations for optimal production of ethanol from starchy materials.17 To control the freezing point of frozen foods maltotetraose syrup was used, which is produced by starch with amylase.31 Smitaand co-workersreported on Lactobacillus plantarum producing cold active amylase best at 35°C on 36 h of incubation.32 Munagantiand co-workers33 reported about α-amylase produced by Arthrobacterkerguelensis VL-RK_09 which showed maximum amylase activity induced by yeast extract at pH 7.0 on 4th day of incubation at 35°C whereas maximum cold active α-amylase production at 4°C was reported from Antarctic psychrophile Alteromonas haloplanktis.3
Inoculum size is a most important factor for initialization of enzyme production. Lower or much higher concentrations of inoculum size is destructive for enzymes, as an increase in inoculum size leads to increase in the duration of the initial lag phase.34 In contrast, lower inoculum size needs more time to grow and have slower log rate leads to lower production of enzymes.35
Amylase is an inducible enzyme and is generally induced in the presence of starch or its hydrolytic product, maltose and organic nitrogen sources, have been preferred for the production of α-amylase.36-38 Similar result of getting organic nitrogen sources for better amylase production was found in this present study. Aiyer (2004) reported about different carbon and nitrogen sources impact on production of amylase by Bacillus licheniformis SPT 27 who reported about more production using organic nitrogen sources than inorganic sources and optimum was achieved by peptone.39 Amylase is regarded as metallo-enzyme, therefore production is induced by the introduction of metal ions. Sivaramakrishnanand co-workersreported aboutα-amylase by Bacillus cereus MTCC 1305, revealed glucose showed enhanced enzyme production.40 Roohiand co-workersreported cold active amylase produced by Microbacteriumfoliorum GA2 isolated from Gangotri glacier, Western Himalaya, India.38 They reported about media factors for optimal production of amylase and reported 1% w/v yeast extract, and metal ion Mg2+ regarded as good producers whereas Ca2+ and Hg2+ showed inhibitory effects on amylase production. It corroborates with our present study.
Initial pH of the production medium affects the growth rate of microorganisms, leads to enzyme production. Inappropriate pH in the production medium alters the 3-D shape of the enzyme which in turn reform protein recognition and the enzyme might become inactive41. Present study reveals a neutral pH for both the isolate Y-37 and N16. Besides, N16 showed a wide range of pH tolerance from pH 5-12 indicating the amylase as novel enzyme, which can be used in clothing and dishwasher detergents to dissolve starches from fabrics and dishes, respectively. Cold active and alkaline amylases protect the colour of delicate clothes. There are many reports of getting more amylases at pH 7.032,33,42,43 which endorse our results.
Conclusions
From this study, it can be concluded that NellaLake isolates have the potentiality to produce cold active amylase and various production parameters were investigated for the hyper amylase production. It successfully produced 5.72 fold increase in amylase production using Rhodotorula sp. Y-37 and 3.74 fold using ArthrobacteralpinusN16 from un-optimized conditions. The studied cold and alkaline active amylase can be used in various industries like food and beverages, detergents, textiles, paper and bakery industries. Besides, this is the first report on the microbiological study from Nella Lake, Antarctica as per best of our knowledge, which may lead to get isolates having novel metabolites for the usefulness of mankind. Furthermore, biodegradation of starchy materials at cold regions by the application of cold active amylas es make it a harmless application as enzymes are non-toxic in nature, which contribute a greater role in cleaning of environment.
Acknowledgements
This study was financed by institutional project “Microbial Diversity of Antarctica” at National Centre for Antarctic & Ocean Research, Vasco-da-Gama, Goa, India. Authors are grateful to the Director, NCAOR, Goa, for facilities and Ms.SimantiniNaik for technical help. Authors want to thankDr.Alok Kumar Srivastava, Principle Scientist, Plant Pathology, ICAR-NBAIM, Kushmaur, U.P. and Dr. G.S. Prasad, Chief Scientist, Institute of Microbial Technology, Chandigarh for molecular identification of isolates.
Conflicts of Interest
The authors declare that they have no conflict of interest. This research was funded by Ministry of Earth Sciences, National Centre for Antarctic and Ocean Research.
References
- Aghajari N, Feller G, Gerday C, Haser R. Structures of the psychrophilic Alteromonashaloplanctis α-amylase give insights into cold adaptation at a molecular level. Structure. 1998;6(12):1503-16.
CrossRef - Amico D.S, Gerday C, Feller G. Temperature adaptation of proteins: engineering mesophilic-like activity and stability in a cold-adapted α-amylase. Journal of Molecular Biology. 2003;332(5):981-8.
CrossRef - Feller G, Bussy O.L, Gerday C. Expression of psychrophilic genes in mesophilic hosts; assessment of the folding state of a recombinant α-amylase. Applied and Environmental Microbiology. 1998;64:1163-5.
- Feller G, Lonhienne T, Deroanne C, Libioulle C, BeeumenV.J, Gerday C. Purification, characterization, and nucleotide sequence of the thermolabile alpha-amylase from the antarcticpsychrotrophAlteromonashaloplanctis A23. Journal of Biological Chemistry. 1992;267(8):5217-21.
- Zhang J.W, Zeng R.Y. Purification and characterization of a cold-adapted α-amylase produced by Nocardiopsissp. 7326 isolated from Prydz Bay, Antarctic. Mar. Biotechnol. 2008;10(1):75-82.
CrossRef - Männistö M.K, Häggblom M.M. Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst. Appl. Microbiol. 2006;29(3):229-43.
CrossRef - Smith M.R, Zahnley J.C. Production of amylase by Arthrobacterpsychrolactophilus. J. Ind. Microbiol. Biotechnol. 2005;32(7):277-83.
CrossRef - Mot D.R, Verachtert H. Regulation of the amylase secretion by the yeast Filobasidiumcapsuligenum and a 2-deoxy-D-glucose resistant mutant. Applied Microbiology and Biotechnology. 1987;26(3):258-62.
CrossRef - Wanderley K.J, Torres F.A, Moraes L.M, Ulhoa C.J. Biochemical characterization of α-amylase from the yeast Cryptococcus flavus. FEMS Microbiol.Lett. 2014;231(2):165-9.
CrossRef - Carrasco M, Villarreal P, Barahona S, Alcaíno J, Cifuentes V, Baeza M. Screening and characterization of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiology. 2016;16(21):1-9.
CrossRef - Akpan I, Adelaja F.A. Production and stabilization of amylase preparations from rice bran solid medium. World Journal of Microbiology and Biotechnology. 2004;20:47-50.
CrossRef - Maharana A.K, Ray P. Low temperature degradation of various substrates by psychrotolerantFusarium spp. isolated from soil of Jammu city. J. Adv. Microbiol. 2014;1:52-6.
- Maharana A.K, Ray P. Screening of psychrotrophic micro-fungi for cold active extracellular enzymes isolated from Jammu city, India. J Pure Appl. Microbiol. 2014;8:2369-75.
- Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilicBacillus sp. isolate ANT-6. Process Biochemistry. 2003;38(10):1397-403.
CrossRef - Haki G.D, Rakshit S.K. Developments in industrially important thermostable enzymes: a review. Bioresource Technology. 2003;89(1):17-34.
CrossRef - Tonkova A. Microbial starch converting enzymes of the α-amylase family. In: Microbial Biotechnology in Horticulture (Ray CR & Words OP, ed). Enfield, New Hampshire, USAScience Publishers. 2006;421-472.
- Kuddus M, Roohi J.M, Ramteke P.W. An overview of cold-active microbial α-amylase: adaptation strategies and biotechnological potentials. Biotechnology. 2011;10(3):246-58.
CrossRef - Ramteke P, Bhatt M.K. Cold active polysaccridases and their potential industrial applications. Res. Signpost. 2007;37:661-73
- Maharana A, Ray P. A novel cold-active lipase from psychrotolerantPseudomonas sp. AKM-L5 showed organic solvent resistant and suitable for detergent formulation. J. Mol. Cata. B Enzy. 2015 120:173-8.
CrossRef - Abe J.I, Nakajima K, Nagano H, Hizukuri S, Obata, K. Properties of the raw-starch digesting amylase of Aspergillus sp. K-27: a synergistic action of glucoamylase and alpha-amylase. Carbohydrate Research. 1988; 175(1):85-92.
CrossRef - Swain M.R, Kar S, Padmaja G, Ray R.C. Partial characterization and optimization of production of extracellular-amylase from Bacillus subtilis isolated from culturable cow dung microflora. Pol. J. Microbiol. 2006;55(4):289-96.
- Maharana A.K, Ray P. Isolation and screening of cold active extracellular enzymes producing psychrotrophic bacteria from soil of Jammu City. Biosci.Biotech. Res. Asia., 2013;10:267-73.
CrossRef - Pathan A.A.K, Bhadra B, Begum Z, Shivaji S. Diversity of yeasts from puddles in the vicinity of midrelovenbreen glacier, arctic and bioprospecting for enzymes and fatty acids. Curr.Microbiol. 2010;60(4): 307-14.
CrossRef - Zhang D.C, Schumann P, Liu H.C, Xin Y.H, Zhou Y.G, Schinner F, Margesin R. Arthrobacteralpinussp. nov., a psychrophilic bacterium isolated from alpine soil. Int. J. Syst. Evol. Microbiol. 2010;60(9):2149-53.
CrossRef - Maharana A.K, Singh S.M. A cold and organic solvent tolerant lipase produced by Antarctic strain Rhodotorula sp. Y-23. J. Basic. Microbiol. 2018;58:1-12. https://doi.org/10.1002/jobm.201700638.
CrossRef - Hamid B. Cold-active α-amylase from psychrophilic and psychrotolerant yeast. Journal of Global Biosciences. 2015;4(7):2670-7.
- Sahay S. Thermo-tolerant cold-active amylolytic activity from a psychrotolerant yeast isolate, Rhodotorulamucilaginosa BPT1. Scholars Acad. J. Biosci. 2015;3(4):406-12.
- Carrasco M, Rozas J.M, Barahona S, Alcaíno J, Cifuentes V, Baeza M. Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiology. 2012; 12(251):1-9.
CrossRef - Buzzini P, Martini A. Extracellular enzymatic activity profiles in yeast and yeast‐like strains isolated from tropical environments. Journal of Applied Microbiology. 2002;93(6):1020-5.
CrossRef - Hatha A.A.M, Rahima M.K.M, Krishnan K.P, Saramma A.V, Saritha G. Characterization and bio-prospecting of cold adapted yeast from water samples of kongsfjord, Norwegian Artic. Indian Journal of Geo-Marine Sciences. 2013;42(4):458-65.
- Aiyer P.V. Amylases and their applications. African Journal of Biotechnology. 2005;4(13):1525-9.
- Smita H.P, Mnas R.S, Shaktimay K, Ramesh C.R, Dider M. Statistical optimization of α-amylase production from probiotic Lactobacillus plantarum MTCC1407 in submerged fermentation. Pol. J. Microbiol. 2008; 57(2):149-55.
- Munaganti R.K, Muvva V, Naragani K. Production of amylase by ArthrobacterkerguelensisVL-RK_09 isolated from Mango Orchards.British Biotech. J. 2015;8(4):1-10.
CrossRef - Singh A.K, Maharana A.K, Masih H, Kumar Y, Mishra S.K. Production, optimization and purification of bacterial cellulase by solid state bio-processing of agro-biomass. Res. J. Pharm. Biol. Chem. Sci. 2012;3:977-89.
- Maharana A.K, Ray P. Optimization and characterization of cold-active endoglucanase produced by Aspergillusterreus strain AKM-F3 grown on sugarcane bagasse. Turk. J. Biol. 2015;39:175-85.
CrossRef - Coronado M.J, Vargas C, Hofemeister J, Ventosa A, Nieto J.J. Production and biochemical characterization of an α-amylase from the moderate halophile Halomonasmeridiana. FEMS Microbiology Letters. 2000;183(1): 67-71.
- Pedersen H, Nielsen J. The influence of nitrogen sources on the α-amylase productivity of Aspergillusoryzae in continuous cultures. Appl. Microbiol. Biotechnol. 2000;53(3):278-81.
CrossRef - Kuddus R.M, Ahmad I.Z, Arif J.M. Production of cold-active extracellular α-amylase by newly isolated Microbacteriumfoliorum GA2 from Gangotri glacier, Western Himalaya, India. Asian J. Biotechnol. 2011;3: 449-59.
CrossRef - Aiyer P.D. Effect of C:N ratio on alpha amylase production by Bacillus licheniformis SPT 27. African Journal of Biotechnology. 2004;3(10):519-22.
CrossRef - Sivaramakrishnan S, Gangadharan D, Nampoothiri K.M, Soccol C.R, Pandey A. Alpha amylase production by Aspergillusoryzae employing solid-state fermentation. J. Sci. Ind. Res. 2007;66(8):621-6.
- Tortora G.J, Funke B.R, Case C.L. Microbiology: An introduction. San Francisco, CA: Benjamin Cummings. 2004.
- Ikram-Ul-Haq N.S, Shamim N, Ashraf H, Ali S, Qadeer M. Effect of surfactants on the biosynthesis of alpha amylase by Bacillus subtilis GCBM-25. Pakistan Journal of Botany. 2005;37:373-9.
- Tanyildizi M.S, Özer D, Elibol M. Optimization of α-amylase production by Bacillus sp. using response surface methodology. Process Biochem. 2005;40(7):2291-6.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





