How to Cite | Publication History | PlumX Article Matrix
M. Edalati Nasab1 , A. A. Naserian2, A. R. Vakili2 and A. M. Tahmasbi2
, A. A. Naserian2, A. R. Vakili2 and A. M. Tahmasbi2
1Ruminant Nutrition, Ferdowsi University of Mashhad International Campus, Mashhad, Iran.
2Department of Animal Sciences, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran.
Corresponding Author E-mail: Abasalin@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2625
ABSTRACT: The purpose of Present study was conducted to evaluate the effects of adding essential oils (Ziziphora clinopodioides and Mentha pulegium) on alfalfa silage on the rumen degradation parameters with in vitro technique. Present study was performed by utilizing an in vitro gas production method at various incubation intervals. Rumen fluid taken from three lactation, rumen-fistulated Holstein cows. The gas production rate was measured at standard times from 0, to 96 hours. The outcomes of this experiment show that Ziziphora clinopodioides and also Mentha pulegium essential oils had a positive influence on gas production rate. Silage content with Mentha pulegium had more decrease effects than Ziziphora clinopodioides in Gas production compare with control silage and it was significant. Gas production values (at 96 h incubation) in silage with no added essential oils, 30ML of Ziziphora clinopodioides and 30ML of Mentha pulegium were 68.82, 56.12 and 49.74, respectively. Compared with control, aerobic stability had a significant difference and it was developed in silage treated with essential oils. The findings of their findings showed that these essential oils could be used to increase the performance of ruminants. In addition, adding essential oils could change the rumen fermentation in ruminant, however, more research is still needed to proving this conclusion.
KEYWORDS: Essential Oils;Mentha Pulegium; Silage Ziziphora Clinopodioides;
Download this article as:| Copy the following to cite this article: Nasab M. E, Naserian A. A, Vakili A. R, Tahmasbi A. M. Effect of using Essential Oils of Ziziphora Clinopodioides and Mentha Pulegium As Additive on In Vitro Study. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Nasab M. E, Naserian A. A, Vakili A. R, Tahmasbi A. M. Effect of using Essential Oils of Ziziphora Clinopodioides and Mentha Pulegium As Additive on In Vitro Study. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29542 |
Introduction
Ruminants have an especial digestive trace that consists microorganisms who degrade feed contents and supply energy and protein for the animals. Ruminant nutritionists have at length been intrigued by modulating those rival microorganisms among different microbial populaces with the destination from claiming moving forward that effectiveness for the vitality of protein utilization in the rumen. This need to be been attained via those streamlining for the formulation of ration and using feed additives which change the environment and upgrade or harness particular microbial populaces (Calsamiglia et al. 2006).
According to Calsamiglia et al. (2006), for this reason, researchers have ended up intrigued by assessing additives with regulating rumen fermentation, such as the utilization of yeasts, natural acids, plant extracts, probiotics, as well as antibodies. EOs (essential oils) have a great impact on health, for example, on cardiovascular diseases, tumors, inflammation, and the gradual elimination of free radicals (Harborne and Williams, 2000; Reddy et al. 2003; Trouillas et al. 2003). These properties rely on upon their capacity to discover free radicals, harness peroxidation of lipids that find a structure of membrane cell. Also, these properties raised the movement of cell reinforcement proteins (Gutierrez et al. , 2003; Lee et al. 2003). Antiseptics and antimicrobials are considered as the most important activities of these compounds. disinfecting properties of many plants have been known from many years ago. For the first time, Borchers (1965) mention the possible advantage of using essential oils on microbial fermentation in the rumen. Borchers (1965) also in an in vitro study viewed that eke out thymol to rumen fluid caused the aggregation of amino acid Nitrogen (AA-N) and the decreased from claiming Ammodytidae N concentrations, offering that thymol prevents amino acid catabolism. After that, Oh et al. (1967, 1968) guessed that maybe the slight palatability of some plants by ruminants is related to both of organoleptic effects, and their negative impact on fermentation by rumen microbial as well as digestion of nutrient.
Considering these cases, the purpose of the current research was investigating impacts of two essential oils (Ziziphora clinopodioides and Mentha pulegium) on rumen fermentation with gas production method.
Materials and Methods
Rumen fluid was taken from four lactating, Holstein cows added with rumen-fistulate (body weight 620± 8.9kg; day in milk 45 ± 13). The cows were fed a total blended diet (chemical analysis mention in table 1) containing silage of barley (46.5%), corn (6.8%), silage of hay alfalfa (4.5%), steam rolled barley (17.7%), dairy supplement pellets (24.5%). Formulating The ration to supply nutritious needs of rumen fluid donor cows according to NRC, 2001 nutrition requirement table, and became feeding two times each day (9:00 and 16:00) ad libitum. After achieving Rumen liquid before they fed morning meal, the rumen fluid was filtered with cheesecloth and then transferred into a completely thermo insulated flasks. Because of the method of Menke et al. (1979), a tight anaerobic condition was used in time of rumen fluid collection. Later on, it was transported to the laboratory.
To harvesting Alfalfa forage with 28-30% DM, a New Holland harvester (New Holland North America, New Holland, PA) were used. Chop length might have been situated on accomplishing the cut of 0.95cm. Three piles of chopped forage (10kg chopped forage in each pile) were treated with the following: 1) 0, 10, 20 and 30 mL of Ziziphora clinopodioides essential oil, 2) 0, 10, 20 and 30 mL of Mentha pulegium essential oil. Alfalfa was ensiled in each trial (500g of DM/kg) from October 1 to November 12, respectively. Silos were stored at 20°C temperatures at the dark and opened 42 days after ensiling.
Aerobic Stability
Each silo was completely blended then 1kg sample was kept from each silo. Each sample transferred to 1 Liter capacity containers (3 containers for each treatment) after growing the silos. Each container has been installed with three Thermochron buttons (Embedded Data Systems, Lawrenceburg, KY) in the top, mid and bottom layers of the silage container to keep the temperature every 20 minutes. Each container was impenetraded with a cheesecloth and kept at the temperature of 20°C up to 7d. Moreover, the temperature of surrounding environment was estimated every 20min at this stage. After 1, 3 and 7 days of aerobic exposure, silages were sampled from each container for chemical investigation, and pH measuring (Tables 2,3).
Compounds Identification
The identification of the parts might have been dependent upon correlation of their mass spectral with those of NIST mass spectral library (Masada, 1976 and NIST, 2002), also, the individuals portrayed by Adams (2001), and comparing their maintenance indices either with those of accurate mixture or with written works qualities (Adams, 2001).
Chemical Analysis
Dry matter (DM) specified with drying each sample for 24 hours in an oven drier at 105°C, to estimating ash content each sample was burned with muffle furnace, at 500°C for 9 hours. Also, by measuring Nitrogen content, utilizing the Kjeldahl method was used (AOAC, 1990). Acid detergent fiber (ADF) and neutral detergent fiber (NDF) estimation were with respect to the Van Soest et al. (1991) by applying an ANKOM fiber analyzer. Two EOs were purchased of a commercial mill in Kashan (Iran). Also, all chemical analyses were repeated in triplicate.
Gas Production Technique
The in vitro gas production procedure was according to the method of Menke et al. (1979). Approximately 200mg dry weights of samples (Alfalfa silage non-essential oils and Alfalfa silage with 10, 20 and 30ML of each essential oils) were estimated in triplicate into 100ml glass syringes with respect to the processes of Menke and Steingass (1988).
First of all, each syringe was pre-warmed at 39°C, later 30ml of rumen liquor buffer mixture (1:2) injected to all syringes, then heated to 39°C in a bain marie. artificial saliva was Prepared with respect to the method of Menke and Steingass (1988). The artificial saliva was made of 237ml buffer solution and 237ml important element solution plus 0.12ml solution of trace element and 1.22 ml resazurin was prepared and stored at 39°C, one day before incubation. Then, the reduction solution (Na2S.9H2O, 0.625g; NaOH 1N, 4ml; distilled water, 95ml) was being added to the incubation of the samples.
The ratio of artificial saliva to ruminal liquor was 2:1 (v/v). In each test and level, three repetitions were utilized, whereas they were mildly shaken for 30min after starting the incubation and for every 1 hour in the first 10h after the incubation. Gas production was evaluated in the calibrated syringes in 2, 4, 6, 8, 12, 16, 24, 48, 72 and 96 hours of incubation.
McIntosh et al. (2003) stated that if levels of essential oils are less than 100 p.p.m, could not change rumen performance. The study of Cardozo (2005) also was in agreement with this findings. In order to specify the impacts of essential oils on the kinetics of gas production on in vitrostudies, data were adjusted to the Orskov and McDonald (1979) model formula as well as the following:
Y= a +b (1-e-ct).
Y= gas product.
a+ b= potential of gas production.
a= rate of gas production of the fast soluble fractions.
b= rate of gas production of the insoluble fractions.
c= constant rate of gas production for the insoluble fractions (ml/h).
t= time of incubation (hours).
Statistical Data Analysis
The data of gas production and related parameters were restricted to the one-way analysis of variance by using variance model ANOVA in SAS software (2006). Although, multiple comparison tests or Duncan’s multiple-t-test (1980) has been used in similar studies. Significant differences were tested by the multiple range Duncan’s method. Mean differences were significant at p<0.05 level. The standard error of the means was estimated by using the method of the residual mean square in the analysis of variance. Therefore, all data collected from three times repetition tests (n= 3).
Results
The chemical combination of alfalfa silage, concentrate and wheat straw of ingredients used in the diet of animals, which rumen liquor was taken, is showed in table 1. The chemical composition of alfalfa and their silage (%) treated with various doses of Mentha pulegium and Ziziphora clinopodioides is seen in table 2 and 3, respectively. As shown in these tables, there were positive differences between silages. In addition, a considerable difference was observed between the forages in terms of chemical composition. According to table 2, the content of crude protein in forages ranged from 20.05 to 20.40%. The silage that treated with 30ML of Mentha pulegium had higher crude protein than the other doses of Mentha pulegium.
Table 1: Analysis of concentrate and forages supply to dairy cows (g/kg DM)
| Item | concentrate | Wheat straw | Alfalfa silage |
| DM | 910.65 | 89.84 | 320.55 |
| CP | 276.37 | 44.96 | 110.42 |
| NDF | 275.32 | 830.11 | 467.22 |
| ADF | 95.51 | 574.20 | 289.54 |
| EE | 43.22 | 15.81 | 30.50 |
| Ash | 65.59 | 87.53 | 76.48 |
DM: dry matter; CP: crude protein; NDF: neutral detergent fiber; ADF: acid detergent fiber; EE: ether extract.
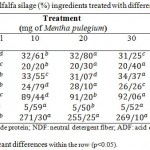 |
Table 2: Composition of alfalfa silage (%) ingredients treated with different doses of Mentha pulegium
|
Aerobic stability had a positive difference and it was developed in silage treated with Mentha pulegium in comparison with control. The data demonstrates the chemical compositions improved by adding various doses of Ziziphora clinopodioides in table 3. pH difference was not positive, but it will diminish slightly in doses of 20 and 30ML of Ziziphora clinopodioides.
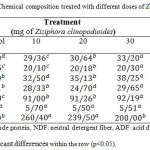 |
Table 3: Alfalfa silage (%) Chemical composition treated with different doses of Ziziphora clinopodioides
|
The effect of incubating the materials in vitro during 2, 4, 6, 8, 12, 24, 48, 72 and 96 hours by a various dose of essential oils of Ziziphora clinopodioides and Mentha pulegium on gas production from the gas test is listed in the tables 4 and 5. The result of the findings indicated adding essential oils on alfalfa silage reduced gas production. It seem reasonable that part of this activity is due to the hydrophobic nature of the cyclic hydrocarbons, which let them associate with cell membranes as well as amass in the Two-layer lipid from bacteria, taking a space between the fatty acids chains (Sikkema et al. 1994; Ultee et al. 1999). This action and reaction Cause structural changes and changes in the structure of the membrane, resulting in its fluidity and enlargement (Griffin et al. 1999). In this situation, lack of membrane stableness brings about the discharge of ions over the cell membranes, that leads to a reduction in the transmembrane ionic difference. In many ways, bacteria can balance these effects with utilizing ionic pumps To prevent cell death, although the great quantity of energy is turned into this mechanism and bacterial growth was restricted (Griffin et al. 1999; Ultee et al. 1999; Cox et al. 2001). With respect to increasing the time of incubation, the cumulative content of gas production will be enhanced. According to Table 4, there are significant differences between control treatment and silages treated with various doses of Ziziphora clinopodioides with respect to gas production at each time of incubation. After 96 h incubation, gas produced ranged between 68.82 and 56.12 ml per 200 g of substrate.
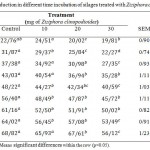 |
Table 4: Gas production in different time incubation of silages treated with Ziziphora clinopodioides
|
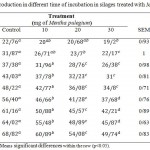 |
Table 5: Gas production in different time of incubation in silages treated with Mentha pulegium
|
Table 5 shows that gas production of silage content with 30ML of Mentha pulegium was significantly and positively (p<0.001) lower than the other. In addition, as it can be seen, there were positive and significant (p<0.001) differences between silages with respect to gas production at all incubation times. Silage content with mentha pulegium had more reduction impact than Ziziphora clinopodioides in gas production in comparison with control silage and it was significant and positive at (p<0.001) level. At 96 h incubation, gas production values of silage no added essential oils, 30ML of Ziziphora clinopodioides and 30ML of Mentha pulegium were 68.82, 56.12 and 49.74, respectively. All incubation times presented that gas production in experimental silages was lower than control silage, but also silages treated with essential oils had higher protein content than control silage. In alfalfa silage, protein was defectively used, especially when the diet was low in energy. (Buxton, 1996). within harvesting and storage of silage (Albrecht and Muck, 1991), extensive degradation happens, and it was followed by more microbial depression in the rumen (Buxton, 1996). On in vitro study, gas is achieved not only due to fermentation (CO2 and CH4) but also consequentially, from the acidify impact of VFAs (volatile fatty acids) on CO2 which released from bicarbonate buffer solution (Getachew et al. 1998). The breakdown of protein is produced ammonia, combining with H+ that released from the buffer that remains in the solution produced NH4+ and indirectly inhibits the production of gas. The antimicrobial activity of essential oils in other treatments is one reason that why gas production value of control silage was lower than other treatments. Also, Nagy and Tengerdy (1968) assessed the ruminal microorganisms sensitivity to the essential oils.
Forty three Mentha pulegium essential oil compounds were identified, suggesting 99.53% of the total mass of essential oil, of which 29 compounds were clarified. The major components were pulegone (38.83%), menthone (19.24%), pipériténone (16.53%), pipéritone (6.35%) and isomenthone (6.09%), Limonène (4.29%), Octanol (1.85%). The major component of the Ziziphora clinopodioides essential oil was monoterpene hydrocarbon and among that, the important ingredients were pulegone (79.34%), Limonene (6.77%) and piperitenone (4.21%). Findings of present study showed that essential oils and doses had positive and significant impacts on gas production. The results of the experiments were different based on each essential oil, doses, incubation time and feed substrate.
Discussion
In the background of continuous the rumen flow, a change in development rate result changing in the rumen bacterial populations portion, also result changing in the fermentation profile (Griffin et al. 1999; Davidson and Naidu, 2000; Dorman and Deans, 2000; Cox et al. 2001). These actions could be more advantageous against gram-positive bacteria, in which the cell membrane can communicate directly with hydrophobic ingredients of EOs (Smith-Palmer et al. 1998; Chao and Young, 2000; Cimanga et al. 2002).
In this study, essential oils reclined gas production of all feed samples. These results of the findings are in agreement with the Cardozo et al. findings (2004). reduced in vitro gas production by essential oils show more effective energy utilizing because of the waste of energy as well as methane. Effects of EOs on rumen fermentation were significantly different. Ziziphora clinopodioides and Mentha pulegium against a wide range of gram-positive bacteria and gram-negative bacteria had bactericidal effects. Both are found in ORE (origanum essential oils) (Sivropoulou et al. 1996). Castillejos et al. (2006) presented that small amount of oregano (less than 50mg/l) didn’t effect on microbial fermentation. however, a higher amount of thymol or oregano decreased total VFA (Castillejos et al. 2006) and reduced gas production (Akkan et al. 2006; Benchaar et al. 2007; Kamalak et al. 2011). Moreover, some researches suggested that oregano has an effect on diet and pH-dependent (Cardozo et al. 2005; Castillejos et al. 2006).
Although mainly emphasized on the antibacterial properties of essential oils, this is not their only effect. Gustafson and Bowen (1997) also represented that among other effects of essential oils, it’s known they are capable to coagulation of some of the cellular components through the denaturation of the proteins. In addition, many types of research had revealed the capability of some phenolic and non-phenolic ingredients of essential oils to act reciprocally with chemical groups in protein structure and other active molecules, such as enzymes (Juven et al. 1994). Generally speaking, phenolic compounds communicate with protein ingredients via hydrogen bonds and electrostatic or hydrophobic bridges (Prescott et al. 2004), while non-phenolic ingredients communicate via other functional groups including the carbonyl group of cinnamaldehyde (Ouattara et al. 1997). In this study, silages treated with essential oils had higher protein content than control silage. Busquet et al. (2005) stated as the essential oil dose increased, the gas production was reduced. These results are in consistent with the present study.
Oh et al. (1967, 1968) reported that the low palatability of some plants to ruminants could be for both organoleptic effects, and their negative impact on rumen microbial fermentation, and nutrient digestion. In another study, Oh et al. (1967) examined the antibacterial activity of the EOs of Pseudotsuga menziesii and related ingredients on 24-h in vitro bath cultures by using of the ruminal fluid of deer and sheep. Their findings showed that doses of injection (4 to 8mL/L of fluid) were low and had not any positive impact on rumen fermentation performance, although higher doses (12mL/L of liquor) decreased gas yield during incubation time.
When the fundamental compounds isolated from the EO was utilized in same levels (3mL/L of liquor), those circular hydrocarbons (limonene and pinene) didn’t change whether seldom motivated microbial activity, yet the cyclic hydrocarbons enrich with oxygen and particular alcohols (as terpinene and α-terpineol) hindered microbial activity in the rumen. It is famous that there is a strong link between gas production in laboratory studies and other in vivo experiments on rumen fermentation and microbial activity (Menke et al. 1979). The outcome of their findings indicated that essential oils could have a positive impact on the rumen microbial fermentation and nutrient digestion.
This is reported by benchaar et al. (2007) that gas production of carvacrol, thymol, and eugenol treatments reduced in comparison with control. These outcomes of their findings are not in agreement with the findings of oregano in the present experiment. Oregano possesses more carvacrol and eugenol compounds respectively than other essential oils. Therefore, garlic and oregano reduced gas production in barley on in vitro test. These results of the findings indicated that essential oils can be used to enhance digestion of leisurely starch degradation and monitor rate of releasing rapidly degradable starch to keep ruminal pH in the physiological range in the rumen.
Recently, the researchers have studied the impacts of active components of EOs on the performance of rumen microbial population. It is worth noting that the first challenge is to specify and determine which potential impacts are studied, and this may be varied with respect to the ration, cows, and production stage. Nevertheless, it is rational, to begin with recognizing and determining additives which develop propionic acid production and decline acetic acid and methane yields without any diminishing effect on VFA production. Also, the EOs which decrease microorganism activities such peptidolysis, proteolysis, deamination, and their association.
A series of in vitro short-term batch culture researchers have been utilized for monitor of capability helpful EOs (Cardozo et al. 2005; Busquet et al. 2006; Castillejos et al. 2006), and chosen essential oils and their important ingredients have been studied in long-term rumen fermentation researches (Cardozo et al. 2004; Busquet et al. 2005a, b, c; Castillejos et al. 2006).
The outcome of findings showed that essential oil of garlic and cinnamaldehyde, and eugenol that is the active element of the clove bud, and also capsaicin (important element of pepper), and anethol (main element of anise oil) enhance the profile of fermentation in continuous culture bath of microorganisms are located in rumen, and they Have been investigated in several in vitro, and some cases of in vivo studies (Cardozo et al. 2006). gas production on In vitro tests was reduced positively with essential oils. Most of the essential oils can be utilized to increase cellulose digestion and it can be regarded as a feed additive. One of the most important elements of Mentha pulegium and Ziziphora clinopodioides (up to79.33% in Ziziphora clinopodioides) is pulegone. In this present study, the pulegone content in essential oils is in consistent with the outcomes of Davidson and Naidu study (2000), that proposed, by using optimal doses, the efficiency of nutrient compound utilization in the rumen would be enhanced. Also, eugenol could increase production of VFA, and utilization of N in lactating animals rumen (Castillejos et al. 2006).
In a commercial form of essential oils, their main ingredients are thymol, eugenol, vanillin, carvacrol, and limonene, which can alter rumen fermentation. (McIntosh et al. 2003; Benchaar et al. 2007). the present experiment, CUM indicated the greatest gas production in comparison to control treatment at each test. ORE (origanum essential oils) also demonstrated the lowest gas production in all in vitro tests. In addition, observed essential Interactions related to the type of feeds, the duration of incubation and the dose that contradicts with findings of McIntosh et al. (2003). In this respect, Benchaar et al. (2007) claimed that observed variations and manipulation in the fermentation of rumen created by EOs ingredients e.g carvacrol, eugenol and thymol may not be useful in lactation cows.
And also proposed that type and amount of essential oils and related ingredients should be meticulously specified and determined. In recent years, considerable knowledge has been achieved on the capability use of EOs to change microbial activity in the rumen. Although, before specifying recommendations for commercial use, several problems need to be addressed to be established. Most of these limitations of the present knowledge required to be resolved. For instance, the number of active ingredients in EOs Extensively depend on the variety, developing situations, and also technical method of extraction.
Conclusions
According to the present study, it can be found that estimated EOs and their compounds have a positive effect on rumen degradation due to relying on the essential oils and feeds applied. Although in vitro studies is still required to monitoring and checking new findings, then specifying functions of behavior, and also an important requirement to perform in vivo research so as to specify and specify the optimum doses in active element unit, the adaptation ability of rumen microorganisms to the action of this essential oils, the destiny of these additives in the gastrointestinal trace and the existence of residues in some products as milk and meat, and the impacts on performance of dairy cattle.
Acknowledgments
We gratefully acknowledge the authorities of the branch of Animal Sciences at the Ferdowsi University of Mashhad for their cooperation. This work was conducted as a Ph.D. thesis in Animal Sciences at Ferdowsi University of Mashhad (Iran). The authors gratefully thanks to Ferdowsi University of Mashhad for their endorsement, and they also especially like to appreciate Prof. A. Naserian, Prof. A. Tahmasbi and Dr. A. Vakili for their comments.
Conflict of Interest
There is no conflict of interest.
References
- Adams R.P. Identification of essential oil components by gas chromatography and quadrupole mass spectrometry. Allured Publ. Corp., Carol Stream IL. 2001.
- Albrecht K. A and Muck R. E. Proteolysis in ensiled forage that vary in tannin concentration. Sci. 1991;31:464-469.
- Association of Official Analytical Chemists, Official Methods of Analysis. 15th Edition.Washington. DC 1990;1.
- Balandrin M. F andKlocke J. A. Natural plant chemicals: Sources of industrial and medicinal materials. Science. 1985;228:1154–1160.
CrossRef - Borchers R. Proteolytic activity of rumen fluid in vitro. Anim. Sci. . 1965;24;1033–1038.
CrossRef - Broudiscou L. P,Papon Y and Broudiscou A. F. Effects of dry plant extracts on feed degradation and the production of rumen microbial biomass in a dual outflow fermenter. Anim. Feed Sci. Technol . 2002;101:183–189.
CrossRef - Broudiscou L. P, Papon Y and Broudiscou. A.F. Effects of dry plant extracts on fermentation and methanogenesis in continuous culture of rumen microbes. Anim. Feed Sci. Technol. 2000;87:263–277.
CrossRef - Busquet M, Calsamiglia S, Ferret A and Kamel C. Plant extracts affect in vitro rumen microbial fermentation. Dairy Sci. 2006;89:761–771.
CrossRef - Busquet M, Calsamiglia S, Ferret A, Kamel C. Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. Dairy Sci. 2005;88:2508-2516.
CrossRef - Busquet M, Greathead H, Calsamiglia S, Ferret A and Kamel C. Efecto del extracto de ajo y el cinemaldehido sobre la produccion, composicion y residuos en leche en vacas de alta produccion. ITEA. 2003;24(Vol. Extra): 756–758.
- Buxton D. R. Quality related characteristics of forages as influenced by plant environment and agronomic factors. Feed Sci. Technol. 1996;59:37-49.
CrossRef - Calsamiglia S, Castillejos L, Busquet M. Alternatives to antimicrobial growth promoters in cattle. in Recent Advances in Animal Nutrition. ( C. Garnsworthy, and Wiseman J , ed. Nottingham University Press, Nottingham, UK). 2006;129–167.
- Cardozo P. W, Calsamiglia S, Ferret A, Kamel C. Effects of alfalfa extract, anise, capsicum and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high concentrate diet. J. Anim. Sci. 2006;84:2801–2808.
CrossRef - Cardozo P.W, Calsamiglia S, Ferret A, Kamel C. Effects of natural plants extracts on ruminal protein degradation and fermentation profiles in continuous culture. Anim. Sci. 2004;82:3230-3236.
CrossRef - Cardozo P.W, Calsamiglia S, Ferret A, Kamel C. Screening for the effects of natural plants extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle. Anim. Sci. 2005;83: 2572-2579.
CrossRef - Castillejos L, Calsamiglia S, Ferret A. Effect of essential oil active compounds on rumen microbial fermentation and nutrient flow in in vitro J. Dairy Sci. 2006;89:2649-2658.
CrossRef - Castillejos L, Calsamiglia S,Ferret A and Losa R. Effects of dose and adaptation time of a specific blend of essential oils compounds on rumen fermentation. Anim. Feed Sci. Technol. 2007;132:186–201.
CrossRef - Castillejos L, Calsamiglia S,Ferret A and Losa R. Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim. Feed Sci. Technol. 2005;119:29–41.
CrossRef - Chao S.C, Young D. G and Oberg C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. Essential Oil Res. 2000;12:639–649.
CrossRef - Cimanga K, Kambu K, Tona L, Apers S , Bruyne T,Hermans N,Totte J, Pieters L and Vlietinck A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. Ethnopharmacol. 2002;79:213–220.
CrossRef - Cox S. D, Mann C. M and Markam J.L. Interaction between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001;91:492–497.
CrossRef - Cowan M. M. Plant products as antimicrobial agents. Microbiol. Rev. 1999;12:564–582.
CrossRef - Davidson P. M and Naidu A.S. Phyto-phenols. in Natural Food Antimicrobial Systems. Naidu S , ed. CRC Press, Boca Raton F.L. 2000;265–293.
- Davidson P.M, Naidu A.S. Phyto-phenols. In: Natural Food Antimicrobial Systems. Naidu A.S. 152 POUR et al. , Biotech. Res. Asia. 2017;14(1):145-152. (Editor). CRC Press. Boca Raton F.L 2000;265-293.
- Dorman H.J.D, and Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Appl. Microbiol. 2000;88:308–316.
CrossRef - Gershenzon J and Croteau R. Terpenoids. in Herbivores: Their Interactions with Secondary Plant Metabolites G. Rosenthal A and Berenbaum M. R, ed. Academic Press, Diego S.C.A. 1991;(1):165–219.
- Griffin S.G, Wyllie S.G, Markham J.L and Leach D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999;14:322–332.
CrossRef - Gustafson R. H and R.E. Bowen. Antibiotic use in animal agriculture. Appl. Microbiol. 1997;83:531–541.
CrossRef - rrez G.M.E, Garcı A.F.a, deMadariaga M. A, Sagrista M.L,Casado F.J and Mora M Interaction of tocopherols and phenolic compounds with membrane lipid components: Evaluation of their antioxidant activity in a liposomal model system. j. Life Sci. 2003;72:2337–2360.
CrossRef - Fraser G. R, Chaves A.V, Wang Y, McAllister T. A, Beauchemin K. A and Benchaar C. Assessment of the effects of cinnamon leaf oil on rumen microbial fermentation using two continuous culture systems. Dairy Sci. 2007; 90:2315–2328.
CrossRef - Greathead H. Plants and plant extracts for improving animal productivity. Nutr. Soc. 2003;62:79–290.
CrossRef - Hristov A.N, Ropp J.K, Zaman S and Melgar A. Effects of essential oils on in vitro ruminal fermentation and ammonia release. Feed Sci. Technol. 2008;144:55-64.
CrossRef - Harborne J. B and Williams C. A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504.
CrossRef - Hristov A.N, McAllister T.A, Herk V.F.H, Cheng K.J, Newbold C.J and Cheeke P.R. Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. Anim. Sci. 1999;77:2554–2563.
CrossRef - Juven B.J, Kanner J, Schved F.and Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. Appl. Bacteriol. 1994;76:626–631.
CrossRef - Kamalak A, Canbolat O, Ozkan C.O, Atalay A.I. Effect of thymol on in vitro gas production, digestibility and metabolizable energy content of alfalfa hay. Kafkas Univ. Vet. Derg. 2011;17:211-216.
- Kamel C. Tracing modes of action and the roles of plant extracts in nonruminants. in Recent Advances in Animal Nutrition P. C. Barnsworthy and J. Wiseman, ed. Nottingham University Press, Nottingham U.K. 2001;135–150.
- Kung L.Jr, Williams P, Schmidt R.J, and Hu W. A blend of essential plant oils used as an additive to alter silage fermentation or used as a feed additive for lactating dairy cows. Dairy Sci. 2008;91:4793–4800.
CrossRef - Lee S.E, Hwang H. J,Ha J. S,Jeong H. S, Kim J. H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–179.
CrossRef - Mahmoud A. L. E. Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett. Appl. Microbiol. 1994;19:110–113.
CrossRef - Masada Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. New York (N.Y.): Wiley. 1976;:334-335.
- McGuffey R.K, Richardson L. F, Wilkinson J. I. D. Ionophores for dairy cattle: current status and future outlook. Dairy Sci. 2001;84:194-203.
CrossRef - McIntosh F.M, Williams P, Losa R, Wallace R. J, Beever D. A, Newbold C. J. Effects of essential oil on ruminal microorganism and their protein metabolism. Environ. Microbiol. 2003;69:5011-5014.
CrossRef - Menke K.H, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. Agr. Sci. 1979;93:217-222.
CrossRef - Menke K.H, Steingass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Develop. 1988;28:7-55.
- Nagy J.G and Tengerdy R.P. Antibacterial action of essential oils of Artemisia as an ecological factor. II. Antibacterial action of the volatile oils of Artemisia tridentata (big sagebrush) on bacteria from the rumen of mule deer. Microbiol. 1968;16:441–444.
- NIST, Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library, vers. 2.0. fiveash data, USA. 2002.
- Nutrient Requirements of Dairy Cattle. 7th rev. ed. Natl. Acad. Sci. 2001.Washington, DC.
- Oh H.K, Jones M.B and Longhurst W.M. Comparison of rumen microbial inhibition resulting from various essential oils isolated from relatively unpalatable plant species. Microbiol. 1968;16:39–44.
- Oh H.K, Sakai T, Jones M.B and Longhurst W.M. Effect of various essential oils isolated from Douglas fir needles upon sheep and deer rumen microbial activity. Microbiol. 1967;15:777–784.
- Ørskov E.R, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. Agr. Sci. 1979;92:499-503.
CrossRef - Ouattara B.R, Simard E, Holley R. A.G,Piette J.-P and Be A.g. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. Food Microbiol. 1997;37:155–162.
CrossRef - Patra A.K, Kamrai D.N, Agarwal N. Effect of spices on rumen fermentation, methanogenesis and protozoa counts in in vitro gas production test. Congress Ser. 2006;1293:176-179. (DOI:10.1016/j.ics.2006.01.025).
CrossRef - Prescott L.M, Harley J.P and Klein D. A. Control de microorganismos por agentes fı´sicos y quı´micos. in Microbiologı´a. McGraw-Hill-Interamericana de Espan˜ a, Madrid, Spain. 2004;145–162.
- Reddy L, Odhav B and Bhoola K.D. Natural products for cancer prevention: A global perspective. Therap. 2003;99:1–13.
CrossRef - SAS Users Guide. Cary, Statistical Analysis Systems Institute, USA. 2006.
- Sikkema J.J, Bont A.M and Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994;269:8022–8028.
- Sivropoulou A, Papanikolaou E, Nicolaou C, Kokkini S, Lanaras T, Arsenakis M. Antimicrobial and cytotoxicaktivities of origanum essential oils. Agr. Food Chem. 1996;44:1202-1205.
CrossRef - Smid E.J and Gorris L.G.M. Natural antimicrobials for food preservation. in Handbook of Food Preservation. M. S. Rahman ed. Dekker M, New York, NY. 1999;285–308.
- Smith-Palmer A, Stewart J and Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998;26:118–122.
CrossRef - Szumacher-Strabel M, Cieoelak A. Potential of phytofactors to mitigate rumen ammonia and methane production. Anim. Feed Sci. 2010;19:319-337.
CrossRef - Trouillas P, Calliste C. A, Allais D.P, Simon A, Marfak A, Delage C and Duroux J.L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003;80:399–407.
CrossRef - Ultee A.M, Bennik H. J and Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. J. Appl. Environ. Microbiol. 2002;68:1561–1568.
CrossRef - Ultee A, Kets E.P and Smid E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. J. Appl. Environ. Microbiol. 1999;65:4606–4610.
- Voda K, Boh B, Vrtacnik M, Pohleven F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot tramatesversicolor and the brown-rot Coniophoraputana. Biodeter. Biodegrad. 2003;51:51-59.
- Wang Y.G,Douglas B, Waghor G.C,Barry T.N, Foote A.G, Purchas R.W. Effects of condensed tannins upon the performance of lambs grazing Lotus corniculatus and lucerne (Medicago sativa). Agric. Sci. (Camb.). 1996; 126:87–98.
CrossRef - Wang Y, McAllister T. A, Newbold C.J, Rode L.M, Cheeke P.R and Cheng K.J. Effects of Yucca schidigera extract on fermentation and degradation of steroidal saponins in the rumen simulation technique (Rusitec). Anim. Feed Sci. Technol. 1998;74:143–153.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





