How to Cite | Publication History | PlumX Article Matrix
Khalid M. Al-ghamdi, Abdulrahman A. Faragallah, M. S. Saleh, Jazem A. Mahyoub and Habeeb M. Al-Solami
Department of Biological Sciences, Faculty of Science, King Abdul Aziz University, P.O. Box 80203, Jeddah, 21589, Saudi Arabia.
Corresponding Author Email: hmalsolami@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2615
ABSTRACT: The main goal of this study was the monitoring of the population fluctuation of the prevalent dipterous fly species complex and to generate a baseline data in Jeddah Governorate and it’s surroundings using malaise and yellow sticky traps in animal pens including sheep, cow, cattle and vegetable market. Data recovered indicated the continued presence of four prevalent fly species including the tachinid species complex, the house fly Musca domestica L., the flesh fly Sarcophaga carnaria and the hover fly (Sphaerophoria) at the rate of 59.91%, 23.55%, 16.14% and 0.41% respectively. It is noteworthy to report the continued presence throughout the year of the tachinid species complex which might indicate it’s efficiency in suppressing the fly population, the fluctuation of other prevalent species that are present extantly.
KEYWORDS: Monitoring; Population Fluctuation; Dipterous Fly Species; Animal Pens; Malaise Traps and Yellow Sticky Traps
Download this article as:| Copy the following to cite this article: Al-ghamdi K. M, Faragallah A. A, Saleh M. S, Mahyoub J. A, Al-Solami H. M. Monitoring the population Fluctuation of the Prevalent Dipterous Fly Species Complex (Order: Diptera) by Using Malaise and Yellow Sticky Traps in Animal Pens in Jeddah Governorate, Western KSA. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Al-ghamdi K. M, Faragallah A. A, Saleh M. S, Mahyoub J. A, Al-Solami H. M. Monitoring the population Fluctuation of the Prevalent Dipterous Fly Species Complex (Order: Diptera) by Using Malaise and Yellow Sticky Traps in Animal Pens in Jeddah Governorate, Western KSA. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29326 |
Introduction
The order Diptera is made of a large well-known group of notorious true fly species and most of their life cycle are completed in short period of time in variegated diverse environments that attract these flies. These environments are considered suitable utopias for the fly population multiplication to reach epidemic levels. These suitable environments include waste dumps rich in decomposed and partially decomposed organic matter of domestic; wild animals and poultry manures, decayed fruits and vegetables, decomposing dead animal bodies, stagnant drainage water, open sewers and cesspools. (Pedigo, 1989; Olkowski et al., 1991; Saunders and Hayward, 1998).
Based on their unwelcomed ubiquitous presence and their being utterly nuisance plus their active role in the transmission of harmful pathogens to humans and animals alike, many approaches have been adopted to curve their population build up and outbreaks to tolerable and sub epidemic levels. (Miller et al., 1993; Amoudi, 1993; Grasswitz and Burst, 1995; Demilo et al., 1997; Kocisova et al., 2000).
A wide range of control measures including sanitation procedures and abatement programs using baited traps, chemical approaches embracing organochlorines and organophosphates, insecticides where all applied, however some appreciable success has been achieved through fly integrated pest management practices. Traditional methods have also been inactive including mechanical, physical, and cultural control, biological pest suppression, use of promising novel third generation insecticides including hormones, insect growth regulators, chitin inhibitors have led to some successful management program, in curbing fly population from reaching epidemic levels and serious outbreaks. (Scott, 1964; Clausen, 1972; Coppel and Mertins, 1977; Silva et al., 2002; Rina et al., 2010).
Critical observance was strengthened by sanitation and hygienic practices, regular cleaning and cleansing of cattle and animal holdings. Vigilant observation of waste water treatment facility to inhibit fly population and to create awareness of citizens through community services to abide by rules and regulations. In addition to campaigns combating serious infestation have been reported. (Buttiker, 1981; Banaja and Madbouly, 1981; Drummond et al.,1988; Amoudi, 1993; Hijazi et al., 1996; Faragalla et al, 2003; Tomberlin et al., 2007; Al-Ghamdi et al., 2010).
The Aim of Study
The objectives of this field study was to monitor the population fluctuation of a prevalent dipterous fly species complex and to generate a solid baseline data on dominant fly species through using malaise and yellow sticky traps in different locations within the premises of Jeddah governorate and it’s surroundings.
Material and Methods
To fulfill the goals of the proposed monitoring study of the fluctuation of the dominant fly species in and around animal pens including (sheep, cattle, camel and vegetable market) malaise and yellow sticky traps were operated to recover fly species in the chosen collection sites. The expertise of Jeddah municipality staff has been solicited and adopted in the choice of suitable selection of these sites based on their infestation records and combat campaigns that has been previously executed.
Different monitoring devices and techniques have been adopted to determine the population density fluctuation in different localities which include light traps, bated traps, daily first collections from household waste, dump sites, garbage disposal sites, plastic bags and garbage containers.
Malaise Traps
The Malaise traps were installed and established in animal holdings (pens) and the sheep and vegetable market in Northern Jeddah butchery and slaughter house facility where the animals are temporarily kept for selling to the citizens.
Weekly data were recovered from Malaise traps kept in 70% alcohol then taken to the lab for further investigations including sorting and identification.
Yellow Sticky Trap
The yellow stick traps has proved to be reliable in the fluctuation studies and monitoring of smallsized flying insects. Each yellow sticky trap is made up of a cardboard having the size of (35×25 inches). Each covered with yellow paint on both sides then covered with resinous glue that is not affected by high temperature or humidity. Each trap (board) is nailed to a vertical stick or hanged from horizontal bar with wired string. Weekly yellow sticky traps, (cardboards) were replaced by new ones and the old ones will be taken to the lab for further investigations, sorting and categorization to the respective family and generic levels. Each yellow sticky board was carefully handled and the number of glued or stuck flying dipterous specimens were all recorded. Special attention and scrutinizing was devoted to the very minute flies.
The unidentified specimens of both traps(Malaise and Yellow sticky) were carefully marked, labeled, wrapped or kept in alcohol or preserved and sent to specialists in the National Museum of Plant Protection in Egypt for correct identification. Some voucher specimens were kept in the lab to be part of the insect collection.
Results and Discussion
After the completion of the field data recovery a fairly rich complex of approximately 60 species made of prevalent species include the true flies, (Tachinia spp. (family :Tachinidae), the house fly Musca domestica L. family: Muscidae), the flesh fly (Sarcophaga carnaria) and the hoover fly (Sphaerophoria flavicauda) family: Syrphidae (Table 1) .
Table 1
| (Robineau-Desvoidy) | ||
| 28 | Exorista larvarum (Linnaeus) | “ |
| 29 | Phytosorolidi squama Villeneuve | “ |
| 30 | Phyto abbreviata villeneuve | “ |
| 31 | * Tachinid sp. | “ |
| 32 | Gymnoparia aegyptiaca | “ |
| Villeneuve | ||
| 33 | Actia crasicornis (Meigen) | “ |
| 34 | Strobliomyia aegyptia villeneuve | “ |
| 35 | Actia sp. | “ |
| 36 | Physiphora demandata | Otitidae |
| 37 | Physiphora smaragdina (Loew) | “ |
| 38 | Villa circe (Klug) | Bombylliidae |
| 39 | Pipunculopsis sp. | “ |
| 40 | Adia Schnable Dziediziki Anthomyiidae | |
| 41 | Fucellia sp. | “ |
| 42 | Stichopogon albellus Loeus | Asilidae |
| 43 | Anisopogon pulchrum Effatoun | “ |
| 44 | *Stichopogon chrysostoma Loeus | “ |
| 45 | Dacus ciliatus Loew | Tephritidae |
| 46 | Ceratitis capitata | “ |
| 47 | Dacus longistylus Loew | “ |
| 48 | Drosophila melanogaster | Drosophilidae |
| 49 | Drosophilid sp. | “ |
| 50 | Psilopa sp. | Ephydridae |
| 51 | Lonchaeid sp. | Lonchaeidae |
| 52 | Therevid sp. | Therevidae |
| 53 | Tethina pallipes Becker | Tethinidae |
| 54 | Fucellia sp. | Anthomyiidae |
| 55 | Tabanus taeniola | Tabanidae |
| 56 | Tabanus sufis | “ |
| 57 | Coratitis capitat | Trypetidae |
| 58 | Hippobosca camelina | Hippoboscidae |
| 59 | Conops nubeculipennis (Bezzi) | Conopidae |
| 60 | Physocephala sp. | “ |
* most prevalent fly species in Jeddah
The most prevalent fly species include (Tachinia spp. (family :Tachinidae), the house fly Musca domestica L. family: Muscidae), the flesh fly (Sarcophaga carnaria) and the hover fly (Sphaerophoria flavicauda) family: Syrphidae) and the asilid fly, Stichopogon chrysosotoma Loues family: Asilidae.
Fig. 1 showed the monthly population fluctuation of three dipterous flies including the highest was Tachinid species complex, the mild population was the flesh fly and the lower population was the house fly. The Tachinid spp., complex showed continued availability throughout the year by showing three peaks. The highest peak during February followed by two others during June and October and then the population crashed down towards the end of the year which might be due to the decrease in temperature and relative humidity. The flesh fly population showed three intermediate peaks during February, June and August and there was a gradual decrease in the population density noticed towards the end of the year (Fig. 2). The house fly population has showed continued availability with three similar low peaks during March, May and December (Fig. 1).
 |
Figure 1: The monthly fluctuation population of the prevalent dipterous fly species between sheep pens recovered by malaise trap from the above ground sheep waste-Jeddah Governorate 2008- 2009
|
Fig.2 showed the population fluctuation of both the predaceous fly Stichopgon chrysostoma and Sphaerophoria flavicauda throughout the year. The population of the asilid fly S.chrysostoma showed a high peak during June and the population gradually decreased towards the end of the year whereas the population of the hoover fly showed three mild peaks during June, August and January. The overall population fluctuation of the five major fly species complex gave the following percentages including the Tachinid complex, the flesh fly, and the house.
 |
Figure 2: The monthly population fluctuation of the hover fly, S.flavicauda and the asilid fly S. chrysostoma from sheep pens recovered by malaise traps. Jeddah Governorate Feb. 2008- Jan. 2009
|
Fly.(37.79%, 24.41%, and 18.15% respectively) (Table 2 and 3). However, Fig. 3 showed the overall annual total percentage of the prevalent dipterous fly species between animal pens recovered by malaise trap in Jeddah Governorate (Fig. 3) . Malaise traps has shown pronounced efficiency in the following up of the population fluctuation of prevalent fly species in sheep pens and have been used for many occasions in biodiversity studies especially of small and medium sized insects. (Goulet and Huber, 1993; Masner 1972, 1980). The sheep pens have the highest population due to the accumulation of solid waste, urine, and decomposed organic matter. Moreover, the irregular removal and disposal of garbage and not unabiding hygienic procedures of the routine cleansing and cleaning of the ground sheep pens grounds might contributed to this high population. (Richardson, 1994; Faragalla and Al-Ghamdi, 2003).
Table 2: The percentage population density of the prevalent dipterous flies between the sheep pens recovered by Malaise traps. 2008 – 2009 Jeddah Governorate
| Fly species | Total/year | % |
| S.flavicauda | 604 | 9.57 |
| S.chrysostoma | 637 | 10.09 |
| M.domestica | 1146 | 18.15 |
| S.carnaria | 1541 | 24.41 |
Table 3: The frequent variation in the number of most prevalent house species between sheep pens recovered by malaise trap. Jeddah Governate 2008 – 2009
| Range | Species | Family | Status |
| 0-1000 | S.flavicauda | Syrphidae | Less Frequent |
| S.chrysostoma | Asillidae | ||
| 1001-2000 | M. domestica | Muscidae | Frequent |
| S. carnaria | Sarchophagidae | ||
| 2001-2500 | Tachinid sp. | Tachinidae | More Frequent |
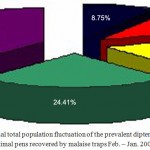 |
Figure 3: The annual total population fluctuation of the prevalent dipterous fly species between animal pens recovered by malaise traps Feb. – Jan. 2008 -2009
|
Data recovered from the yellow sticky traps from around sheep pens recovered four prevalent dipterous fly species including the Tachinid fly complex, the house fly, the flesh fly and the hover fly. It is clearly evident that the Tachinid species complex represented the dominant dipterous flies followed by the house fly then the flesh fly and finally the hover fly which all gave the percentages 59.91%, 23.55%, 16.14% and 0.14% respectively (Table 4.) . Moreover the population fluctuation numbers were categorized according to their frequent presence and availability (Table 5).
Table 4: The percentage prevalence of dipterous fly species recovered from sheep pens by yellow sticky traps. Jeddah Governorate Feb. 2008 – Jan. 2009
| Fly species | Total/year | % |
| S.flavicauda | 39 | 0.41 |
| S.carnaria | 1541 | 16.14 |
| M.domistica | 2249 | 23.55 |
Table 5: The frequent variation in numbers of prevalent dipterous fly species recovered by yellow sticky traps installed in the sheep pens. Jeddah Governate Feb. 2008 – Jan. 2009
| Range | Species | Family | Status |
| 0-100 | S.flavicauda | Syrphidae | Less Frequent |
| 101-2000 | S. carnaria | Sarcophagidae | Frequent |
| 2001-6000 | M. domesticaTachinid sp. | MuscidaeTachinidae | More Frequent |
Fig.4 showed the monthly population fluctuation of the Tachinid complex as a major dipterous fly species with continued presence in high population numbers starting from March – November then the population gradually declined during Dec. with slight increase during Jan. The other two dipterous species, the house fly, M. domestic and the flesh fly, S. carnaria showed steady presence with low population density with the flesh fly being the lowest.
 |
Figure 4: The monthly population fluctuation of the house fly, flesh fly and the Tachinid species complex recovered by yellow sticky trap from the sheep pens. Jeddah Governate Feb. 2008 – Jan .2009
|
The Tachinid complex has two pronounced peaks, the first during March and the second during August, the house fly has 2 low peaks during April and October. The population of the flesh fly was also continuous throughout the year and this might be due to the availability of organic matter, exposed blood and the presence of the preferred host with high activity during March (Fig. 4).
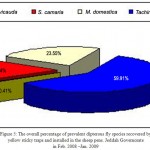 |
Figure 5: The overall percentage of prevalent dipterous fly species recovered by yellow sticky traps and installed in the sheep pens. Jeddah Governorate in Feb. 2008 –Jan. 2009
|
It is evident that the tachinid larval parasites played an important role in keeping the population of other dipterous species viz. M. domestica and S.carnaria at low levels which indicated their biological effectiveness in the suppression of their population buildup. (Fig. 5). Hence the percentage presence of prevalent dipterous fly species from the sheep pens included tachinid species complex, M. domestica, S. carnaria and S.flavicauda gave 59.91%, 23.55%, 16.4 and 0.41% respectively. It is noteworthy to observe the highest population of the Tachinid species complex which exhibited their biological role as important parasites in these fly area build up domains. (Seymour and Campbell, 1993; Klunker, 1994; Faragalla and Al-Ghamdi, 2003; Olkowski et al., 1991) .More extensive field investigations are needed to include other parameters for the continuous dipterous population fluctuation throughout the year in these Jeddah localitys and its surroundings including studies of biology, ecology environmental conditions, pest/prey parasite interactions, behavior and suitable methods of control and suppression (Fig. 5) .
References
- Al-Ghamdi KM, Faragalla AA, Al-Solami MH (Evaluation of two conventional insecticides and a chitin synthesis inhibitor against immature stages of the house fly Musca domestica L. (Diptera: Muscidae). Biosciences, Biotechnology Research Asia. 2010; 7(2): 597-588.
- Amoudi MA, New records of some of sarcophagid flies with distribution of all known flesh flies (Diptera: sarcophagidae) of Saudi Arabia. J. Egypt. Soc. Parasitol. 1993; 23(91): 297-304.
- Banaja A, Madbouly MH., Field and laboratory observation on three dipterous larvae causing myiasis in livestock. JKAC. 1981; 1: 1-10.
- Buttiker W., Fauna of Saudi Arabia; Observation on urban mosquitoes in Saudi Arabia. In : Fauna of Saudi Arabia. W. Wittmer & W. Buttiker. (Eds.) 1981; 3: 472-479.
- Clausen CP., Entomophagous Insects, Hafiner Publishing Company 1972; 688.
- Coppel HC, Mertins JW., Biological insect pest suppression. Springer-verlag, Berlin. Heidelberg, 1977.
CrossRef - Demilo AB, Gelman DB, Bordas B., Benzoylbiuret insect chitin inhibitors: Strucuture activity correlations derived from an invitro clasper assay and an in vivo mosquito about emergence assay. J. of Entomolo. Science. 1997; 32(2): 212-228.
CrossRef - Drummond RO, Geroge JE, Kunz SE., Control of Arthropod pests of Livestock: a review of technology. CRC. Press, Boca Raton, FL. 245 pp.
- Faragalla AA, Al-Ghamdi MS, Tahaer MO, Kurdi M., Effects of the chitin inhibitor (Dimilin) on larvae of the house fly Musca domestica L. 1988; (In press).
- Goulet H, Huber JT., Hymoenoptera of the world: an identification guide to families, Research branch agriculture Canada publications 1993.
- Grasswitz TR, Burts EC., Effects of native natural enemies and augmentative releases of Chrysoperia rufitabris Burmeister and Aphidoletes aphidimyza (Rondani) on the population dynamics of the green apple aphid, Aphis pomi De Geer, International Journal of pest Management, 1995; 41(3): 176-183.
CrossRef - Hijazi MN, Yasir AA Saeed AA., Pest control Abu Atahma for Books and Stationery, AlMadinah, K.S.A. 1996; 596 P.
- Klunker R., The occurrence of puparium parasitoids as natural enemies of house flies. Appl. Parasitol. 1994; 35 (1): 36-50.
- Kocisova A, Venglovsky,J, Para L, Petrovsky M., Control of the house flies (Musca domestica) using the insect growth regulator diflubenzuron. Czech. Journal of Animal Science 2000; 45(10): 475-480.
- Masner L., The classification and interrelationships of Thranini (hymenoptera: Proctotrupoidea, Scelionida) Canadian entomdogist 1972; 104 : 833-849.
- Masner L., Key to the holarctic genera of Scelionidae, with descriptions of new genera and species (Hymenoptera: Practotrupoidia). Memoirs of the Entomological society of Canada 1980; 113. 54 pp.
- Miller RW, Rutz DA, Pickens LG, Geden CJ., Evaluation of traps and the parasitoid Muscidifurax raptor Girault and Sanders to manage house flies and stable flies on dairy farms. J. Agric Entomol. 1993; 10: 9-19.
- Olkowski W, Daar S, Olkowski H., Common-sense pest control: least-toxic solutions for your home garden, Pets and community. Taurton Press, Newtown, CT. 1991; 715 pp.
- Pedigo LP., Entomology and pest management. McMillian Publishing Co. New York. 1989; 646.
- Richardson J., Echoes from our network. Echo Development Notes (46), October. North Fort Myers, Fl. 1994; 6.
- Rina T, Verma AK, Urmila B, Wankhade., Effectiveness of diflubenzuron in control of house flies. J. Vector Borne Dis. 2010; 47: 97-102
- Saunders DS, Hayward SA., Geographical and diapause-related cold tolerance in the blow fly, Calliphora vicina. J. Insect Physiol. 1998; 44(7-8): 541-551.
CrossRef - Scott HG., Human myiasis in North America, Fla. Ent. 47. house fly Musca domestica (Diptera. Muscidae). J. Am. Mosq. Control Assoc. 1964; 8: 268-272.
- Seymour RC,Campbell JB., Predators and parasitoids of house flies and stable flies (Diptera: Muscidae) in cattle confinement in west central Nebraska. Environ. Entomol. 1993; 22: 212-219.
CrossRef - Silva JJ, Mendes J., Effect of diflubenzuron on immature stages of Haematobia irrituans (L.) Diptera: Muscidae) in UberLandia, State of Mians gerais brazil. Men. Inst. Oswaldo. Cruz. Jul. 2002; 97(5): 679-82.
CrossRef - Tomberlin JK, Lohmeyer KH, Kattes D., Treatment of pastures with diflubenzuron suppresses horn fly Haematabia irritans (Diptera: Muscidae) development. J. Agric. And Urban Entomel. 2007; 24(2): 95-101.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






