Manuscript accepted on : 01 March 2018
Published online on: --
K. G. Akpomie1, C. C. Ezeofor1, O. L. Alum1, U. A. Augustine2 and F. N. Ibeanu3
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria.
2Department of Chemistry, Federal University Lafia, Lafia, Nigeria.
3National Institute for Hospitality and Tourism (NIHOTOUR), Enugu Campus, Enugu, Nigeria.
Corresponding Author E-mail: kovo.akpomie@unn.edu.ng
DOI : http://dx.doi.org/10.13005/bbra/2608
ABSTRACT: The effect of Dioscorea rotundata peel environmental waste as an insulating additive for local clay was studied. The potential of a low cost indigenous clay mineral obtained from Akpugu-Ezedike in Nigeria for industrial application was also evaluated. The clay was characterized by the Fourier transform infrared spectroscopy, X-ray diffraction and Scanning electron microscopy (SEM). Prepared slurry mixture of the clay and peels were shaped, dried and fired. The chemical composition of the clay was determined by classical methods. Physical properties such as linear shrinkage, total shrinkage, modulus of rupture, apparent density, bulk density, apparent porosity,s welling index and water absorption, modulus of plasticity and refractoriness of the fired samples were determined. The clay was found to have a refractoriness of 12000C. SEM analysis revealed an increase in porosity of the fired clay bodies with increase in dosage of Dioscorea rotundata added. This result was corroborated by the high apparent porosity 49.67 – 60.02% and water absorption recorded 30.92 – 48.44% for the mixed fired samples. The result of this study clearly indicated the potential of Dioscorea rotundata peel as additive in enhancing insulating properties of fired clay as well as the usefulness of the local clay mineral for industrial purposes.
KEYWORDS: Akpugu-Ezedike Clay; Dioscorea Rotundata; Environmental Waste;Insulation Refractory;
Download this article as:| Copy the following to cite this article: Akpomie K. G, Ezeofor C. C, Alum O. L, Augustine U. A, Ibeanu F. N. Performance of Dioscorea Rotundata Peel As An Environmental Waste Additive in Enhancing the Insulating Properties of A Local Clay Mineral. Biosci Biotech Res Asia 2018;15(1). |
| Copy the following to cite this URL: Akpomie K. G, Ezeofor C. C, Alum O. L, Augustine U. A, Ibeanu F. N. Performance of Dioscorea Rotundata Peel As An Environmental Waste Additive in Enhancing the Insulating Properties of A Local Clay Mineral. Biosci Biotech Res Asia 2018;15(1). Available from: https://www.biotech-asia.org/?p=29406 |
Introduction
Clays can be defined as a solid rock matter with very fine particles which are plastic when wet and undergo changes to become hard when heated. Clay is composed of silica, alumina and water with appreciable concentration of oxides of iron, alkali and alkaline earth and contains groups of crystalline substances called clay minerals such as feldspar, silica and mica.1 The minor oxide component of clays is seen as impurities which occur in variable quantities are very important. This is because their presence imparts some properties to clay which are of technical value.2 Clay is used in the production of various important products such as refractory brick, ceramics, paints, pottery ware, and tiles and so on. Clay has been found to be useful for producing insulating materials for refractory kiln and other metallurgical purposes. Insulators refer to materials that resist the flow of heat or electric current and are applied in situations where high temperature applications are required in most industrial processes. Therefore materials that are able to withstand high temperatures are said to be refractory.3 The raw material mostly consumed in the production of refractory is clay. However, despite the abundance of clays in Nigeria, most refractory materials are been imported and this has a negative effect on the Nigerian economy.3,4 Therefore it is important to utilize the local clay minerals available in abundance for the manufacture of refractory or insulating materials. Many researchers have harnessed local clay minerals in various areas of research which also includes the manufacture of refractory or insulating bodies.5-13 Despite this, a large amount of clay deposits in various parts of Nigeria are yet to be harnessed. One of such is the abundance of clay deposits in Akpugu-Ezedike in Uzo-Uwani local government area of Enugu state, Nigeria. This clay obtained from this area called Akpugu-Ezedike Clay (AEC) was thus utilized in this study. Also apart from fire clays which can naturally be used in the manufacture of refractory, other clays are utilized with the presence of other additives such as silica and feldspar in order to improve the desired properties. The insulating properties of such clay products when desired is achieved by the addition of biomass materials such as the commonly used sawdust and rice husk.12 For the product to have good insulating properties it must be highly porous, have low thermal conductivity, and possess low solid density with low shrinkage.3 They should be highly porous and have low thermal conductivity and high thermal insulating properties suitable for minimizing heat losses and maximizing heat conservation in furnaces.14 The insulating effect is simply the result of achieving series of air spaces between the sintered or vitrified clay bodies. They derive their low thermal conductivities from their pores, while their heat capacity is determined by the entire solid body.15 Also important to note is that the material used as additive to achieve insulation most be low cost, readily available and locally sourced for economic purposes. The most commonly used materials are sawdust and rice husk. A previous study revealed the good potentials of cassava peel in enhancing the insulating properties of a local clay deposit.4 The white yam (Dioscorea rotundata) is present and cultivated in abundance in West Africa and Nigeria in particular, the peels are readily available, found in large amounts as solid environmental wastes, but have not been utilized as additives in enhancing the insulating properties of clay. In this regard, this study therefore investigates for the first time the effect of Dioscorea rotundata Peel (DRP) in enhancing the insulating properties of AEC. The characterization of AEC was first determined and the effect of DRP on its insulating properties was evaluated.
Experimental
AEC Preparation and Moulding
AEC was collected by random sampling at different points at depths of 1.55 m from Akpugu-Ezedike in Uzo-Uwani local government area of Enugu state, Nigeria. The samples were mixed properly to obtain a homogenous sample. The cone and quartered method was used to obtain a representative sample as described previously by Abubakar et al.16 Thereafter, it was dispersed in excess water in a plastic container and stirred properly. The dispersed sample was then filtered through a 0.425 mm mesh sieve in order to remove plant materials and unwanted particles. The obtained filtrate was kept to settle and then excess water was decanted. The residue (AEC) was sundried, followed by oven drying at 100 0C for 3 hr, pulverized and passed through a mesh sieve of size 0.18 mm to obtain the clay without DRP additive (100AEC). Dioscorea rotundata was obtained from Orie market, Emene, Enugu, Nigeria and washed properly with tap water. The peels were removed with the help of a kitchen knife and washed further. The DRP were sundried for 23 days, then oven dried at 600C for 4 hrs, pulverized and passed through mesh sieve of size 0.18 mm to obtained particles as those of AEC.
The influence of DRP on the insulating properties of AEC was performed by mixing both materials in the following percentage proportions by weight respectively; 10 to 90% (90AEC-10DRP), 20 to 80% (80AEC-20DRP) and 30 to 70% (70AEC-30DRP). 2.0 kg of each sample was mixed with about 0.5 L of water to make it plastic enough for the molding. The samples were molded to obtain cylindrical and rectangular test pieces and the physical properties at various firing temperatures determined as described in a previous study.4,13
Determination of Physical Properties
The making moisture of AEC was obtained by weighing the cylindrical test pieces immediately after molding and recorded as the wet weight (Wo) . The test pieces were then air-dried for 24 hr and then oven dried at 1050C until constant weight and then weighed as dried weight (Wi). The making moisture of AEC was then calculated:
Making Moisture (%) = 100[Wo-Wi]/Wo (1)
The modulus of plasticity of AEC was determined by taken the original height (Ho) in cm of the cylindrical test pieces by the use of a venier caliper taking the average of the three sides. A manual plastometer machine was then used to deform the test pieces. The deformation height obtained by taking the average of the three sides was recorded as Hi in cm. The modulus of plasticity was calculated from:
Modulus of Plasticity = Ho/Hi (2)
The Modulus of Rupture (MOR) of the samples was determined as described previously17; five long rectangular test pieces were utilized and air dried for 7 days after which they were oven dried at 1050C until a constant weight was obtained. Four of the pieces were fired to their respective temperatures of 900, 1000, 1100 and 12000C in a laboratory kiln (Fulham Pottery). The electrical transversal strength machine was used to determine the breaking load, p (Kg). A vernier caliper was used to determine the distance between support L (cm) of the transversal machine. The height, h (cm) and the width, b (cm) of the broken pieces were determined and the average value obtained from the two broken parts was recorded. The modulus of rupture was then calculated:
Modulus of Rupture (KgF/cm2) = 3pL/2bh2 (3)
The linear and total shrinkage were also determined. A venier caliper was used to pierce a 5.0 cm mark on the long rectangular test pieces immediately after molding and recorded as original length Lo (cm). The test pieces were then air dried for 7days and then dried in an oven at 1050C until a constant weight was obtained. The shrinkage from the 5cm mark was then determined and recorded as the dried length, Ld (cm). Four of the dried samples were then fired to temperatures of 900, 1000, 1100 and 12000C each temperature for a particular test piece. The shrinkage of the 5cm mark was then determined as the fired length, Lf (cm) and the linear and total shrinkage were then calculated:1
Linear Shrinkage (%) = 100[Ld – Lf]/ Ld (4)
Total Shrinkage (%) = 100[Lo – Lf]/ Lo (5)
The water of absorption (WA) was determined as described.12 The fired rectangular test pieces were weighed as dried weight M1 (g). The test pieces were then soaked in water for one hour, then removed, cleaned and weighed immediately and recorded as soaked weight, M2 (g) and the WA was calculated:
WA (%) = 100 [M2 – M1]/ M1 (6)
The suspended weight of the test pieces were then determined by the use of a lever balance and recorded as M3 (g). The apparent porosity and bulk density were calculated:
Apparent Porosity (%) = 100 [M2– M1]/ [M2 – M3] (7)
Bulk Density (g/cm3) = M1/[M2 – M3] (8)
The Loss on Ignition (LOI) of AEC was determined by the method described previously.4 The weight of an empty porcelain crucible was determined as W1 (g), 2.0 g of the dried pulverized AEC was added and the weight determined, W2 (g). The sample was then ignited in the laboratory kiln at 12000C and cooled in a dessicator and the weight of the crucible + sample after ignition was determined, W3 (g). The LOI of AEC was then calculated:
LOI = 100[W2 – W3]/ [W2 – W1] (9)
The swelling index test was performed by the method described by Abuh et al,1 10 g of oven dried AEC was passed through a mesh of size 0.425 µm and placed in 100 mL transparent graduated measuring cylinder containing distilled water. The mixture was stirred continuously for thorough mixing, stopped and allowed to sand for 24 hrs. The sediment volume (V) was measured against the graduations of the measuring cylinder (Vo). The free swelling index was calculated from the equation:
FSI (%) = 100[(V-Vo)/Vo] (10)
Material Characterization
The chemical composition of AEC was determined by the Atomic Absorption Spectrophotometer (AAS) (Buck Scientific Model, 210VGP) after digestion of the sample with nitric acid. The Fourier transform infrared (FT-IR) spectrum of AEC was determined with the Fourier Transform Infrared Spectrophotometer (Shimadzu FT-IR 8400s). X-ray diffraction (XRD) analysis was determined using a model MD 10 Randicon diffractometer operating at 25kv and 20mA. The scanning regions of the diffraction were 20-800 on the 2Ɵ angle. The morphology of AEC was analyzed by the Scanning Electron Microscope (SEM) (Hitachi S4800 model).
Results and Discussion
Chemical Characterization
The result of the chemical analysis of AEC is shown in Table 1. The table showed the quantitative analysis of the clay, revealing the percentages of the principal elemental oxides present. The major elemental oxides present in AEC are silica (SiO2) and alumina (Al2O3) which constitute over 60% of the clay. The other oxides present in appreciable quantity are Fe2O3 (6.11), K2O (4.03%) and CaO (3.76%), while some metal oxides are present in very small quantities. The low concentrations of MgO and MnO do not constitute any treat to the expected performance of the fired body at high temperature.12 However, most of the major minerals will enhance the desired properties of the clay body. The higher the alumina content the more refractory the clay.16 The alumina content of 12.48% was lower than the standard required for the manufacture of ceramics (> 26.5%) and refractory bricks (25-44%)18 this may indicate a low or moderate refractoriness of AEC. Silica concentration of 52.65% was lower than the requirement for high melting clays (53-73%).18 The high Fe2O3 concentration of 6.11% is not desirable for insulating products as it tends to conduct when present in clay reducing the insulating property of the clay minerals and also tend to impact a reddish color to the fired body as seen in the Table. High Fe2O3 concentration may act as fluxes reducing the refractoriness of AEC.14 Furthermore, the presence of alkali oxides acts as mild fluxes reducing the refractoriness of clay. The presence of appreciable amounts of CaO, K2O and Na2O suggest again a low or moderate refractoriness of AEC and this indicates why a refractoriness of 12000C was obtained (Table 1). At higher temperature than this signs of failure were observed, which implied that AEC was below the standard of 1580 – 17500C for refractory materials.1 Similar results have been reported previously for some clay.1,10 The loss on ignition (LOI) of 17.87% was within the requirement for ceramics (> 8.18%) and refractory bricks (8-18%) but higher than that for high melting clays (5-14%).19 The free swelling index examines the tendency to swell over a period of time. The FSI of AEC was 38.4% which was high and below the requirement (>60%) needed for drilling mud as most bentonite clays have. The moderately high FSI may indicate that AEC should have good modulus of plasticity as seen in Table 1 where the clay recorded a modulus of plasticity of 1.32. This implies that the clay can easily be molded into shape.
Table 1: Physicochemical properties of Akpugu-Ezedike clay
| Parameters | Value |
| Al2O3 (%) | 12.48 |
| SiO2 (%) | 52.65 |
| Fe2O3 (%) | 6.11 |
| CaO (%) | 3.76 |
| K2O (%) | 4.03 |
| Na2O (%) | 1.81 |
| MgO (%) | 0.92 |
| MnO (%) | 0.13 |
| LOI (%) | 17.87 |
| Colour before firing | Dark brown |
| Colour after firing | Reddish |
| Refractoriness (0C) | 1200 |
| Modulus of Plasticity | 1.32 |
| Making moisture (%) | 18.73 |
| Free Swelling Index (%) | 38.4 |
FTIR, XRD and SEM Characterization
The Fourier Transform Infrared (FTIR) spectrum of AEC is shown in Fig.1, The broad bands around 3600 cm-1 represents the inner OH stretching vibration of clays while the weak band around 3200 cm-I corresponds to the outer OH stretch vibration.9 Absorption bands around 1620 cm-I correspond to the OH bending vibration of water and can also be attributed to the symmetric –COO- stretching vibration. The presence of the outer –OH and the symmetric –COO- vibration indicated the presence of the smectite structure in AEC. Absorption bands around 900 cm-1 was attributed to the Al-O bending vibration of clays.11 The presence of OH in general may correspond to inner water molecules which would be removed during firing but influences the plastic properties of the clay which is desirable.
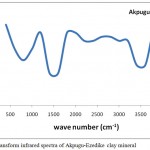 |
Figure 1: Fourier transform infrared spectra of Akpugu-Ezedike clay mineral
|
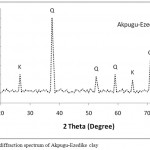 |
Figure 2: X-ray diffraction spectrum of Akpugu-Ezedike clay
|
The XRD spectrum of AEC is shown in Fig. 2, the spectrum showed the occurrence of sharp and high peaks indicating the crystalline nature of the material, and 2θ values at 38.5, 52.7, 59.3, 71.8 corresponding to the presence of quartz as the principle mineral and kaolinite as minor or accessory material at 26.7 and 65.3. The type and quantities of the minerals present influence the refractory performance. For refractory materials, the presence of quartz is not desirable because it reduces refractoriness. The preferred minerals are kaolinites and illites.12 Therefore the low refractoriness recorded for AEC may also be as a result of the high concentration of quartz as revealed in the XRD and chemical composition in Table 1. Fig. 3 showed the SEM morphology of the fired samples, it is clearly observed that an increase in the porosity of the clay body with increase in concentration of DRP was recorded which is desirable in enhancing the insulating properties of AEC. The formation of porous structure is responsible for low thermal conductivity of the product formed after firing.15
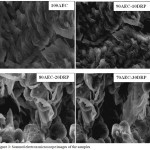 |
Figure 3: Scanned electron microscope images of the samples
|
Physical Analysis
The influence of the addition of DRP on the insulating properties of AEC at firing temperatures of 900-12000C is shown in Figs 4 – 10. Fig. 4 showed the effect of temperature on the linear shrinkage of the samples. As observed, increase in linear shrinkage with increase in firing temperature was recorded for the samples. With increase in firing temperature from 900-12000C the linear shrinkage of 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP increased from 1.81-2.79%, 1.89-3.0%, 2.34-3.20% and 2.67-3.3% respectively. This increase is attributed to the compression of the clay particles as a result of the decomposition of DRP components and the removal of the combustible components which resulted in sintering and vitrification. It was also observed that an increase in linear shrinkage with increase in the dosage of DRP added was also recorded. This is attributed to the decomposition of more DRP component during firing with increase in its concentration in the fired body. A reasonable low linear shrinkage (<10%) is needed for a clay to have good insulating properties.3 The range of linear shrinkage for all the samples at all firing temperatures were within this recommended range for insulating products.13
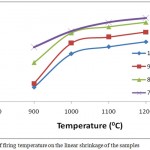 |
Figure 4: Effect of firing temperature on the linear shrinkage of the samples
|
The result for the effect of firing temperature and DRP addition on the total shrinkage of the samples is shown in Fig. 5. Similar trend of increase in total shrinkage with firing temperature and with DRP addition was also recorded as in the case of the linear shrinkage. This could be attributed to the same reasons as those of linear shrinkage. With increase in firing temperature from 900 – 12000C the total shrinkage increased from 2.91-4.12%, 3.42-4.42%, 3.83-4.38% and 4.03-5.05% for 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP, respectively. Total shrinkage in general is of less importance as the linear shrinkage as the total shrinkage is usually affected greatly by the making moisture applied during molding. Similar result was obtained when the effect of cassava peel on the insulating properties of ogugu clay deposit was investigated.4
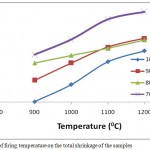 |
Figure 5: Effect of firing temperature on the total shrinkage of the samples
|
Fig.6 shows the influence of firing temperature and DRP addition on the modulus of rupture of AEC. An increase in MOR with increase in temperature for all the samples was recorded. Increase in firing temperature from 900 – 12000C gave an increase in MOR from11.67-21.45 kgF/cm2, 21.45-25.98 kgF/cm2, 20.51-24.76 kgF/cm2 and 18.43-20.43 kgF/cm2 for 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP respectively. The increase is attributed to bond formation during sintering and vitrification of the clay body and the coming together of the particles during shrinkage. An increase in MOR with addition of DRP was obtained for 90AEC-10DRP after which it decreased steadily with further addition for 80AEC-20DRP and 70AEC-30DRP. The initial increase with the addition of about 10% DRP indicated the suitability of this dosage in blending the clay components for enhanced strength during vitrification. The decrease in MOR with further increase in dosage of DRP is attributed to the excessive burning off of DRP component which induced much porosity thereby reducing the strength. Similar result has been reported previously.12
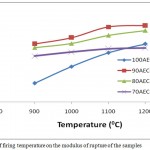 |
Figure 6: Effect of firing temperature on the modulus of rupture of the samples
|
The influence of firing temperature and DRP addition on the bulk density of the samples is shown in Fig.7. It was observed that an increase in the bulk density of the fired bodies with increase in firing temperature was recorded. The increase is attributed to an increase in shrinkage which resulted in the bodies becoming more compact and dense. It is also attributed to bond formation as a result of sintering and vitrification. With increase in firing temperature from 900 – 12000C the bulk density of 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP increased from 1.58-1.60 g/cm3, 1.52-1.54 g/cm3, 1.47-1.50 g/cm3 and 1.44-1.47 g/cm3 respectively. A corresponding decrease in the bulk density with increase in the dosage of DRP in the clay body was also observed. This is expected due to pores formation which resulted from the burning off of DRP component during firing. As the components are removed the clay body tends to become lighter, this implies that as the dosage of DRP in the sample is increase more would be removed during firing from the total weigh thereby making the sample lighter. This low solid density is desirable for a good insulating refractory.3 bA similar result was obtained by Sutcu et al.15 in the production of anorthite refractory insulating firebrick from mixtures of clay and recycled paper waste with sawdust addition.
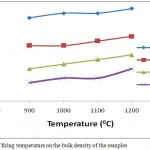 |
Figure 7: Effect of firing temperature on the bulk density of the samples
|
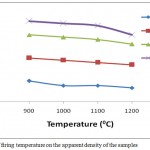 |
Figure 8: Effect of firing temperature on the apparent density of the samples
|
The apparent density which is the weight of the material divided by its exterior volume less the volume of its open pores was determined. The apparent density is very important as it is related greatly to the porosity of the material. The higher the apparent density the more porous the material and the smaller the apparent density the less porous or more compact the material. The apparent density always records an opposite trend to the bulk density. The opposite trend of decrease in apparent density with increase in firing temperature for all the samples was obtained in this study as shown in Fig. 8. This indicated a decrease in porosity of the fired body due to increase in shrinkage with firing temperature. In fact with increase in firing temperature from 900 – 12000C the apparent density decreased from 2.09-2.06 g/cm3, 2.19-2.16 g/cm3, 2.29-2.25 g/cm3 and 2.35-2.29 g/cm3 for 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP respectively. Similarly a reverse trend to the bulk density in which an increase in the apparent density with increase in dosage of DRP added to the clay body was recorded at all firing temperatures. This indicated that the samples became more porous and lighter with DRP addition and is attributed to the burning off of the added DRP components as stated earlier.
The influence of firing temperature and DRP addition on the apparent porosity of the fired bodies are shown in Fig.9. It was observed that decrease in apparent porosity with increase in firing temperature was recorded for all the samples. With increase in firing temperature from 900 – 12000C the apparent porosity decreased from 36.21-33.03%, 53.84-49.67%, 57.21-53.01% and 60.02-56.51% for 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP respectively. This decrease is as a result of increase in shrinkage which led to decrease in porosity with increase in firing temperature. The result is corroborated by the results discussed earlier on the increase in linear and total shrinkage and decrease in apparent density with firing temperature. Also an increase in the apparent porosity with increase in the dosage of DRP added to the fired clay body was recorded. It is clearly seen that addition of DRP to the clay body greatly increased the porosity which is desirable compared to the sample where it was absent (100AEC). This result was corroborated by the SEM morphology discussed previously. The porosity was induced by the burning off of more DRP components from the clay body as its concentration in the sample increased. The presence of pores helps insulation by serving as air spaces to trap heat and prevent flow or conduction. A major requirement for good insulating refractory is that it must be highly porous.3,13 The apparent porosity of all the fired clay bodies containing DRP was within the standard requirement (> 45%) for the production of insulating firebricks.20 This indicated excellent insulating potential of DRP in clay body which is desired in this study. The porosity values obtained were much greater than those obtained when cassava peel was utilized in enhancing the insulating properties of ogugu clay.4
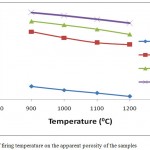 |
Figure 9: Effect of firing temperature on the apparent porosity of the samples
|
The effect of firing temperature and DRP addition on the water absorption of AEC fired body is shown in Fig. 10. Similar trend as the apparent porosity was obtained for the water of adsorption, as these two parameters are directly proportional to each other. A decrease in the water absorption of all the fired clay bodies with increase in firing temperature was obtained. This increase is due to decrease in porosity of the samples with increase in firing temperature, as the pores are responsible for water uptake. In fact with increase in firing temperature from 900 – 12000C the water of absorption decreased from 22.67-20.11%, 33.81-30.92%, 40.21-37.62% and 48.44-45.23% for 100AEC, 90AEC-10DRP, 80AEC-20DRP and 70AEC-30DRP respectively. The high water of absorption indicated a highly porous material suitable for insulating purposes. The water absorption also showed a corresponding increase with increase in dosage of DRP added to the clay body. This is expected due to the burning off of more of the DRP component during firing and consequently increases in porosity of the fired body. Similar result was reported when the effect of olive mill waste addition on the properties of porous fired clay bricks using Taguchi method was studied.21
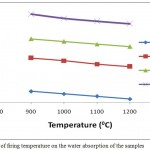 |
Figure 10: Effect of firing temperature on the water absorption of the samples
|
Conclusion
The experimental result of this study showed the usefulness of Akpugu-Ezedike clay as a local low-cost available material for industrial purposes such as bricks or ceramics production. Also Dioscorea rotundata peel was found to have very good ability in enhancing the insulating properties of the clay minerals as reported from its high apparent porosity (> 45%) and water absorption (> 30%) induced in the fired clay bodies.
Acknowledgements
We acknowledge the support of Mr. Isa of the Department of Chemical Engineering, Ahmadu Bello University (ABU), Zaria, Nigeria for the XRD and SEM characterization. The Authors are also grateful to the management of the Ceramics Research and Development Department (CRPD) of the Projects Development Institute (PRODA), Enugu, Nigeria for the Conducive laboratory provided for this research work.
References
- Abuh M. A, Abia-Bassey N, Udeinya T. C, Nwannewuihe H. U, Abong A. A, Akpomie K. G. Pacific J. Sci. Technol. 2014;15:63.
- Folorunso D.O, Olubambi P, Borode J.O. IOSR .J. Appl. Chem. 2014;7:40.
CrossRef - Manukaji J. U. Inter. J. Eng. Res. Appl. 2013;3 06.
- Etukudoh A. B, Akpomie K. G, Okereke O. C. B, Abuh M. A. Inter. J. Adv. Eng. Res. Technol. 2016;4 273.
- Elueze A. A, Ntekin E. E, Ekwere J. J. J. Min. Geol. 1999;35:117.
- Nnuka E. E, Agbo U. J. E. NSE Tech. Transact. 2000;35 34.
- Gbadebo A. M. Niger. J. Eng. Res. Develop. 2002;1:20.
- Igwe I. O, Ezeamaku L. U. Malaysian Poly. J. 2010;5:81.
- Dawodu F. A, Akpomie K. G, (2014). J. Mater. Res. Technol. 2014;3:129.
CrossRef - Hassan M. A, Yami A. M, Raji A, Ngala M. J. Inter. J. Eng. Sci. 2014;3 40.
- Akpomie K. G, Dawodu F. A, Adebowale K. O. Alexandria Eng. J. 2015;54:757.
CrossRef - Folorunso D. O, Aramide F. O, Olubambi P, Borode J. O. J. Miner. Mater. Character. Eng 3. 2015;309.
CrossRef - Mathew G. O, Owoeye S. S. Leonard. Elect. J. Pract. Technol. 2016;29:115.
- Kefas H. M, Patrick D. O, Chiroma T. M. Leonard. Elect. J. Pract. Technol. 2007;11:123.
- Sutcu M, Akkurt S, Bayram A, Uluca U. Ceramics Inter. 2012;38:1033.
CrossRef - Abubakar I, Birnin-Yauri U. A, Faruq U. Z, Moma S. S, Sharif N. Afr. J. Environ. Sci. Technol. 2014;4:234.
- Awilapo L. D, Wiik K. Bull. Chem. Soc. Ethiopia. 2003;17:147.
- Chester J. H. Refractory, Production and Properties, Iron and Steel Institute, London. 1973;4.
- Grimshow R. W. The Chemistry and Physics of clay and Alhed Ceramics Materials, 4th Edition, Revised, Wiley in Conscience, New York. 1971;15.
- ASTM, American Society for Testing and Materials, Pittsburg, (1984) pp 133.
- Sutcu M, Ozturk S, Yalamac E, Gencel O. J. Environ. Manag. 2016;181-185.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





