How to Cite | Publication History | PlumX Article Matrix
Shazmin Mohiuddin Hafiz1, Smital Sameer Kulkarni1 and Mansee Kapil Thakur2
1Department of Medical Biotechnology - MGM School of Biomedical Sciences, MGMIHS, Kamothe, Navi Mumbai, Maharashtra, India.
2Department of Medical Biotechnology and Central Research Laboratory, MGM School of Biomedical Sciences, MGMIHS and MGMCET, MGM Medical College building, MGM Institute of Health Sciences, Kamothe, Navi Mumbai 410209, Maharashtra, India.
Corresponding Author E-mail: mansibiotech79@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2645
ABSTRACT: Nanoparticles are increasingly used for biomedical purposes. In the past decades, much attention has been paid to toxicity assessment of nanoparticles prior to clinical and biological applications. Iron oxide nanoparticles (IONPs) are being introduced into the consumer market significantly. Effects and accumulation patterns of IONPs were studied using zebrafish embryos. Nanoparticles of iron were synthesized by biological reduction of spinach, using 0.1 M Ferric Chloride. These nanoparticles were characterized by SEM-EDS, XRD, FTIR and AAS were found to have the size range of 100 to 250 nm. Fish (n=12/group) were exposed from 8hpf to 7dpf to 1, 5, 10, 50 and 100 mg/L IONPs. The results showed that LC50 was found to be10 mg/L concentration of iron oxide nanoparticles. 50 mg/L and 100 mg/L concentrations showed developmental toxicity in these embryos, causing mortality, and hatching delay. This study is one of the first on developmental toxicity in zebra fish caused by using biologically synthesised iron oxide nanoparticles.
KEYWORDS: FTIR; Iron Oxide Nanoparticles; SEM; Toxicity XRD; Zebrafish;
Download this article as:| Copy the following to cite this article: Hafiz S. M, Kulkarni S. S, Thakur M. K. In-Vivo Toxicity Assessment of Biologically Synthesized Iron Oxide Nanoparticles in Zebrafish (Danio Rerio). Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Hafiz S. M, Kulkarni S. S, Thakur M. K. In-Vivo Toxicity Assessment of Biologically Synthesized Iron Oxide Nanoparticles in Zebrafish (Danio Rerio). Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=29828 |
Introduction
Nanotechnology is one of the newest and rapidly growing fields of science and technology, which applies the manufacturing of new materials at the molecular level at the nanoscale size.1 One of the upcoming technology in today’s biological research is “Nanotechnology” which deals with nanosized particles with a wide variety of applications in science and technology.2 Nanoparticles (NP) are characterized by their minute sizes, ranging between 1 and 100 nanometres (nm) and various diameters and forms. 3The synthesis and characterization of nanoparticles is an important area of research as a selection of size and shape of nanoparticles provides an efficient control over many of the physical and chemical properties. The current chemical methods for synthesizing nanoparticles are energy comprehensive i.e. they employ the use of toxic chemicals which produce hazardous wastes that preclude them for any biomedical application. Therefore, the use of bio-compatible, non-toxic, eco-friendly, as well as cost-effective methods, is becoming a necessary requirement for the production of nanoparticles. Biological methods are considered safe and ecologically sound for the production of nanomaterial and alternative to conventional physical and chemical methods.4 Biological systems such as yeast, fungi, bacteria, plants etc., considered as biomimetic for the synthesis of nanostructures.4
Use of plants for the synthesis of metallic nanoparticles offers a cost-effective and regulated alteration in its size and shape.5 Inactivated plant tissues, exudates, plant extracts and other living plant parts serve as a substitute for the production and synthesis of metallic nanoparticles.6 Nanoparticle synthesis using various plant parts and their extracts offers benefit in their ease of accessibility and management. It also possesses various metabolites such as antioxidants, nucleotides, and vitamins. Spinach, a green leafy vegetable with high iron content and vast medical applications have been chosen for the synthesis of iron oxide nanoparticles. It contains several essential nutrients phytonutrients, minerals, vitamins and therapeutic properties. It has many essential mineral components which include manganese, zinc, potassium, magnesium, calcium, and iron. It also contains essential vitamins like niacin, foliate, vitaminB6, vitamin A, and vitamin C. It has an abundant supply of riboflavin and thiamine. Pigments present in spinach such as lutein, beta-carotene, chlorophyll, and xythene are thus useful for maintenance of healthy eyes, cardiovascular system, and nervous system. It is recommended for a variety of disorders like constipation, anemia, high blood pressure, obesity, tumors etc.7,8 In the present research program, IONPs have been synthesized by using an aqueous extract of spinach plants at room temperature.
With the increasing environmental exposure of these nanoparticles, evaluation of their toxicity level has become very important. There are several routes by which these nanoparticles may impose toxicity. One of which may be, access to the intracellular metabolism through the passage of cell membrane.9 Dimension, dose, and durability such as size, shape, charge, aggregation, concentration, composition, etc are the most crucial parameters in determining adverse health effects of nanoparticles and thus thinking about their solution. Proper assessment of the toxicological aspects of nanomaterials is extremely important, especially if intending to administer them to patients. This is important because of the increase in the number of hazardous products produced and released into the environment and the threat of potential exposure to them naively. Many mammalian models for toxicology are expensive and difficult to manipulate and assess during the embryonic stage. Zebrafish is among the few vertebrate model organism’s that has expeditiously gained popularity.10 The important benefits of using zebrafish as a toxicological model over other vertebrate species are with regards to their size, husbandry, and early morphology. Zebrafish eggs remain transparent from fertilization to when the tissues become dense and pigmentation is initiated at approximately 30–72 h post fertilization (hpf)), this allows unobstructed observations of the main morphological changes.11 High level of resemblance exists between the human and zebrafish genomes (more or less 75 % similarity) making it a feasible animal model for analytical studies. They have high genomic homology.
Material and Methods
Preparation of Spinacia Oleracea Leaves Extract
The fresh leaves of spinach (Spinacia oleracea) were washed several times with distilled water to remove the dust. 20-25g of leaves were weighed, washed thoroughly with double distilled water, cut into small pieces and added in a flask containing 100 ml of distilled water. It was boiled for 15-20 min at 70oC, the colour of the solution changes from watery to light green. The extract was filtered using Whatman filter paper no. 42 and stored at 4°C for further use.
Green Synthesis of Iron Oxide Nanoparticles
In a conical flask, 0.1 M FeCl3 was added to plant extract in the ratio 1:1. This arrangement was kept at room temperature for 1 hour with continuous stirring on a magnetic stirrer. Centrifugation was done at 3000 rpm for 15 minutes. The supernatant was removed and ethanol was added to the pellet for washing. Again centrifugation was done for 5 minutes at 3000 rpm. This procedure was repeated thrice. The supernatant was removed and to the pellet, ethanol was added and kept on sonication for 30 minutes. The solution was poured into a beaker and oven dried for 24-48 hrs. This resulted in the formation of solid particles which were iron oxide nanoparticles which were confirmed by characterization.
Characterization of the Synthesized Nanoparticles
The brown colored solid particles obtained were characterized using various sophisticated instruments. The powdered sample was analyzed for the structure and morphology using JSM-7600F scanning electron microscope with an accelerating voltage between 10 and 20 KV under vacuum conditions. The structural characterization of biosynthesized nanoparticles was carried out by XRD analysis using Seifert XRD 3003 diffractometer. FTIR analysis for identification of functional groups of active components was done using 3000 Hyperion Microscope with Vertex 80 FTIR system model, IIT Bombay. The quantitative analysis of iron content present in the sample was estimated by Atomic absorption spectroscopy using PE Analyst 800.
In Vivo Toxicity Assessment of Biosynthesized Ionps
Zebrafish Housing
All the experimental protocols were approved by the Animal Institutional Ethics Committee of MGM Medical College, Navi Mumbai (Approval No: 2017/10/SC/22). Wild-type adult zebrafish obtained from a known zebrafish dealer were housed at zebrafish facility of Central research lab (CRL), MGMIHS Kamothe, Navi Mumbai. The zebrafish were kept in 6 lt of glass aquariums having chlorine-free water and aeration, at a density of 5-6 fishes per aquarium for 2 weeks before the experiment. Newly hatched brine shrimp and dry flake food diet were given three times a day. Breeding pairs were specifically fed brine shrimp half an hour before breeding. The water temperature was maintained at 27.0 ±0.5°C. The light cycle was set at 14h:10h Light: dark. Newly hatched embryo (stable chorion, highly transparent, non-sticky) eggs were collected by means of a plastic pipette and housed in containers filled with E3 medium. Approximately, 100 larvae were kept in each container. At the early developmental stages, zebrafish consumed paramecium as a source of nourishment.12
Breeding and Collection of Eggs
Females and males were kept at a ratio of 1:2 in a chamber filled with filtered tap water. The breeding chamber consists of one circular container that held a smaller circular container wish mesh bottom. The bottom of the inner container is rested above the bottom of the outer container to allow expelled eggs to fall on the inner side to prevent predation of eggs by parent fish. After the first light exposure in the morning, spawning and fertilization take place within 30 minutes. About 30-60 minutes after spawning, the egg traps were removed, and the eggs were collected by means of a plastic pipette.
Exposure Process
Test solutions were prepared immediately prior to use by diluting the stocks of Fe2O3 with the E3 medium. The embryotoxicity assay was commenced on the selection of 72 ( 12 each group) intact fertilized eggs at 8 hpf. These eggs were transferred to a 24 well multi-plate carrying 3 12ml each of five Fe2O3 test solutions and one control group. Four wells were allotted for control group containing the E3 medium, whereas remaining twenty wells were used by the five concentration gradients. Embryos were exposed to five different concentrations of IONPs which included 1, 5, 10, 50, 100 mg/L. The experiment was performed thrice. These plates were kept in a controlled environment with 14 h light and 10 h dark cycle in the fish laboratory. All the dead embryos and larvae were discarded at the end of each day.
Statistical Analysis
All the experiments were performed in triplicates. Data was recorded statistically with mean, standard deviation and standard error. Statistical significance of the control and treated groups were estimated using paired t-test. P<0.05 was considered statistically significant.
Results and Discussion
The powdered sample was analyzed for the structure and morphology of the synthesized iron oxide nanoparticles using SEM. SEM images revealed that the synthesized iron oxide nanoparticles were in the size range of 100- 250 nm. It also represented the varied morphological diversity of the iron nanoparticles including rod shape, spikes, cube and spherical (Figure 1 a, b, c, d). There may be a probability of various plant metabolites, including terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins, play an important role in the bioreduction of metal ions, yielding a heterogeneous population of nanoparticles. The diversity in the morphology and size of nanoparticles synthesized from a variety of metal ions in extracts of various plants has been described in detail in the reviews.13,14 EDS analysis was carried out to determine the elemental composition and stoichiometry of the synthesized iron oxide nanoparticles. Iron, oxygen signals and other signals also have been detected. The other signal may be coming from the bioactive molecules present in Spinach leaves extract. It implies that the nanoparticles are mainly indeed made up of only Fe and O. The EDS study has identified various elements (C, O, Al, Si, P, S, Cl& Fe) with its composition. The elemental composition of Fe was found to be 16.17% (Figure 2).15,16
 |
Figure 1: The SEM images of IONPs a) Nano Rods b) Spine like Nanowires c) Cubes d) Sphere |
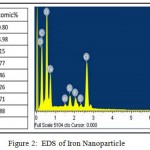 |
Figure 2: EDS of Iron Nanoparticle |
The XRD pattern of synthesized IONPs is given in Figure 3, the peaks obtained at 2θ = 24.14°, 41.76°, 50.41°, 54.09°, 57.61°, corresponding to hematite (Fe2O3), respectively. Moreover, our studies also reported that the synthesized IONPs by plant extracts are crystalline in nature.15,16,17,18,19
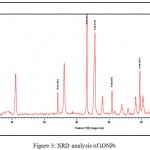 |
Figure 3: XRD analysis of IONPs
|
FTIR analysis was carried out to identify the possible biomolecules responsible for the reduction of IONPs and capping of the bio‐reduced IONPs synthesized by the leaf extract. The strong absorption peak at 3234 cm for FTIR of IONPs is assigned to O-H stretching of alcohol and phenolic compounds simultaneously. The peak at 1638.61 cm is due to stretching vibration of CO groups in the ketones, aldehydes and carboxylic acids. The formation of Fe2O3 is characterized by the absorption bands at 637 cm corresponds to the Fe-O bond (Figure 4A). These peaks were absent in their corresponding plant extracts which indicate the formation of iron oxide nanoparticles (Figure 4B). FTIR analysis confirmed that the bioreduction of ferric chloride into iron oxide nanoparticles is due to the reduction by capping material of spinach leaf extract [15,16,17,18,19].
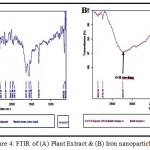 |
Figure 4: FTIR of (A) Plant Extract & (B) Iron nanoparticles
|
Atomic absorption spectroscopy is a quantitative estimation of elements using the absorption of optical radiation (light) by free atoms in the gaseous state. The IONPs synthesized from spinach extract has estimated 38.63% of Fe.
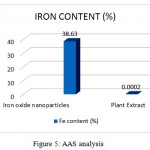 |
Figure 5: AAS analysis
|
Table 1: AAS analysis
| Sample | Fe content (%) |
| Iron oxide nanoparticles | 38.63 |
| Plant Extract | 0.0002 |
In vivo toxicity screening
These iron nanosystems presented good in vivo toxicity evaluations and its dose-dependent effects on embryonic development in zebrafish. The survival of zebrafish embryos and larvae exposed to different concentrations of Fe2O3 was determined at specific time points (8hpf to 7dpf). 1 mg/L to 5 mg/L of Fe2O3 exhibited no toxicity to zebrafish embryos or larvae. Both 50 and100 mg/L of Fe2O3 demonstrated toxicity, killing 100% of the zebrafish embryos/larvae at the end of the exposure (168 hpf). As a result, the 168-h NOEC of Fe2O3 was less than 10 mg/L. Moreover, survival of the embryos was more than 100% at 168 hpf in 1 mg/L and 5 mg/L, but it declined sharply to 0% in 50 mg/L and 100 mg/L, it might because of particle aggregation of IONPs, at least to some extent, involved in the toxicity observed in the embryos, as aggregates trapped in the chorion pores might obstruct oxygen transport between the medium and the embryos as represented in Figure 6. This result suggests that the zebrafish development toxicity of Fe2O3 is time and dose-dependent. In our experiment, Fe2O3 aggregates were found to be toxic to zebrafish embryos and larvae, causing a dose-dependent mortality and hatching inhibition. The most critical and sensitive stage of development is indeed suggested to be 1 day after fertilization (i.e. 24 hpf) when organogenesis is taking place.
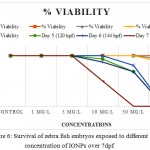 |
Figure 6: Survival of zebra fish embryos exposed to different concentration of IONPs over 7dpf
|
At this phase, rapid differentiation of organs occurs and the extensive rate of cell proliferation makes them particularly prone to teratogenicity factors and leading to most of the structural malformations. No developmental abnormalities, such as pericardial edema, malformation or tissue ulceration were found. Delay in hatching period was observed in 50 mg/L & 100 mg/L concentrations respectively. According to the previous studies, the evaluation of iron oxide toxicity was in debate. It is widely identified that iron oxide NPs does not contain any toxic material, thus it has been significantly preferred by the researchers. Kim et al. 2005 proposed the findings and stated that iron oxide particles doesn’t hinder the toxicity to mice in vivo.20 But, in several other studies, iron oxide had shown drastic change by increasing its toxicity level in a biological system (in vivo model of rodents).21
Conclusion
Biosynthesis of nanoparticles is considered a green technology that involves the exploitation of different plant materials and debars the involvement of any harmful chemicals. In this study, iron oxide nanoparticles (Fe2O3) size range 100-250nm were synthesized using Spinacia oleracea leaves extract. With the gradual rise in the use of iron oxide nanoparticles in targeted therapy, catalysis, sensors, MRI, tumor therapy, drug and gene transfer to cells, wastewater treatment, etc. It has become an immediate concern to verify its toxicological effects on humans. Our results in this study incorporate that Fe2O3 causes decreased motility, hatching delay and eventual death in the early life stages of zebrafish. This result can be partially explained by nanoparticle aggregation, as particles remained undissolved in water, saline or PBS. Properly dissolved particles and particles with small diameters can easily pass through the egg chorion pores. Whereas, large particles may get trapped in the chorion pores thus causing blockage of oxygen transport into embryos and eventually causing mortality.
Conflict of Interest
There is no conflict of interests.
Acknowledgement
The authors gratefully acknowledge financial support and infrastructure of MGMIHS
References
- Jothirethinam A, Prathiba S, Shanthi N, Arunkumar K. Green synthesized silver nanoparticles prepared from the antimicrobial crude extracts of two brown seaweeds against plant pathogens. American Journal of Nanotechnology. 2015;6(2):31.
CrossRef - Brundo M.V, Pecoraro R, Marino F, Salvaggio A, Tibullo D, Saccone S, Bramanti V, Buccheri M.A, Impellizzeri G, Scuderi V, Zimbone M. Toxicity Evaluation of New Engineered Nanomaterials in Zebrafish. Frontiers in physiology. 2016;7.
CrossRef - Teuşan A, Baranov N, Dmour R, Vizitiu A, Jelihovschi I, Teuşan V. The Effects Of Metal Nanoparticles On Embryos Of Different Animal Species. A Review. International Journal of Medical Dentistry. 2015;5(3).
- Zhu X, Tian S, Cai Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Daniorerio) early life stages. PLoS One. 2012;7(9).
CrossRef - Duan J, Yu Y, Shi H, Tian L, Guo C, Huang P, Zhou X, Peng S, Sun Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PloS one. 2013;8(9).
CrossRef - Kumar V, Sharma N, Maitra S.S. In vitro and in vivo toxicity assessment of nanoparticles. International Nano Letters. 2017;1-4.
CrossRef - Tiwari D.K, Behari J, Sen P. Application of nanoparticles in waste water treatment 1.
- Ghobadian M. Zebrafish as an in vivo Vertebrate Model for Nano EHS Studies. Journal of Clinical Developmental Biology. 2016;1(2).
CrossRef - Hill A.J, Teraoka H, Heideman W, Peterson R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicological sciences. 2005;86(1):6-19.
CrossRef - Reynolds M. A Toxicological Study using Zebrafish (Daniorerio) as a Model.
- Chakraborty C, Sharma A.R, Sharma G, Lee SS. Zebrafish. A complete animal model to enumerate the nanoparticle toxicity. Journal of nanobiotechnology. 2016;14(1):65.
CrossRef - Thakur M, Dayal N, Pai G, Joshi D. Toxicity Assessment of Silver Nanoparticles in Zebrafish Embryos.
- Raveendran P, Fu J, Wallen S.L. Completely “green” synthesis and stabilization of metal nanoparticles. Journal of the American Chemical Society. 2003;125(46):13940-1.
CrossRef - Haverkamp R.G, Marshall A.T. The mechanism of metal nanoparticle formation in plants: limits on accumulation. Journal of Nanoparticle Research. 2009;11(6):1453-63.
CrossRef - Monalisa Pattanayak and P.L. Nayak. Green Synthesis and Characterization of Zero Valent Iron Nanoparticles from the Leaf Extract of Azadirachta indica (Neem). World Journal of Nano Science & Technology. 2013;2(1): 06-09.
- Monalisa Pattanayak and P. L. Nayak. Ecofriendly Green Synthesis of Iron Nanoparticles from Various Plants and Spices Extract. International Journal of Plant, Animal and Environmental Sciences. 2013;3(1).
- Matheswaran Balamurugan, Shanmugam Saravanan, Tetsuo Soga. Synthesis of Iron Oxide Nanoparticles by Using Eucalyptus Globulus Plant Extract. e-Journal of Surface Science and Nanotechnology. 2014;12:363-367.
CrossRef - Akram Omnidvari, Faranak Manteghi, Beheshteh Sohrabi, Yasereh Afra. A herbal extract for the synthesis of magnetite nanoparticles. The 18th International Electronic Conference on Synthetic Organic Chemistry. 2014.
- Mahnaz Mahdavi, Farideh Namvar, Mansor Bin Ahmad and Rosfarizan Mohamad. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum muticum) Aqueous Extract. Molecules. 2013;18:5954-5964.
CrossRef - Kim J.S, Yoon T.J, Yu K.N, Kim B.G, Park S.J, Kim H.W, Lee K.H, Park S.B, Lee J.K, Cho M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicological Sciences. 2005;89(1):338-47.
- Zhu M.T, Feng W.Y, Wang B, Wang T.C, Gu Y.Q, Wang M, Wang Y, Ouyang H, Zhao Y.L, Chai Z.F. Comparative study of pulmonary responses to nano-and submicron-sized ferric oxide in rats. Toxicology. 2008;247(2-3):102-11.

This work is licensed under a Creative Commons Attribution 4.0 International License.





