How to Cite | Publication History | PlumX Article Matrix
Gorantla Sri Charitha, Kurmeti Sudhakar and K. Pratap Reddy
Department of Zoology, University college of Science, Osmania University, Hyderabad -500007, Telangana, India.
Corresponding Author E-mail: pratapkreddyou@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2652
ABSTRACT: Fluoride naturally occurs in the earth’s crust and ground water and it causes fluorosis when it is consumed in high levels. The fluorosis also affects soft tissues like liver, kidney, heart, brain etc., in addition to skeletal and dental systems. The present study reports the protective effects of selenium against sodium fluoride induced neurotoxic effects. Three months old (around 250 – 280 g weight) wistar rats were randomly categorized into four groups viz. Group I (control) which received normal tap water, Group II (sodium fluoride, NaF) treated with 20 ppm of fluoride through IP, Group III treated with (NaF 20 ppm) + Selenium (5 mgkg-1 body wt./day/rat) and Group IV treated with Selenium (5 mgkg-1 body wt./day/rat) alone. The doses were continued for a period of 15 days and after that they were used for recording behavioral (rota rod, hot plate), anti-oxidant (LPO, SOD, CAT and GSH-Px) and histological (Golgi cox staining) observations. The rats treated with NaF showed the decreased motor coordination, thermal pain response, decreased CAT and SOD activity and increased LPO levels and GSH-Px activity with compared to control group. Moreover, NaF received rats also showed the decreased number of dendrites, synaptic connections and neural networks. These all alterations were reversed on administration of selenium towards fluoride toxicity and the results were significant (p<0.01). The results of selenium alone treated group of rats is comparable to control group. Based on these observed results, the present study evidenced the protective role of selenium against fluoride induced neurotoxicity.
KEYWORDS: Neural Networks; Neurodegeneration; Selenium; Sodium fluoride; Synaptic Connections
Download this article as:| Copy the following to cite this article: Charitha G. S, Sudhakar K, Reddy K. P. Protective Effects of Selenium Against Sodium Fluoride Induced Behavioral, Anti-Oxidant and Neurohistological Alterations in Wistar Rats. Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Charitha G. S, Sudhakar K, Reddy K. P. Protective Effects of Selenium Against Sodium Fluoride Induced Behavioral, Anti-Oxidant and Neurohistological Alterations in Wistar Rats. Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=30294 |
Introduction
Fluoride is a trace element that widely distributed in various natural resources. The primary sources are earth’s crust and untreated water resources. Moreover, it is also present in tooth paste, mouth rinses, tea, processed food, beverages, soft drinks, pesticides, medication for osteoporosis etc. Even though it is beneficial to bone and teeth health in lower concentrations, at high level it is toxic to human and animal health. At higher ppm (> 1 ppm) it primarily causes skeletal and dental fluorosis. Furthermore, the continuous exposure to fluoride leads to damage of soft tissues including brain, heart, liver, kidney etc., and also leads to various cancers and reproductive abnormalities (Bhatnagar et al., 2003; Dhar and Bhatnagar, 2009). It also affects the health of collagen tissue by inhibiting its synthesis and increase its breakdown in bone, muscle, tendon, skin, cartilaginous tissue, lungs and kidney (Gupta et al., 2013). The fluoride in the form of fluorine generates excess amount of free radicals which cause oxidative stress and excito-toxicity. Brian tissue is more vulnerable to free radicals as it consumes more amount of oxygen which in turn produce more ROS which enhance the oxidization of vital cellular components including membrane lipids, proteins and DNA (Bradford and Gupta, 2005; Aliev et al., 2008). ROS produced from fluoride leads to oxidative stress which in turn causes cellular damage by oxidation of membrane lipids, proteins and DNA oxidation (Temple et al., 2005; Valko et al., 2004). The inhibition of some enzymes associated with free radical metabolism affects brain and muscle, energy production and transfer, membrane transport and synaptic transmission (Vani and Reddy, 2000). Fluoride disturbs neurochemical milieu of brain including oxidative stress markers, neurotransmitters and overall microenvironment of brain. The fluoride initiate and increase oxidative stress in cerebral cortex as well as in hippocampus region of brain (Zhang et al., 2007; Zhang et al., 2008). A very recent reports showed the elevated oxidative damage in nuclear and mitochondrial DNA in brains of human subjects with mild cognitive impairments who were exposed to fluoride (Scott and Pandita, 2006; Wang et al., 2006; Zhang et al., 2008). In recent years, many researchers attempted to treat fluorosis with various natural compounds including plant secondary metabolites. For instance, Tamarind fruit pulp (Khandare et al., 2000; Reddy and Reddy, 2015), curcumin (Nabavi et al., 2012), quercetin (Nabavi et al., 2012; Mesram et al., 2017), resveratrol (Reddy and Reddy, 2016), vitamin E (Al-Hayani et al., 2013), Okra seed extract (Sudhakar et al., 2017), vitamin C (Yilmaz and Erkan, 2015) and calcium (Mohamed, 2016). These compounds are rich in polyphenolic profile which help in reduction or elimination of free radicals thus produced at least some positive results against fluoride toxicity.
Selenium (Se) is a nutritionally trace element regarded as an antioxidant in animals and plants (Djanaguiraman et al., 2005). Selenium exerts its biological functions through seleno-proteins, which contain the amino acid seleno-cysteine. Selenium is essential for regulating the activity of the enzymes involved in protection against oxidative stress and this element also contributes in various biological processes such as antioxidant defense, thyroid hormone production, and immune responses (Ognjanovic et al., 2008; Tinggi, 2008). Previous studies reports state that Se protects the cells from harmful effects of high free radicals (El-Boshy et al., 2015). Antioxidant enzymes particularly, glutathione peroxidase which eliminate free radicals contain selenium. Selenium maintains the antioxidant elements like vitamin C and E in the body and decreases the damage caused by free radicals (Kaur and Kaur, 2000). The selenium has strong free radical scavenging activity (Chen et al., 2013; Gunes et al., 2017). Moreover, selenium also inhibits cell apoptotic mechanism, prevent mitochondrial disfunction (Ognjanovic et al., 2012) and protect against lipid peroxidation (Xia et al., 2016; Hasanvand et al., 2017). Some studies demonstrated the efficacy of Se on cognitive deficits and oxidative damage in ICZ-STZ rats (Ishrat et al., 2009). Reddy et al., (2009) reported the protective role of selenium to fluoride induced alterations in certain enzymes in brain tissue of mice. Furthermore, Selenium also prevents apoptosis and cell cycle changes in renal tissue of rats exposed to fluoride (Yu et al., 2013). The restoration of Neurobehavioural activities and histological activities was marked by pre-treatment of Selenium in Cerebral ischemic rats (Yousuf et al., 2007).
In view of this, the present study evaluate the protective role of selenium against sodium fluoride induced behavioral, antioxidants and histological alterations in wistar rats.
Material and Methods
Albino male rats, Wistar strain (around 250-300 g) were purchased from NIN, Hyderabad. They were maintained under standard laboratory conditions like temperature 22-24o C, relative humidity was 40-60%. Light and dark cycles were maintained at 12 h interval (6.00 AM to 6.00 PM). Under these conditions they were housed in polythene cages for one week for acclimatization. After that the healthy rats were randomly grouped into four, five rats for each. The rats were allowed to feed on rat dry pellet diet and tap water ad libitum throughout the experimental period. According to guidelines of CPCSEA (CPCSEA No: 383/01/a/CPCSEA) the rats were maintained and IAEC, Dept. of Zoology permission was taken to conduct experiments on rats. They were exposed to doses as follows:
Group I – control rats (received normal tap water)
Group II – sodium fluoride (NaF) treated rats (20 ppm of NaF was given through IP)
Group III – NaF (20 ppm; through IP) +Selenium (5 mgkg-1 body wt; through orally with plastic gavage)
Group IV – Selenium (5 mgkg-1 body wt; through orally with plastic gavage)
The doses were administered for each animal on every day for a period of 15 days. After the completion of experimental duration rats were used for behavioral tests and the same rats’ brain was used for assess of various oxidative stress markers and histological examinations.
Chemicals
Thiobarbituric acid (TBA), Pyrogallol, Cumene hydroperoxide, NADPH, reduced GSH,Diethylene triamine penta acetic acid (DETPA),EDTA, H2O2, Potassium dichromate, potassium chromate, mercury and others were purchased from local chemical suppliers.
Behavioral Parameters
Rota Rod Test
The rota rod test is commonly used for small laboratory animals like rodents (rats and mice) to assess anxiety, motor coordination and other behavioral responses. Rats try to cope up on rotating rod and balance themselves on it. For assessing rats’ motor ability, they were kept on rotating rod at initial speed is of 4 rpm and observed their retention time on rod, falling frequency off the rod and falling speed are considered. The rota rod instrument (DolphinTM instruments) had four compartments and incremental fixed-speed (about 30rpm) rotating rod. Rats were trained for three consecutive days (10 minutes for each day). Before they exposed to the setup, they were acclimated in test environment for half an hour. Recordings were noted down on fourth day to compare motor coordination among groups (Saunders et al., 2012).
Hot Plate Test
The Eddy’s hot plate test is generally used for small laboratory animals like rats to assess pain response to heat stimulus. This response is because of foot pad contact with heated metal surface which heated with electrical thermode. It was maintained at 52±0.5oC and rat was introduced into hot plate. Due to foot pad contact it was showed various responses like licking of a hind paw or fore paw, face washing, jumping etc. On observing these behaviors either individually or in combination, rat returned to home cage and latency time to exhibiting these behaviors is taken as response time and it was noted in seconds (Gunn et al., 2011).
Oxidative Stress Markers
Lipid Peroxidation (LPO)
According to the method of Bhuyan et al., (1981), lipid peroxidation in brain tissue was assessed. The malondialdehyde (MDA), end product of lipid peroxidation which isreacts with TBA to produce a pink colored condensation. Absorbance was measured at 533 nm on spectrophotometer and its levels were expressed as µmol of TBARS/gr. weight of tissue.
Superoxide dismutase (SOD)
Marklund and Marklund (1974) method was used to estimate the SOD activity in the rat brain tissue. Pyrogallol undergoes auto-oxidation in the presence of EDTA. The OD was recorded at 420nm on spectrophotometer and activity is measured in Units/mg of protein.
Catalase
The rate of decomposition of H2O2 corresponds to the Catalase activity. Catalase degrades H2O2 into H2O andO2 which is the basis of the assay. The activity of Catalase enzyme was measured on spectrophotometer at 260nm. One unit decomposes one micromole of H2O2 per minute (Brannan et al., 1981).
Glutathione Peroxidase (GSH-Px)
GSH-Px was measured using Rotruck et al., (1973) method. GSH-Px reduces Cumene hydroperoxide while oxidizing GSH to GSSG. Glutathione Reductase reduces GSSG to GSH using NADPH as co-factor.The GSSG thus generated is reduced to GSH in the presence of glutathione reductase with consumption of NADPH. The decrease of NADPH corresponds to GSH-Px activity and it was measured on spectrophotometer at 340 nm. The activity of GSH-Px was expressed as μMol of GSH consumed/min/mg protein.
Histological Studies
Golgi cox Stain
Golgi cox staining method is used to study neural connections, circuitry, and neuronal networks. The staining solution was prepared by mixing of equal volumes of 5% potassium chromate, 5% potassium dichromate, 5% mercuric chloride and equal volume of distilled water. The animals were sacrificed and brains were dissected out and preserved in this solution for 10 days in dark room. After that they were transferred to 30% sucrose solution and kept for 6 – 8 hours at 40C. Then the brain was fixed on vibrotome stage with cyanoacrelate glue and made into sections about 10 µ thick. These sections were transferred to water, hypo, water, and ammonium solution andto graded alcohol baths (30%, 50%, 70%, 90% and 100%). The slides were prepared and observed under digital microscope with 4X (Gibb and Kolb, 1998).
Statistical Analysis
Data is subjected to analyses of variance (ANOVA) to assess the effect of Na-F and t-test was applied for all possible combinations of experimental groups for individual comparison. Data presented as mean±SEM and probability of p<0.01 and p<0.001 was considered significant for all evaluations.
Results
Rota Rod Test
The NaF induced rats showed decreased motor coordination with compared to control and NaF+Se showed reversal of motor coordination. Se alone treated rats showed similar results to that control. The percentage of decreased motor coordination when compared to control groups are about 20% in NaF induced rats, 5.5% decrease in NaF+Se treated rats and -5.8% in Se alone treated rats (Fig.1).
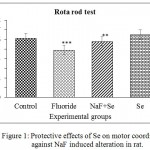 |
Figure 1: Protective effects of Se on motor coordination against NaF induced alteration in rat.
|
Each bar graph representing the mean±SE and sample size is n = 5. Significance of the data is P<0.05 (**) and P<0.001 (***).
Hot Plate Test
The increased latency time in Eddy’s hotplate was observed in NaF treated rats when compared to control rats. It was reverted on exposure to Se towards control. The percent change of sensitivity from control rats was about -32.00% in NaF, -11.53% in NaF+Se, and 1.53% in Se treated rats (Fig. 2)
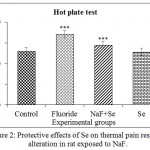 |
Figure 2: Protective effects of Se on thermal pain response alteration in rat exposed to NaF.
|
Each bar graph representing the mean±SE and sample size is n = 5. Significance of the data is P<0.001 (***).
Lipid peroxidation
In the NaF induced rats, the LPO is increased and its levels reverted in NaF+Se treated rats. There is no significant change in the levels of lipid peroxidation in Se treated rats. The percent of difference in LPO levels in NaF received rats from control is by -41.66%, in NaF + Se treated rats is by -8.33% (Fig. 3).
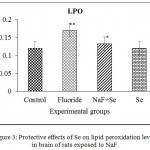 |
Figure 3: Protective effects of Se on lipid peroxidation levels in brain of rats exposed to NaF.
|
Data presented as mean±SE (n = 5). Significance of the data is P<0.01 (*) and P<0.05 (**).
Superoxide Dismutase
In NaF exposed rats, SOD activity is decreased when compared to control rats. Its activity was reverted on administration of Se towards fluoride toxicity and Se received rats displayed the normal SOD activity which is comparable with control rats. The SOD activity was decreased about 30.00% in NaF, 11.53% in NaF+Se and 3.84% in Se treated rats when compared to control group of rats (Fig. 4).
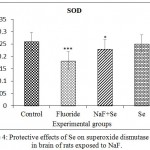 |
Figure 4: Protective effects of Se on superoxide dismutase activity in brain of rats exposed to NaF.
|
Data presented as mean±SE (n = 5). Significance of the data is P<0.01 (*) and P<0.001 (***).
Catalase
The decreased catalase activity was observed in NaF intoxicated rats when compared to control rats and it was decreased about 23.53%. The catalase activity was reverted in NaF+Se treated rats and the percent of change from control is by 11.76%. Se received rats showed the normal catalase activity and there is no significant difference between control and Se treated rats (Fig. 5).
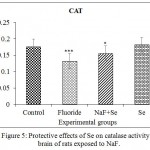 |
Figure 5: Protective effects of Se on catalase activity in brain of rats exposed to NaF.
|
Data presented as mean±SE (n = 5). Significance of the data is P<0.01 (*) and P<0.001 (***).
Glutathione Peroxidase
The increased GSH-Px activity was recorded in NaF received rats when compared to control rats. It was reversed on treatment with Se towards NaF toxicity. Se received rats showed the normal GSH-Px activity. -28.12% of GSH-Px activity was increased in NaF exposed rats with compared to control rats. Furthermore, its activity was increased about -9.37% in NaF+Se treated rats (Fig. 6).
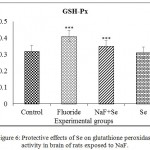 |
Figure 6: Protective effects of Se on glutathione peroxidase activity in brain of rats exposed to NaF.
|
Data presented as mean±SE (n = 5). Significant of the data is P<0.001 (***).
Golgi Cox Staining
The decreased number of dendrites, terminal branches, synaptic connections, axon collaterals and neural networks in NaF received rats was observed when compared to control group of rats. These alterations were reverted on administration of Se towards controls. Free radicals interact with membrane lipids and increase the oxidation process thus LPO levels were increased. Due to membrane destruction the neural cells unable get proper nourishment, maintain normal connections. As a result initiate and increase the apoptotic mechanism and decreased neural connections are resulted in NaF exposed rats (Fig. 7).
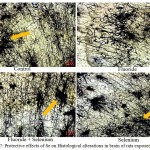 |
Figure 7: Protective effects of Se on Histological alterations in brain of rats exposed to NaF.
|
In control rats, yellow colour arrow mark showing the normal neural cells with connections and networks in comparison with NaF intoxicated rats. Red colour arrow mark depicting the damaged neural cells in terms of connections and networks (Lawrence digital microscope, 10X).
Discussion
The recurrent exposure of fluoride cross the blood brain barrier, generates free radicals in excess amount and lead to the damage of brain tissue. The experimental data represented the Sodium fluoride induced oxidative damage in rat brain leading to biochemical and behavioral alterations and neurodegeneration. The oral administration of Selenium helped in increasing the neuronal connectivity, minimize the oxidative damage and improve motor coordination in the fluoride induced rats. The present study indicates the evidence on minimizing of NaF induced oxidative damage, improving the motor coordination, and alternations in levels of pro and antioxidants by employing Selenium as a protectant.
On the basis of the time of endurance, balance, gripping abilities of rats and frequency of fall off from the rod, the motor performance was assessed (Mullenix et al., 1995). NaF+Se treated rats exhibited enhanced motor abilities than compared to NaF group. Only Se administered rats were observed to have normal forelimb to hind limb coordination and results were equivalent to control rats. This was in accordance with results of Paul et al., (1998). In our earlier report, NaF treated rats showed the decreased motor coordination when compared to control rats and it was reverted on treatment with Abelmoschus moschatus seed extract (Sudhakar et al., 2017). The fluoride treated rats showed decreased sensitivity towards the heat stimulus. This was similar to the studies by Madhavan et al., (2012) and Sudhakar et al., (2017) in which they reported the decreased pain sensitivity particularly to heat stimulus. Fluoride causes increase of the free radicals which oxidizes the membranes of receptors and thus decrease in their number or decrease the sensitivity to stimulus resulted in reduction of pain sensitivity to thermal stimulus. Administration of Se increases the antioxidant enzymes which prevent the production of excess free radicals thus maintain normal status of cell functioning. Hence, the pain sensitivity was reverted on treatment with Se.
Se is reported to maintain the levels of antioxidants and their enzymes near to the normal levels thus preventing the oxidation of the membrane lipids (El-Demerdash, 2004). NaF treated rats shown increased lipid peroxidation and decreased anti-oxidant defense systems thus create an imbalance between oxidant and anti-oxidant status of brain. This is in good accordance with the study of Shivarajashankar et al., (2001). The decreased levels of SOD in fluoride induced rats indicates the abnormal increase in the free radicals (Vani and Reddy, 2000). In agreement with these results, the present study results showed the decreased SOD activity in NaF received rats with compared to control. The activity of GPx was considerably increased in the fluoride induced rats whereas reversal effect of Se showed reduced activity of GPx in the present study. Similar observations are found in the study by Aysel and Kaya (2005). CAT is a heme protein, which protects the tissues from highly reactive hydroxyl radicals by decomposing H2O2 (Chance et al., 1982). The Catalase activity was decreased in fluoride treated rats leading to the build up of H2O2 in the tissues leading to massive oxidative stress. The decreased CAT activity was reported in NaF treated rats with compared to control rats and the similar results were previously reported by Shanthakumari et al., (2004).
Fluoride treated rats showed decrease in the neuronal connections due to decrease in the number of dendrites, terminal branches, axon collaterals and synaptic connections. This leads to the reduction in neuronal networks in the brains of fluoride treated rats. Fluoride induced rats are known to have less efficient neuronal and cerebrovascular integrity, in the hippocampal and cerebral cortex regions of the brain. (Shivarajashankara et al., 2002). Due to oxidation of myelin lipids, myelin sheath was destructed which leads to neurodegeneration. Se has antagonistic effects towards the oxidative stress which maintains the antioxidant status of the brain. Thus Se negates the free radicals and prevents the damage of myelin in the neurons in the brain.
Selenium protect against fluoride toxicity by preventing free radical production. In earlier reports, Flora et al., (2009) demonstrated three possible mechanisms for selenium to eliminate fluoride viz. i) by directly neutralizing free radicals, (ii) by reducing peroxide concentrations and repairing oxidized membranes, and (iii) by quenching iron to decrease ROS production (via lipid metabolism, short-chain free fatty acids and cholesterol esters neutralize ROS).
In summary, fluoride produces high amount of free radicals which interact with membrane lipids and increase their oxidization. Due to increased oxidation LPO levels and GSH-Px activity were increased while decreased SOD and catalase activity were observed and thus overall biochemical milieu was disturbed on NaF exposure. Increased oxidation mechanism initiate the myelin destruction, neural cell death, synaptic connections and neural networks in brain. Selenium is a potential free radical scavenger, eliminates excess free radicals and/or reduce the production of free radicals from fluoride. Thus selenium maintains a balanced oxidant-antioxidant status and it is crucial for normal health of brain. Hence, selenium protects the brain from fluoride toxicity.
Conclusion
Fluoride in the brain produces high levels of free radicals which altered the antioxidant status of brain. Selenium helps in elimination of excess free radicals and thus maintain the healthy neural cells. Hence, the present study conclude that the remarkable reversal effects against fluoride induced damage by maintaining the antioxidant status of the brain.
Acknowledgements
Authors acknowledge to UGC-DSA-II for financial assistance.
Conflict of Interests
There is no conflicts of interest.
References
- Al-Hayani A, Elshal E.B, Aal I.H, Al-Shammer E. Does vitamin E protect against sodium fluoride toxicity on the cerebellar cortex of albino rats?. Middle East J. Sci. Res. 2013;16(7):1019-26.
- Aliev G, Obrenovich M.E, Reddy V.P, Shenk J.C, Moreira P.I, Nunomura A, et al. Antioxidant therapy in Alzheimer’s disease: Theory and practice. Rev. Med. Chem. 2008;8:1395–406.
CrossRef - Aysel G and Kaya N. Effect of fluoride intoxication on lipid peroxidation and reduced glutathione in TUJ sheep. Rep. 2005;38(2):139–42.
- Bhatnagar M, Rao P, Bhatnagar C, Bhatnagar R. Trace element concentration in various tissues following fluoride administration to female mice. Indian J. Exp. Biol. 2003;41:652–4.
- Bhuyan K.C, Bhuyan D.K, Johansen N. Estimation of Malondialdehyde. IRCS Med. Sci. 1981;9:126-7.
- Bradford F and Gupta S. A review of antioxidant and Alzheimer’s disease. Clin. Psychiatry. 2005;37:269–86.
- Brannan T.S, Maker H.O, Raess I.P. Regional distribution of catalase in adult rat brain. Neurochem. 1981;86:307-9.
CrossRef - Chance B, Green Stein D.S, Roughton R.J.W. The mechanism of catalyse action steady state analysis. Biochem. Biophys. 1982;37:301–39.
CrossRef - Chen K, Shu G, Peng X, Fang J, Cui H, Chen J, Wang F, Chen Z, Zuo Z, Deng J, Geng Y, Lai W. Protective role of sodiumselenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B(1). Food Chem. Toxicol. 2013;59:446–54.
CrossRef - Shanthakumari D, Srinivasalu S, Subramanian S. Effect of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. 2004;204:219–28.
- Dhar V.and Bhatnagar M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009;20:350–5.
CrossRef - Djanaguiraman M, Devi D.D, Shanker A.K,Sheeba J.A, Bangarusamy U. Selenium – an anti-oxidative protectant in soybean during senescence. Plant Soil. 2005;272(1-2):77–86.
CrossRef - El-Boshy M.E, Risha E.F, Abdelhamid F.M, Mubarak M.S, Hadda T.B. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepato-renal damage in rats. Trace Elem. Med. Biol. 2015;(29):104-10.
- El-Demerdash,F.M. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. Trace Elem. Med. Biol. 2004;18(1):113-21.
CrossRef - Flora S.J.S, Mittal M, Mishra D. Co-exposure to arsenic and fluoride on oxidative stress, glutathione linked enzymes, biogenic amines and DNA damage in mouse brain. Neurolog. Sci. 2009;285:198–205.
CrossRef - Gibb R and Kolb B. A method for vibratome sectioning of Golgi – cox stained whole rat brain. Neurosci. Meth. 1998;79(1):1-4.
CrossRef - Gunes S, Sahinturk V, Uslu S, Ayhanci A, Kacar S, Uyar R. Protective Effects of Selenium on Cyclophosphamide-Induced Oxidative Stress and Kidney Injury. Biolog Trace Elem. Res. 2017;1-8.
- Gunn A, Bobeck E.N, Weber C, Morgan M.M. The Influence of Non-Nociceptive Factors on Hot Plate Latency in Rats. Pain. 2011;12(2):222–7.
CrossRef - Gupta A.R, Dey S, Swarup D, Saini M. Effects of excessive fluoride ingestion on collagen protein and expression of type I collagen gene in skeletal muscles of rats. Fluoride. 2013;46:149-55.
- Hasanvand A, Abbaszadeh A, Darabi S, Nazari A, Gholami M, Kharazmkia A. Evaluation of selenium on kidney function following ischemic injury in rats; protective effects and antioxidant activity. Renal Inj. Prev. 2017; 6(2):93–8.
CrossRef - Ishrat T, Parveen K, Khan M, Khuwaja G, Khan M.B, Yousuf S, Ahmad A, Shrivastav P, Islam F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin induced experimental dementia of Alzheimer’s type. Brain Res. 2009;1281:117-27.
CrossRef - Kaur R and Kaur K. Effects of dietary selenium on morphology of testis and cauda epididymis in rats. Indian J. Pharmacol. 2000;44(3):265–72.
- Khandare A.L, Kumar P.U, Lakshmaiah N. Beneficial effect of tamarind ingestion on fluoride toxicity in dogs. Fluoride. 2000;33(1):33-8.
- Madhavan, S., Nayak, M., Shenoy, A., Shetty, R., Prasad, K. Dentinal hypersensitivity: A comparative clinical evaluation of CPP-ACP F, sodium fluoride, propolis, and placebo. Conservat. Dentistry.2012; 15(4): 315-8.
- Mesram, N., Nagapuri, K., Banala, R.R., Nalagoni, C.R., Karnati, P.R. Quercetin treatment against NaF induced oxidative stress related neuronal and learning changes in developing rats. King Saud Univ. Sci., 2017; 29: 221-9.
CrossRef - Mohamed, N.E. The Role of Calcium in Ameliorating the Oxidative Stress of Fluoride in Rats. Trace Elem. Res.,2016; 170(1): 128-44.
CrossRef - Mullenix, P.J., Denbesten, P.K., Schunior, A., Kernan, W.J. Neurotoxicity of sodium fluoride in rats. Teratol., 1995; 17: 169.
CrossRef - Marklund, S., and Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. J. Biochem., 1974; 47(3): 469-74.
- Nabavi, S.F., Moghaddam, A.H., Eslami, S., Nabavi, S.M. Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys. Trace Elem. Res. 2012; 145(3): 369-74.
CrossRef - Nabavi, S.F., Nabavi, S.M., Mirzaei, M., Moghaddam, A.H. Protective effect of quercetin against sodium fluoride induced oxidative stress in rat’s heart. Food Funct., 2012; 3(4): 437-41.
CrossRef - Ognjanovic, B., Markovic, S.D., Pavlovic, S.Z., Zikic, R.V., Stajn, A.S., Saicic, Z.S. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats; protective effect of selenium. Res., 2008; 57: 403–11.
- Ognjanovic, B.I., Djordjevic, N.Z., Matic, M.M., Obradovic, J.M., Mladenovic, J.M., Stajn, A.S., Saicic, Z.S. Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys: a possible protective effect of selenium. J. Mol. Sci., 2012; 13(2): 1790–1803.
CrossRef - Reddy, C.H., and Reddy, P.K. Protective effects of resveratrol against neuronal damage through oxidative stress in cerebral hemisphere of aluminum and fluoride treated rats. Toxicol., 2016; 9(2): 101-5.
- Reddy, K.P., Sailaja, G., Krishnaiah, C. Protective effects of selenium on fluoride induced alterations in certain enzymes in brain of mice. Environ Biol., 2009; 30(5): 859-864.
- Reddy, M.M., and Reddy, K.P. Protective effects of aqueous extract of fruit pulp of Tamarindus indica on motor activity and metabolism of the gastrocnemius muscle of rats treated with fluoride. J. Toxicol. Pharmcol. Res., 2015; 7(5): 241-8.
- Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G., Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973; 179(4073): 588-90.
CrossRef - Saunders, N.R., Liddelow, S.A., Dziegielewska, K.M. Barrier mechanisms in the developing brain. Frontiers in Pharmacol., 2012; 3: 46.
CrossRef - Scott, S.P., and Pandita, T.K. The cellular control of DNA double-strand breaks. Cell Biochem., 2006; 99: 1463–75.
CrossRef - Shivarajashankara, Y.M., Shivashankara, A.R., Bhat, P.G., Rao, S.H. Brain lipid peroxidation and the antioxidant systems of young rats in chronic fluoride intoxication. Fluoride. 2002; 35: 197-203.
- Shivarajashankar, Y.M., Shivashankara, A.R., Bhat, P.G., Rao, S.H. Effect of fluoride intoxication on lipid peroxidation and antioxidant systems in rats. 2001; 34: 108-13.
- Sudhakar, K., Nageshwar, M., Reddy, P.K. Seed extract of Abelmoschus moschatus medik reverses NaF-induced behavioral changes through neurodegeneration and oxidative stress in brain of rat. Asian J. Pharm. Clin. Res., 2017; 10(10): 165-71.
CrossRef - Temple, M.D., Perrone, G.G., Dawes, I.W., Complex cellular responses to reactive oxygen species. Trends Cell Biol., 2005; 15: 319–326.
CrossRef - Tinggi, U. Selenium: its role as antioxidant in human health. Health Prev. Med., 2008; 13: 102–8.
CrossRef - Valko, M., Izakovic, M., Mazur, M., Rhodes, C.J., Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Cell. Biochem., 2004; 266: 37–56.
CrossRef - Vani, M.L., and Reddy, K.P. Effects of fluoride accumulation on some enzymes of the brain and on the gastrocnemius muscle of mice. Fluoride. 2000; 33: 17-26.
- Paul, V., Ekambaram, P., Jayakumar, A.R. Effects of sodium fluoride on locomotor behavior and a few biochemical parameters in rats. Environ Toxicol Pharmacol., 1998; 6: 187–91
CrossRef - Wang, J., Markesbery, W.R., Lovell, M.A. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Neurochem., 2006; 96: 825–832.
CrossRef - Xia, L., Yunlong, Z., Yuan, Y., Yong, S., Yan, Q., Zeyuan, D., Hongyan, L. Protective effects of selenium, vitamin E, and purple carrot anthocyanins on d-galactose-induced oxidative damage in blood, liver, heart and kidney rats. Trace Elem. Res., 2016; 173(2): 433–442.
CrossRef - Yilmaza, B.O., and Erkan, M. Effects of vitamin C on sodium fluoride-induced oxidative damage in sertoli cells. Fluoride. 2015; 48(3): 241-251.
- Yousuf, S., Atif, F., Ahmad, M., Hoda, M.N., Khan, M.B., Ishrat, T., Islam, F. Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Res.,2007; 1147: 218-25.
CrossRef - Yu, R., Xia, T., Wang, A.G., Chen, X.M. Effects of Selenium and Zinc on renal oxidative stress and apoptosis induced by fluoride in Rats. Environ. Sci., 2013; 19(6): 439-44.
- Zhang, M., Wang, A., Xia, T., He, P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-κB in primary cultured rat hippocampal neurons. Lett., 2008; 179(1): 1-5.
- Zhang, M., Wang, A.G., He, W.H., He, P., Xu, B.Y., Xia, T., Chen, X.M., Yang, K.D. Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. , 2007; 236: 208–16.

This work is licensed under a Creative Commons Attribution 4.0 International License.






