How to Cite | Publication History | PlumX Article Matrix
Recent Trends in Biomedical Applications of Nanomaterials
Khalid E. Ibrahim1, Amel O. Bakhiet2, Ayaat Khan3 and Haseeb A. Khan4
1Department of Zoology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia.
2Sudan University of Science and Technology, Khartoum, Sudan.
3Integral Institute of Medical Sciences and Research, Lucknow 226026, India.
4Department of Biochemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia.
Corresponding Author E-mail: khan_haseeb@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2627
ABSTRACT: In recent years, there have been enormous developments in utilizing the potential of nanotechnology in different fields including biomedical sciences. The most remarkable biomedical applications of nanoparticles (NPs) are in the diagnosis and treatment of various diseases. Functionalization of NPs renders them unique properties so that they can be used as contrast agent for dual or triple modal imaging. The design and synthesis of new generation NPs aiming at targeted drug delivery has revolutionized the safe and effective therapies for complex and difficult to treat diseases. The theranostic NPs possess the dual capabilities for disease diagnosis and treatment. This review highlights the biomedical applications of NPs based on recent reports published in this area of research.
KEYWORDS: Biomedical Applications; Diagnosis; Imaging; Nanomaterials; Treatment; Theranostic
Download this article as:| Copy the following to cite this article: Ibrahim K. E, Bakhiet A. O, Khan A, Khan H. A. Recent Trends In Biomedical Applications of Nanomaterials. Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Ibrahim K. E, Bakhiet A. O, Khan A, Khan H. A. Recent Trends In Biomedical Applications of Nanomaterials. Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=29787 |
Introduction
There is a growing trend about the design, synthesis and use of engineered nanoparticles (NPs) in different areas including medicine, cosmetics, coating, bioremediation, paints, electronics and food industry (Eswar et al 2014, Kang et al 2015, Khatun et al 2015, Zehedina et al 2015, Nafiujjaman et al 2015, Nurunnabi et al 2015, Nafiujjaman et al 2017). Different types of nanoparticles have been formulated and currently in use for biomedical applications. Nanospheres and nanocapsules are widely used as nanoparticles due to their unique properties such as biocompatibility and bio-mimetic character. Liposomes and micelles are lipid based nanoparticles and are made of an aqueous core and one or more concentric phospholipid bilayers (Masserini 2013). Liposomes are used both as an agent in imaging studies as well as drug delivery system, due to their biocompability, biodegradability, low toxicity and the virtue to capsulate both hydrophilic and lipophilic drugs. Important solid core based NPs include gold, silver and iron NPs. Gold nanoparticles (GNPs) possess important physical properties such as surface plasmon resonance and the ability to quench fluorescence (Yeh et al 2012). Dendrimers are a class of NPs that comprise of radially symmetric molecules with homogenous, three-dimensional globular shape and monodisperse structure with branches of atoms (Abbasi et al 2014). Dendrimers have the ability to penetrate the cell wall easily due to their lipophilicity and three-dimensional structure which make them good drug delivery systems.
Carbon nanotubes (CNTs) are hollow materials that can either be a single layer or multiple layers of graphene sheets, named as single walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs) respectively. These nanomaterials possess intrinsic spectroscopic properties that make them efficient for cell tracking and monitoring of targeted drug delivery (Zhang et al 2011). Quantum dots (QDs) range from 2 to 50 nm in size with quantum-confinement property that after excitation can emit fluorescence from visible to infrared regions (Zhang et al 2008). Small sized carbon-QDs have the properties of high conductivity, chemical stability and strong photoluminescence emission than classical QDs (Namdari et al 2017). Graphene oxide (GO) possesses the unique properties of large surface area, hydrophilicity and dispersibility in aqueous solutions and therefore is commonly used for biomedical applications (Sun et al 2014). Nanoparticles possess unique properties that are being explored for their biomedical, engineering and industrial applications. The important biomedical applications of NPs are in imaging, drug delivery, theranostic, and biosensors.
Nanoparticles for Imaging
Nanoparticles are being researched as potential contrast agents in computed tomography (CT) (Cormode et al 2014), optical imaging (Kim et al 2004), photoacoustic imaging (Cai et al 2011), magnetic resonance imaging (MRI) and ultrasonography (Wilson and Burns 2010). Gold nanoparticles (GNPs) have unique X-rays attenuation properties which, combined with their easy surface functionalization, makes them ideal candidates for contrast agents. Surface coating of polyethylene glycol (PEG) on GNPs increases their blood retention. Conjugated with a cancer-specific antibody, PEGylated GNPs have been used for CT imaging in tumor-bearing mice (Nakagawa et al 2016). For CT imaging of a tumor model, small sized polyamidoamine-entrapped GNPs were more easily internalized via endocytosis in the liver, leading to more obvious enhanced contrast (Wang et al 2016). Aziz et al (2017) observed that porous GNPs exhibited higher contrast than solid GNPs for direct CT scanning. For accurate localization and evaluation of atherosclerotic plaques via dual-modal imaging, GNPs were caped with a thin amino-PEGs cover and conjugated with Annexin V and radionuclide Tc-99m simultaneously to form single photon emission imaging probe targeting apoptotic macrophages (Li et al 2016).
Zhao et al (2016) coated SWCNTs with a self-polymerized polydopamine (PDA) shell, which made the nanomaterial highly water soluble and also provided additional features such as chelation with Mn2+ to offer enhanced T1 and T2 magnetic resonance imaging (MRI) contrast. Wang et al (2017a) reported that sub-5 nm iron oxide nanoparticles can easily extravasate from the tumor vasculature and readily diffuse into the tumor tissue compared to larger sized nanoparticles. In-vivo MRI showed that iron oxide NPs exhibited bright T1 contrast when dispersed in the tumor vasculature and peripheral area at 1 h after intravenous administration, followed by emerging dark T2 contrast in the tumor after 24 h. Magneto-plasmonic NPs comprised of magnetic core and gold shell exhibited superparamagnetic and magnetic properties that contributed to the concentration-dependent contrast in MRI. The transmigration study of the magneto-plasmonic NPs using a blood brain barrier (BBB) model proved enhanced transmigration efficiency without disrupting the integrity of the BBB, and showed potential to be used for imaging in brain diseases and neurological disorders (Tomitaka et al 2017). Sun et al (2016) have developed a polymer coated and fluorine-18 labeled of iron oxide NPs for positron emission tomography (PET)/magnetic resonance (MR) dual-modality imaging. Xu et al (2017a) have synthesized a tri-modal imaging agent, composed of gold cluster and gadolinium oxide integrated nanoparticles (AuGds) that exhibit red fluorescence at 660 nm for optical imaging, strong X-ray absorption for CT imaging, and a high r1 value for MR imaging.
Choi et al (2017) developed PEGylated lipid NPs loaded with IR780 iodide as a contrast agent for near infrared (NIR) fluorescence imaging and modified them with RGD (Arg-Gly-Asp) peptide to target cancer specific integrin avβ3. Park et al (2009) designed luminescent porous silicon nanoparticles (LPSiNPs) that are able to self-destruct and excreted in a mouse model with no evidence of toxicity. These larger (100 nm-scale) silicon-based biodegradable nanoparticles overcome many of the disadvantages of smaller (<5.5 nm) nanocrystals (Choi et al 2007). Dextran-coated LPSiNPs (D-LPSiNPs), with a low-toxicity degradation pathway, were successfully applied for tumor imaging in a live mouse model. Injection of the D-LPSiNP formulation (20 mg/kg) into a tumor bearing nude mouse resulted in passive accumulation of the nanomaterial in the tumor, using the near-infrared fluorescence imaging (Fig. 1) (Park et al 2009).
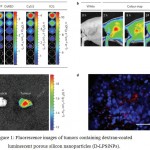 |
Figure 1: Fluorescence images of tumors containing dextran-coated luminescent porous silicon nanoparticles (D-LPSiNPs).
|
(A) Fluorescence images of D-LPSiNPs as a function of concentration using different excitation filters. (b) Representative fluorescence images of a tumor bearing mouse. (c) Ex vivo fluorescence images of tumor and muscle around the tumor from the mouse. (d) Fluorescence images of a tumor slice from the mouse. Red and blue colors indicate D-LPSiNPs and cell nuclei, respectively. The scale bar is 100 µm. Reprinted from Park et al (2009) with permission from Nature Publishing Group.
Nanoparticles for Drug Delivery and Therapy
Liposomes were the first generation of NPs used in drug delivery systems followed by emergence of polymeric NPs that are loaded with compounds to mask the surface to pervading immune cells to prevent immune recognition and consequent elimination. For targeting cancer cells, NPs are engineered to target specific receptors on the cancer cells by attaching complementary ligands on the surface of NPs. Some important receptors that are commonly targeted are transferrin receptor (Daniels et al 2012), folate or folic acid (FA) receptor (Sudimack and Lee 2000) and luteinizing hormone-releasing hormone receptor (LHRH) (Dharap et al 2005). Ferritin NPs loaded with neural drug regulated the microenvironment in pancreatic cancer by specifically targeting the tumor cells via transferrin receptor (Lei et al 2016). Folate-targeted H1 nanopolymer inhibited cell growth, increased apoptosis and enhanced cell cycle arrest in vitro and suppressed tumor growth and increased survival rate of hormone-independent prostate cancer in animal models (Zhang et al 2017). By employing the tumor homing property of cetuximab (EGFR inhibitor) and the drug-loading capability of silica NPs, a targeted drug delivery system (CET-SLN-DOX) co-delivered two drugs with superior tumor-targeting and anti-cancer efficiencies (Wang et al 2017b). Yang et al (2016) demonstrated efficient targeting of breast cancer metastasis in an experimental murine model with nano-GO, which was conjugated to a monoclonal antibody against follicle-stimulating hormone receptor (FSHR), which is a highly selective tumor vasculature marker abundant in both primary and metastatic tumors.
The natural linear polysaccharide, hyaluronic acid (HA), has inherent ability to target the CD44 receptors and internalize into tumor cells via receptor-mediated endocytosis. Sargazi et al (2017) synthesized mitoxantrone (MTX)-conjugated polymeric NPs composed of PEG-HA for MTX delivery toward special tumor cells. Ahmad et al (2017) compared the cellular uptake and bioavailability of anticancer drug docetaxel (DTX) in DTX-loaded chitosan-coated PLGA NPs (DTX-CS-PLGA-NPs) with DTX-PLGA-NPs as well as DTX suspension, in an in-vitro model of intestinal cancer. The pharmacokinetics showed higher oral drug bioavailability from DTX-CS-PLGA-NPs (about 5-folds) and DTX-PLGA-NPs (about 3-folds) as compared with DTX-suspension.
Although NPs are being largely tested for targeted drug delivery in cancer however they may also offer applications in many other disorders such as neurodegenerative, inflammatory and ocular diseases. Functionalized SWCNT restored normal autophagy by reversing abnormal activation of mTOR signaling and deficits in lysosomal proteolysis, thereby facilitating elimination of autophagic substrates in primary glial cells, suggesting SWCNT as a novel neuroprotective approach to Alzheimer’s disease (Xue et al 2014). Intracerebroventricular injection of microRNA (miR-124) NPs increased the number of new neurons in the olfactory bulb and improved the motor function in 6-hydroxidopamine lesioned mice, a model for Parkinson’s disease (Saraiva et al 2016). McMasters et al (2017) reported a hollow NP thermosensitive system as an excellent platform for the delivery of peptide therapeutics into highly proteolytic environments such as osteoarthritis.
The intraocular pressure was attenuated when the drug-nanoparticle complex was administered topically and released in a sustainable rate (Yang and Leffler 2013; Mishra and Jain 2014). Dendrimers have been studied as feasible hydrogel adhesives for cornea wound repair (Oelker et al 2011). Polymethacrylate NPs bound to heparin significantly improved the anti-inflammatory efficiency of the drug in ulcerative colitis model, due to its protection against degradation in luminal environment and selective drug delivery (Yazeji et al 2017). To overcome multiple barriers for oral delivery of insulin, the chitosan-based multifunctional nanocarriers modified by L-valine and phenylboronic acid have shown effective hypoglycemic effect compared with subcutaneous injection of insulin in diabetic rats (Li et al 2017b). Design and synthesis of novel NPs have also extended their application in gene therapy by shielding the genetic material until delivered to the target site (Dorraj et al 2017; Cha et al 2017).
Nanoparticles for Theranostic Applications
Nanoparticles can also be designed with dual capabilities of both diagnostic and therapeutic applications; this combined property is called ‘theranostic’. Nanotechnology has enabled the design and synthesis of smart theranostic NPs that can simultaneously diagnose disease, start treatment, and monitor response (Sneider et al 2017). The advancements in remotely triggered nanotheranostics, using different modes of activators such as photodynamic, photothermal, phototriggered chemotherapeutic release, ultrasound, electrothermal, magnetothermal, X-ray, and radiofrequency has significantly overcome the challenges for successful clinical implementation of this technology (Sneider et al 2017).
Despite the fact that first-generation superparamagnetic iron oxide (SPIO) NPs only had diagnostic capabilities, the new generation SPIO NPs have theranostic applications in image-guided cancer therapies. Polymer-coated theranostic SPIO NPs possess good biocompatibility, biodegradability and versatile functionality rendered by polymeric matrices (Li et al 2017a). Chen et al (2017) developed a dual-modality MRI and near-infrared fluorescence (NIRF) probe comprised of SPIO NPs coated with liposomes to which a tumor-targeted agent (RGD peptide), and a NIRF dye (indocyanine green) were conjugated. This novel dual modality probe showed promising results for accurate tumor detection and resection in a mouse model (Fig. 2).
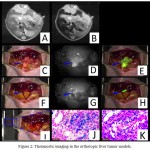 |
Figure 2: Theranostic imaging in the orthotopic liver tumor models.
|
(A) The MRI image before SPIO@Liposome-ICG-RGDs injection. (B)The MRI signal is obviously decreased in normal liver tissue after targeted probe injection. (C) Surgical guidance by intraoperative FMI-NIR (fluorescence molecular imaging system). (D) The implanted liver tumor tissue (blue arrow) exhibits obvious contrast in color and texture with normal liver tissues. (E) The merge image of color and fluorescence demonstrates the excellent contrast. (F) The residual tumor node (blue arrow) after the first operation. (G) The residual tumor node exhibits obvious contrast in color and texture with normal liver tissues. (H) The merged color and fluorescence image demonstrates the excellent contrast in the residual tumor node. (I) Identification of the residual tumor after the initial resection. (J) Prussian blue staining confirmation of the targeting ability of SPIO@Liposome-ICG-RGDs. (K) H&E staining confirmation of the liver tumor tissue. Reprinted from Chen et al (2017) under Creative Commons Attribution License.
Wang et al (2017c) constructed poly(lactic-co-glycolic acid) (PLGA) NPs and loaded them with gold nano-rods (AuNRs) and docetaxel (DTX) and finally coated with ultrathin nanofilms of manganese dioxide (MnO2), offering a unique potential for MR imaging, chemotherapy and RF hyperthermia. Hong et al (2017) reported indocyanine green (ICG)-loaded hollow mesoporous silica NPs that behaved nonfluorescent and nonphototoxic extracellularly but after entering the cancer cells, they become highly fluorescent and phototoxic resulting in an enhanced phototherapy of cancer. Kang et al (2017b) developed a nanocarrier, carbon dot (CD) created mesoporous hollow organosilica (C-hMOS) NPs, to deliver anticancer drug and to perform optical imaging. The DOX-caryying C-hMOS efficiently targeted the cancer cells and induced cellular apoptosis in addition to multi-color visualization. The incorporation of CDs with liposome opens up their application in fluorescence cell imaging studies, which is very well supported with fluorescence microscopic analysis of the liposome skin penetration. These nano-liposomes do not show any cytotoxicity for MCF-7 cells; however, when loaded with drug, they are able to destroy the cancer cells with a high rate (Patra et al 2016). Chen et al (2015) developed 131I- labeled PEG-coated reduced nano-graphene oxide (131I-RGO-PEG) for nuclear imaging guided combined radiotherapy and photothermal therapy that effectively reduced tumors in an animal model.
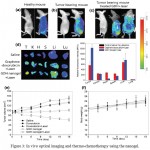 |
Figure 3: In vivo optical imaging and thermo-chemotherapy using the nanogel.
|
(A) Optical imaging of healthy mice. (b) Optical imaging of tumor bearing mice. (c) Light responsive imaging in mice treated with nanogels and laser. (d) Ex vivo imaging and fluorescence intensities of tumors and normal tissues. Organs were arranged in the following order: tumor (T), kidney (K), heart (H), spleen (S), liver (Li), and lung (Lu). (e) Thermo-chemotherapy after treating doxorubicin and the nanogels with and without laser irradiation. (f) Body weight changes of mice after treatment with the nanogels. Reprinted from Khatun et al (2015) with permission from The Royal Society of Chemistry.
Khatun et al (2015) synthesized a nanogel using the combination of light-responsive graphene, chemo-agent doxorubicin and pH-sensitive disulfide-bond linked HA that exerts an activity with multiple effects: thermo and chemotherapeutic, real-time noninvasive imaging, and light-glutathione-responsive controlled drug release. After injection in tumor bearing mice, there was a significant increase in fluorescence intensity in the tumors after laser irradiation and the tumors showed rapid regression and their size was reduced about 30-fold as compared to controls (Fig. 3). Kang et al (2017a) developed a theranostic system, fibrin-targeted imaging and antithrombotic nanomedicine (FTIAN), for imaging of obstructed vessels and inhibition of thrombus formation. In a mouse model of carotid thrombosis, FTIAN specifically targeted the obstructive thrombus and significantly enhanced the fluorescence/photoacoustic signal. FTIAN also remarkably suppressed the thrombus formation when loaded with antiplatelet drug tirofiban, suggesting its translational potential for the diagnosis and treatment of obstructive thrombosis (Kang et al 2017a).
Conclusion
Recent researches have shown the enormous potentials of various NPs in the diagnosis and treatment of human diseases. Specific functionalization of NPs render them excellent properties for their applications in contrast imaging, cellular tracking, and image-guided interventions. The new generation nanomaterials have the potential of dual and triple mode imaging for the diagnosis of various diseases including cancer, cardiovascular and brain disorders. Targeted drug delivery is yet another important biomedical application of NPs. By encapsulating drugs inside a nanocarrier, the solubility and stability of drugs can be improved in terms of favorable pharmacokinetics and minimal toxicity. The emergence of theranostic NPs offers an opportunity of using a single agent for disease diagnosis as well as therapy.
References
- Abbasi E, Aval S.F, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscal. Res. Lett. 2014;9:247.
- Ahmad N, Alam M.A, Ahmad R, Naqvi A.A, Ahmad F.J. Preparation and characterization of surface-modified PLGA-polymeric nanoparticles used to target treatment of intestinal cancer. Artif. Cell. Nanomed. Biotechnol. 2017;1-15.
- Aziz F, Ihsan A, Nazir A, et al. Novel route synthesis of porous and solid gold nanoparticles for investigating their comparative performance as contrast agent in computed tomography scan and effect on liver and kidney function. Int. J. Nanomed. 2017;12:1555-63.
- Cai X, Li W, Kim C.H, Yuan Y, Wang L.V, Xia Y. In vivo quantitative evaluation of the transport kinetics of gold nanocages in a lymphatic system by noninvasive photoacoustic tomography. ACS Nano. 2011;5:9658-67.
- Cha W, Fan R, Miao Y, Zhou Y, Qin C, Shan X, Wan X, Li J. Mesoporous silica nanoparticles as carriers for intracellular delivery of nucleic acids and subsequent therapeutic applications. Molecules. 2017;22(5). pii: E782. doi: 10.3390/molecules22050782.
- Chen L, Zhong X, Yi X, Huang M, Nin P, Liu T, Ge C, Chai Z, Liu Z, Yang K. Radionuclide 131I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials. 2015;66:21-8.
- Chen Q, Shang W, Zeng C, et al. Theranostic imaging of liver cancer using targeted optical/MRI dual-modal probes. Oncotarget. 2017;8:32741-51.
- Choi H.S, Liu W, Misra P, Tanaka E, Zimmer J.P, Itty Ipe B, Bawendi M.G, Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165-70.
- Choi J, Rustique E, Henry M, et al. Targeting tumors with cyclic RGD-conjugated lipid nanoparticles loaded with an IR780 NIR dye: In vitro and in vivo evaluation. Int. J. Pharm. 2017. pii: S0378-5173(17):30175-8.
- Cormode D.P, Naha P.C, Fayad Z.A. Nanoparticle contrast agents for computed tomography: a focus on micelles. Contr. Media. Mol. Imag. 2014;9:37-52.
- Daniels T.R, Bernabeu E, Rodríguez J.A, et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta. 2012;1820:291-317.
- Dharap S.S, Wang Y, Chandna P, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Natl. Acad. Sci. USA. 2005;102:12962-7.
- Dorraj G, José Carreras J, Núñez H, Abushammala I, Melero A. Lipid nanoparticles as potential gene therapeutic delivery systems for oral administration. Curr. Gene. Ther. 2017. doi: 10.2174/1566523217666170510163038.
- Eswar K.A, Rouhi J, Husairi F.S, Dalvand R, Alrokayan S.A, Khan H.A, Mahmood R, Abdullah S. Hydrothermal growth of flower-like ZnO nanostructures on porous silicon substrate. J. Mol. Struct. 2014; 1074:140–3.
- Hong S.H, Kim H, Choi Y. Indocyanine green-loaded hollow mesoporous silica nanoparticles as an activatable theranostic agent. Nanotechnology. 2017;28:185102.
- Kang S.H, Nafiujjaman M, Nurunnabi M, Li L, Khan H.A, Cho K.J, Huh K.M, Lee Y. Hybrid photoactive nanomaterial composed of gold nanoparticles, pheophorbide-A and hyaluronic acid as a targeted bimodal phototherapy. Macromol. Res. 2015;23:474–84.
- Kang C, Gwon S, Song C, Kang P.M, Park S.C, Jeon J, Hwang D.W, Lee D. Fibrin-targeted and H2O2-responsive nanoparticles as a theranostics for thrombosed vessels. ACS Nano. 2017a. doi: 10.1021/acsnano.7b02308.
- Kang M.S, Singh R.K, Kim T.H, Kim J.H, Patel K.D, Kim H.W. Optical imaging and anticancer chemotherapy through carbon dot created hollow mesoporous silica nanoparticles. Acta. Biomater. 2017b. pii: S1742-7061(17):30225-8.
- Khatun Z, Nurunnabi M, Nafiujjaman M, Reeck G.R, Khan H.A, Cho K.J, Lee Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7:10680-9.
- Kim S, Lim Y.T, Soltesz E.G, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004;22:93-7.
- Lei Y, Hamada Y, Li J, Cong L, Wang N, Li Y, Zheng W, Jiang X. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J. Control. Release. 2016;232:131-42.
- Li K, Nejadnik H, Daldrup-Link H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017a. pii: S1359-6446(17):30183-6.
- Li L, Jiang G, Yu W, et al. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017b;70:278-86.
- Li X, Wang C, Tan H, et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials. 2016;108:71-80.
- Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238-428.
- McMasters J, Poh S, Lin J.B, Panitch A. Delivery of anti-inflammatory peptides from hollow PEGylated poly(NIPAM) nanoparticles reduces inflammation in an ex vivo osteoarthritis model. J. Control. Release. 2017.pii: S0168-3659(16):31138-5.
- Mishra V, Jain N.K. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharm. 2014;461:380-90.
- Nafiujjaman M, Khan H.A, Lee Y.K. Peptide-influenced graphene quantum dots on iron oxide nanoparticles for dual imaging of lung cancer cells. J. Nanosci. Nanotech. 2017;17:1704-11.
- Nafiujjaman M, Nurunnabi M, Kang S.H, Reeck G, Khan H.A, Lee Y.K. Ternary graphene quantum dot-polydopamine-Mn3O4 nanoparticles for optical imaging guided photodynamic therapy and T1-weighted magnetic resonance imaging. J. Mater. Chem. B. 2015;3:5815–23.
- Nakagawa T, Gonda K, Kamei T, et al. X-ray computed tomography imaging of a tumor with high sensitivity using gold nanoparticles conjugated to a cancer-specific antibody via polyethylene glycol chains on their surface. Sci. Technol. Adv. Mater. 2016;17:87-97.
- Namdari P, Negahdari B, Ali Eatemadi A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017;87:209-22.
- Nurunnabi M, Parvez K, Nafiujjaman M, Revuri V, Khan H.A, Feng X, Lee Y. Bioapplication of graphene oxide derivatives: drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015;5:42141–61.
- Oelker A.M, Berlin J.A, Wathier M, Grinstaff M.W. Synthesis and characterization of dendron cross-linked PEG hydrogels as corneal adhesives. Biomacromolecules. 2011;12:1658-65.
- Park J.H, Gu L, von Maltzahn G, Ruoslahti E, Bhatia S.N, Sailor M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Na.t Mater. 2009;8:331-6.
- Patra S, Roy E, Madhuri R, Sharma P.K. The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater. Sci. 2016;4:418-29.
- Saraiva C, Ferreira L, Bernardino L. Traceable microRNA-124 loaded nanoparticles as a new promising therapeutic tool for Parkinson’s disease. Neurogenesis (Austin). 2016;3:e1256855.
- Sargazi A, Kamali N, Shiri F, Heidari Majd M. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cell. Nanomed. Biotechnol. 2017:1-10. doi: 10.1080/21691401.2017.1324462.
- Sneider A, VanDyke D, Paliwal S, Rai P. Remotely triggered nano-theranostics for cancer applications. Nanotheranostics. 2017 1:1-22.
- Sudimack J, Lee R.J. Targeted drug delivery via the folate receptor. Adv. Drug. Deliv. Rev. 2000;41:147-62.
- Sun J, Chao J, Huang J, Yin M, Zhang H, Peng C, Zhong Z, Chen N. Uniform small graphene oxide as an efficient cellular nanocarrier for immunostimulatory CpG oligonucleotides. ACS Appl. Mater. Interfaces. 2014; 6:7926-32.
- Sun Z, Cheng K, Wu F., et al. Robust surface coating for a fast, facile fluorine-18 labeling of iron oxide nanoparticles for PET/MR dual-modality imaging. Nanoscale. 2016;8:19644-53.
- Tomitaka A, Arami H, Raymond A., et al. Development of magneto-plasmonic nanoparticles for multimodal image-guided therapy to the brain. Nanoscale. 2017;9:764-73.
- Wang J.K, Zhou Y.Y, Guo S.J, et al. Cetuximab conjugated and doxorubicin loaded silica nanoparticles for tumor-targeting and tumor microenvironment responsive binary drug delivery of liver cancer therapy. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017b;76:944-50.
- Wang L, Huang J, Chen. H, et al. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1-T2 switchable magnetic resonance imaging contrast. ACS Nano. 2017a. doi: 10.1021/acsnano.7b00038.
- Wang W, Li J, Liu R, Zhang A, Yuan Z. Size effect of Au/PAMAM contrast agent on CT imaging of reticuloendothelial system and tumor tissue. Nanoscale. Res. Lett. 2016;11:429.
- Wang Z, Qiao R, Tang N, et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials. 2017c;127:25-35.
- Wilson S.R, Burns P.N. Microbubble-enhanced US in body imaging: what role?. Radiology. 2010;257:24-39.
- Xu C, Wang Y, Zhang C, Ji Y, Luo Y, Gao X. AuGd integrated nanoprobes for optical/MRI/CT triple-modal in vivo tumor imaging. Nanoscale. 2017a;9:4620-8.
- Xue X, Wang L.R, Sato Y, Jiang Y, Berg M, Yang D.S, Nixon R.A, Liang X.J. Single-walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer’s disease. Nano. Lett. 2014;14:5110-7.
- Yang D, Feng L, Dougherty C.A., et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials. 2016;104:361-71.
- Yang H, Leffler C.T. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: an advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J. Ocul. Pharmacol. Ther. 2013;29:166-72.
- Yeh Y.C, Creran B, Rotello V.M. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871-80.
- Yazeji T, Moulari B, Beduneau A, Stein V, Dietrich D, Pellequer Y, Lamprecht A. Nanoparticle-based delivery enhances anti-inflammatory effect of low molecular weight heparin in experimental ulcerative colitis. Drug. Deliv. 2017;24:811-17.
- Zehedina K, Nurunnabi M, Nafiujjaman M, Reeck G, Khan H.A, Cho K.J, Lee Y. A hyaluronic acid nanogel for photo-chemo theranostic of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7:10680–9.
- Zhang H, Yee D, Wang C. Quantum dots for cancer diagnosis and therapy: biological and clinical perspectives. Nanomedicine (Lond). 2008;3:83-91.
- Zhang W, Zhang Z, Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale. Res. Lett. 2011;6:555.
- Zhang X, Liu N, Shao Z, Qiu H, Yao H, Ji J, Wang J, Lu W, Chen R.C, Zhang L. Folate-targeted nanoparticle delivery of androgen receptor shRNA enhances the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomedicine. 2017;13:1309-21.
- Zhao H, Chao Y, Liu, J, Huang J, Pan J, Guo W, Wu J, Sheng M, Yang K, Wang J, Liu Z, 2016. Polydopamine coated single-walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics. 2016;6:1833-43.

This work is licensed under a Creative Commons Attribution 4.0 International License.





