How to Cite | Publication History | PlumX Article Matrix
Study of Y-Chromosome Microdeletions in Azoospermic Infertile Males using Multiplex PCR Analysis
Prafulla S. Ambulkar and Sunil S. Pande
and Sunil S. Pande
Rajiv Gandhi Biotechnology Centre, LIT premises, RTMNU University, Nagpur-33 MS, India.
Crossponding Author E-mail: ambulkarsp@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2639
ABSTRACT: The infertility affects about 15% of couples and male factors being responsible about 40-50%. In male infertility, genetic abnormalities of Y chromosome play crucial role in spermatogenesis defect. Y chromosome q arm having Azoospermia factor region (AZFa, AZFb, and AZFc) are most important for spermatogenesis. Here, we investigated the frequencies of Y-chromosome microdeletions using three sets of multiplex PCR in idiopathic cases of azoospermia. We studied a total of 110 infertile male with non-obstructive azoospermia subjects & 50 fertile control subjects. All DNA samples were used for Y chromosome microdeletions analysis by using 11 STS markers in three different multiplex PCR of AZF regions. Out of 110 infertile azoospermic males, 14 (12.72%) infertile males showed partial deletion of AZF regions using three sets of multiplex PCR group. In the AZF microdeletions of infertile males, individually AZFc region was the most deletions sites (10%) followed by AZFb (6.36%) and AZFa (1.81%). The sites and sizes of microdeletions differ in all infertile azoospermic males who showed at least two or more STS markers microdeletions. The frequency of Y chromosome microdeletions in our azoospermic infertile males is 12.72%. We conclude that Y chromosome microdeletions frequency in azoospermic infertile males is higher than other infertile group due to severe impairment in spermatogenesis. Multiplex PCR screening of microdeletions is very useful and time saving technique when used more number of STS markers. It will be great help to infertility clinics for genetic counseling and assisted reproduction.
KEYWORDS: AZF Factor Deletions; Azoospermia; Infertility; Microdeletions Y-chromosome
Download this article as:| Copy the following to cite this article: Ambulkar P. S, Pande S. S. Study of Y-Chromosome Microdeletions in Azoospermic Infertile Males using Multiplex PCR Analysis. Biosci Biotech Res Asia 2018;15(2). |
| Copy the following to cite this URL: Ambulkar P. S, Pande S. S. Study of Y-Chromosome Microdeletions in Azoospermic Infertile Males using Multiplex PCR Analysis. Biosci Biotech Res Asia 2018;15(2). Available from: https://www.biotech-asia.org/?p=30167 |
Introduction
The infertility affects around 10-15% of married couples who trying to achieve pregnancy and nearly 40-50% of these are accountable to the male partner (Boivin, et al, 2007). The male Infertility is an important reproductive health problem with multi-factorial genetic and non-genetic etiology. Various patho-physiological factors have been identified for male infertility but still more than 60% of cases of infertility the origin of altered testicular function is unknown (Krausz, et al, 2006). A genetic factor plays an important role in spermatogenic failure in infertile males. Spermatogenesis is a highly complex process of germ cell development that regulates more than 2000 genes (Ferlin, et al, 2007a). The percentage of chromosomal abnormalities in the azoospermic infertile male population is around 15-20% (Krausz, et al, 2006). However, in last two decades several researchers have been published the relationship of Y chromosome microdeletions and idiopathic male infertility. The Y chromosome contains three functional AZF regions which involved in the active spermatogenesis. These three AZF regions spanned over the interval 5 and 6 of long arm of Y chromosome. The molecular technique has been used for analysis of microdeletions of Yq spermatogenesis controlling region commonly deleted in azoospermia and oligozoospermia infertile males (Vogt, et al, 1996).
The male sex-determining region (SRY) has been mapped on the short arm of Y chromosome at Yp11. Initial study of the Y chromosome long arm deletions in azoospermic infertile men were carried out through cytogenetic analysis but most of deletions on the Y chromosome are too minute to be detected by standard cytogenetic banding method. However, only large structural rearrangements can observe mainly more than 2 Mb or whole long Yq11 region deletions in infertile males (Tiepolo, et al, 1976). In India, Y chromosome microdeletions testing in diverse classes of infertile men has been reported by different groups and a very wide variation (0–28 %) in the prevalence of Yq microdeletions have been observed (Sen, et al, 2013). In addition, it is important to note that infertile men carrying Yq microdeletions always pass on the defect to their male offspring born after Intra-cytoplasmic Sperm Injections (ICSI) thereby continuing infertility in the offspring of infertile couple (Silber, et al, 2011). The molecular screening through PCR technique has made it easy to identified microdeletions on the Y chromosome for proper genetic counseling of infertile males (Ferlin, et al, 2007b). In the previous studies large number of STS markers have been used for detection of Y chromosome microdeletions among infertile males. The frequency of microdeletions has been found around 0-60% in different categories of infertile males. The wide frequency of microdeletions may be due to different number of STS markers for screening of microdeletions and selection criteria of infertile males on the basis of semen profiles (Kuchukaslan, et al, 2013). In the present study we investigated the Y chromosome microdeletions in non-obstructive azoospermic infertile male population using three multiplex PCR sets of 11 STS markers.
Material and Methods
In the present study 110 azoospermic infertile individuals were studied for Y-chromosome microdeletions, belonging to the age group of 24-41 years (30.24 + 4.61) as cases and 50 ages matched fertile men as controls (WHO, 2010). Chromosomal Analysis was done on phytohemagglutinin-M stimulated whole peripheral blood culture following standard protocol of our lab. All infertile subjects with a normal 46,XY karyotype as shown by GTG banding were included in present study. EDTA containing whole blood was used for genomic DNA extraction process following proteniase-k, phenol chloroform extraction method (Ambulkar, et al, 2014). The purified DNA samples were dissolved in TE buffer for multiplex PCR reactions.
Multiplex PCR used for Defining Microdeletions with Three sets of STS Markers
Total genomic DNAs of 110 non-obstructive idiopathic azoospermic infertile men and 50 normal healthy proven fertile subjects as positive controls and 5 normal female as negative controls were tested using for 14 STS markers of AZF loci which mapped to long arm of the Y chromosome (Yq) particularly interval 5 and 6 spanning over the azoospermia factor (AZF) regions. The STS primers used were 2 primers: sY86, sY84 for AZFa; 4 primers: sY113, sY118, sY127, sY134, for AZFb and 4 primers: sY153, sY255, sY254, sY157 for AZFc (Ambulkar, et al, 2014; Simoni, et al, 2004). All STSs were used in three sets of multiplex PCR reactions (Table 1). The internal control was STS primer sY14 for sex determining region of the Y (SRY). 300 ng DNA samples were subjected to PCR amplification reactions with 1 X PCR assay buffer, 1.5 mmol/l MgCl2, 200 µmol/l deoxy-nucleotidetriphosphate (dNTPs), 1µmol/µl of each primer pair and 0.5 U Taq-DNA polymerase (Merck Biosciences, Bangalore, India) and 1% (DMSO) Dimethyl sulphoxide. The reactions were performed in a Veriti 96 well thermal cycler (Applied Biosystem, Foster city, CA, USA) for 30 cycles using standard protocol. Initial denaturation was done at 94oC for 5 min. & final extension at 72oC for 7 minutes in the last cycle. The subsequent denaturations were set at 94oC for 60 second, annealing temperature at 60oC for 60 second and extension at 72oC for 60 second and same for all the samples. Different annealing temperatures that were used (58oC -60oC for 30 second- 50 second) depending on STS markers sets. PCR product was analyzed on a 2 % agarose gel electrophoresis containing ethidium bromide (0.5 microgram/ml). In the event of detecting a microdeletion of a Y-specific sequence tagged sites (STSs) with a primer, the PCR assay was repeated thrice for confirmation.
Multiplex PCR Sets and AZF Regions with PCR Product Size
The three different multiplex PCR sets were prepared for screening of Y-chromosome microdeletions; each set contains three STS markers. For every reaction, same amplification condition was used (Bor, et al, 2001). Out of eleven STS markers, 10 STS markers span over the euchromatic region of Y-chromosome covered AZFa, AZFb, AZFc regions to be associated with spermatogenesis defects and one STS marker specified sex determination (SRY) gene. Each STS marker specified AZF region and PCR products size were mentioned in (Table 1). Every sets of multiplex PCR was designed like that distance of amplified PCR products at least 25 bp nucleotide.
Table 1: Three multiplex PCR markers sets used for Detection of Y Microdeletions.
| Primer mixture | STS markers | AZF region | PCR products (bp) |
| A | SRY | internal control | 476 |
| sY254 | AZFc | 380 | |
| sY127 | AZFb | 274 | |
| sY153 | AZFc | 139 | |
| B | sY86 | AZFa | |
| sY134 | AZFb | 301 | |
| sY255 | AZFc | 126 | |
| sY118 | AZFb | 218 | |
| C | sY84 | AZFa | 130 |
| sY113 | AZFb | 304 | |
| sY158 | AZFc | 231 |
Results
A total 110 infertile individuals with non-obstructive azoospermia were analyzed for incidence of Y chromosome microdeletions. A total of 14 (12.72%) infertile azoospermic individuals were identified interstitial Y chromosome microdeletions using with multiplex PCR analysis. All fourteen azoospermic infertile males were cytogenetically normal and detected with the presence of either one or more deleted STS loci. Each STS primer amplified the expected size of products in all normal fertile controls and no deletions were identified in any of the 50 fertile men. In normal fertile women as negative control, STS failed to amplify the expected product size of DNA. All the three sets of multiplex PCR were amplified successfully and showed expected band size on corresponding position (Figure 1).
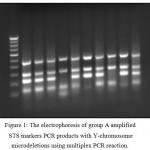 |
Figure 1: The electrophoresis of group A amplified STS markers PCR products with Y-chromosome microdeletions using multiplex PCR reaction.
|
All 14 infertile men have shown deletion with two or more STS primer and total STS primer deletions is presented in (Table 2). Out of 14 Y-chromosome microdeletions of AZF regions, deletion AZFa region was observed in 1 (7.14%) infertile males, deletion AZFb was observed in 1 (7.14%) infertile males and deletion AZFc region was observed in 6 (42.85%) infertile males, along with deletion of AZFb+c region was observed in 5 (35.71%) infertile males and AZFa+b region was observed in 1 (7.14%) infertile males (Table 3). The overall frequency of deletions of AZFa, AZFb, AZFc regions were found 2 (1.81%), 7 (6.36%) and 11(10%) respectively. We found AZFc regions was the most deleted region of Yq microdeletions than AZFa region and AZFb region of microdeletions. In the five azoospermic infertile males microdeletions were found in two overlapping regions AZFb+c and one infertile males had shown deletions of overlapping region AZFa+b (Figure 2). The microdeletions found in multiplex PCR reaction were confirmed by simplex PCR for only deleted STS markers.
Table 2: The 14 infertile males with details of Y-chromosome microdeletions STS markers.
| Patients No. | Types of Infertility | Age | Karyotype | STS deletions | AZF regions |
| P1 | Azoospermia | 37 | 46,XY | sY153, sY255, sY254 | AZFc |
| P2 | Azoospermia | 34 | 46,XY | sY113, sY153, sY148, sY157 | AZFb+c |
| P3 | Azoospermia | 27 | 46,XY | sY86, sY84 | AZFa |
| P4 | Azoospermia | 28 | 46,XY | sY84, sY113 | AZFa+b |
| P5 | Azoospermia | 29 | 46,XY | sY255, sY254 | AZFc |
| P6 | Azoospermia | 36 | 46,XY | sY113, sY118, sY153, sY158 | AZFb+c |
| P7 | Azoospermia | 30 | 46,XY | sY153, sY255, sY254 | AZFc |
| P8 | Azoospermia | 29 | 46,XY | sY153, sY148, sY255, s254, | AZFc |
| P9 | Azoospermia | 30 | 46,XY | sY127, sY134, sY255, sY254 | AZFb+c |
| P10 | Azoospermia | 34 | 46,XY | sY158, sY254, sY255 | AZFc |
| P11 | Azoospermia | 32 | 46,XY | sY127, sY118, sY255, sY254 | AZFb+c |
| P12 | Azoospermia | 29 | 46,XY | sY113, sY118 | AZFb |
| P13 | Azoospermia | 39 | 46,XY | sY255, sY254, sY158 | AZFc |
| P14 | Azoospermia | 37 | 46,XY | sY113, sY118,sY153, sY158 | AZFb+c |
Table 3: The following table showing region-wise frequency of AZF microdeletions
| AZF region | No of infertile males with deletions | Frequency of deletions |
| AZFa | 1 | (7.14%) |
| AZFb | 1 | (7.14%) |
| AZFc | 6 | (42.85%) |
| AZFb+c | 5 | (35.71%) |
| AZFa+b | 1 | (7.14%) |
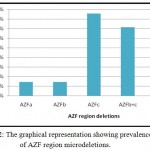 |
Figure 2: The graphical representation showing prevalence of AZF region microdeletions.
|
Discussions
The Y chromosome long arm has contained euchromatic region which divided in three important regions AZFa, AZFb, AZFc. Most of spermatogenesis controlling candidate genes have found in AZF region which actively involved in spermatogenesis and male fertility (Foresta, et al, 2001). Microdeletions in Y-chromosomal AZF regions are known cause of male infertility but the frequency of Y chromosome microdeletions varied with the severity of the spermatogenic impairment from oligozoospermia to azoospermia infertile males (Zhang, et al, 2007). Over all 10-15% of azoospermic and 5-10% oligozoospermic men had shown Y microdeletions in idiopathic conditions and it is due to inclusion criteria and number of STS markers used in screening of AZF regions microdeletions on Y chromosome (Sen, et al, 2013; Sachdeva, et al, 2010). Now, the diagnosis of genetic etiology has important clinical significance to overcome male infertility problem using different sets of STS markers for screening of microdeletions in AZF regions. However, systematic use of STS-PCR markers is beneficial tools for screening of Y chromosome microdeletions and provides counseling to infertile couple effectively, regard to the birth of an infertile male offspring (Lange, et al, 2008; Mitra, et al, 2008).
In present study, 12.72% microdeletions was reported in the Y chromosome in idiopathic azoospermic infertile men using three sets of multiplex PCR amplifications. We have studied microdeletions through three sets of multiplex reaction using 10 STS markers and one internal control SRY (sY14) markers (Table 2). All the STS markers have been recommended by Indian council of Medical research, New Delhi and which covered six STS markers of European andrology Association (EAA). In 110 azoospermic infertile males, 14 (12.72%) azoospermic males had shown Y chromosome microdeletions with different AZF regions. Out of 14 azoospermic males of microdeletions, only AZFc regions had shown in 6 (42.85%) infertile males followed by AZFb+c region in 5 (35.71%) azoospermic infertile males and AZFa, AZFb and AZFa+b region had shown in each 1 (7.14%) azoospermic infertile male. Five azoospermic males had shown overlapping microdeletions of AZFb+c region, it is covered long STS markers microdeletions (Figure 2). Another one azoospermic infertile male had shown continuous deletion of AZFa and AZFb region using STS markers DFFRY, sY113 and sY118. Individually microdeletions in AZF region had shown AZFc was most deleted regions in 11 (10%) infertile males, covered with DAZ and CDY gene family. The AZFb region had shown deletions in 7 (6.36%) infertile males and AZFc region had shown deletions in 2 (1.81%) infertile males. Previous research reported the percentage of microdeletions of Yq in different studies range between 0% and 19% of infertile males with non-obstructive azoospermia or severe oligozoospermia in India (Sen, et al, 2013; Ambasudhan et al, 2003; Kim, et al, 2017). In comparison to the statistical data obtained from all the published report to date, some studies reported 20% of microdeletions in the Y chromosome of infertile males, while others only 2% (Raicu, et al, 2003). Further studies had showed an incidence of microdeletions 5.1% to 9.6% in the infertile males (Ferlin 2008; Tuttelmann, et al, 2007). On the other hand, in Italy 55% microdeletions of Y chromosome were found in infertile males (Simoni, et al, 2012). Results of present study and prevalence of microdeletions are in accordance with the previously reported results and reported between 10-23% (Thangaraj, et al, 2003; Dada, et al, 2004). The studies published on Y chromosome microdeletions have shown a remarkably difference in the microdeletions frequency. This is due to selection criteria of different patients groups and use of different sets of STS marker (Sachdeva, et al, 2010; Motovali-Bashi, et al, 2015).
From last two decades, there have been several studies in which infertile men have been screened for Y chromosome microdeletions and large numbers of STS markers have been developed for different AZF region along with heterochromatic region of Y-chromosome (Lange, et al, 2008; Asadi, et al, 2017). With the development of STS markers, it is not possible to used one reaction for one marker. Hence, it is need to establish technique for amplifying several loci in one reaction, multiplex PCR is a rapid, robust and convenient method for analyzing of many loci in a single reaction with short time (Bor, et al, 2001). Thus, multiplex PCR has been suggested to be the choice for routine screening of microdeletions on the Y-chromosome in infertile men, since analysis of many STSs is impractical by single PCR (Asadi, et al, 2017). In the present study, all three set of multiplex covered EAA recommended markers and also included STS markers mostly used in Indian studies (Simoni, et al, 2004). All multiplex reaction set has been optimized to work under identical conditions for all 11 STSs markers, but it is always difficult to establish of multiplex PCR in making different primer pairs work together.
Y chromosome of AZFc region was most frequently deleted sites in infertile males (Figure 5). DAZ (Deleted in azoospermia) is candidate gene of AZFc region plays important role for spermatogenesis (Massart, et al, 2012). DAZ is multiple copy number genes which contain higher number of pallindromic repetitive sequences in AZFc region (Thangaraj, et al, 2003). Skaletsky et al. (2003) was reported that reduction of copy number to have an increased risk in infertility (Skaletsky, et al, 2003). Deletion of AZFa and AZFb of Y chromosome can cause azoospermia or severe oligozoospermia and these results showed that all AZFa and AZFb microdeletions were present in only azoospermic infertile males (Vogt, et al, 1996; Mitra, et al, 2008). The present study showed that microdeletions in spermatogenesis involving genes of AZF region cause various abnormal phenotypes in infertile males. On Yq region of AZF microdeletions was directly related with the phase in which spermatogenesis was arrested. Microdeletions of each locus cause spermatogenic arrest at a particular stage because each candidate genes of AZF locus specified a different phase of spermatogenesis (Geoffroy, et al, 2007). Testicular histology of Y chromosome microdeletions in azoospermia and oligozoospermia infertile male had shown variable phenotypes of spermatogenesis (Singh, et al, 2005). These results confirmed that before the infertile couple have adapted Intra cytoplasmic sperm Injection or other assisted reproductive techniques, sets of STS markers of AZF regions should be used routinely as diagnostic genetic test to screen for Y chromosome microdeletions.
Conclusion
The present study demonstrated that a microdeletion of Y-chromosome is a important genetic cause of non-obstructive azoospermia which is not observed through cytogenetic technique. Especially multiplex PCR approach is very useful method for screening of Y-chromosome microdeletions in short time with large number of STS markers. The screening of Y chromosome microdeletions and genetic counseling is powerful exercises for infertile men prior to opting of assisted reproduction techniques.
Conflict of Interest
There is no conflict of interest
Acknowledgement
Authors are thankful to ICMR, New Delhi for financial assistance of this research work.
References
- Boivin J, Bunting L, Collins J. A & Nygren K. G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human Reproduction. 2007;22: 1506–1512.
CrossRef - Krausz C & Degl’Innocenti S. Y chromosome and male infertility: update. Frontier Biosciences. 2006;11: 3049–3061.
CrossRef - Ferlin A, Arredi B & Foresta C. Genetic causes of male infertility. Reproduction Toxicology. 2007a;22:133–141.
CrossRef - Vogt P. H, Edelmann A, Kirsch S, Henegariu O, Hirschmann P & Kiesewetter F. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Human Molecular Genetics. 1996;5:933-943.
CrossRef - Tiepolo L & Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Human Genetics. 1976;34:119-124.
CrossRef - Sen S, Pasi A.R, Dada R, Shamsi M.B & Modi D. Y chromosome microdeletions in infertile men: prevalence, phenotypes and screening markers for the Indian population. Journal of Assisted Reproduction and Genetics. 2014;30:413-422.
CrossRef - Silber S.J. The Y chromosome in the era of intracytoplasmic sperm injection: a personal review. Fertility & Sterility. 2011;95:2448e1-2448e5.
- Ferlin A, Arredi B, Speltra E, Cazzadore C, Selice R & Garolla A. Molecular and clinical characterization of Y chromosome microdeletions in infertile men: a 10-year experience in Italy. Journal Clinical Endocrinology Metabolism. 2007b;92:762–770.
CrossRef - Kuchukaslan A. S, Cetintas V.B, Altintas R, Vardarli A.T, Mutlu Z & Ulukas M. Identification of Y chromosome microdeletions in infertile Turkish men. Turkish Journal of Urology. 2013;39:170-174.
CrossRef - World Health Organization.WHO laboratory manual for the examination and processing of human semen. 5th ed. United Kingdom, Cambridge University Press. 2010.
- Ambulkar P.S, Singh R, Reddy M.V.R, Varma P.S, Gupta D.O, Pal A. K. Genetic Risk of Azoospermia Factor (AZF) Microdeletions in Idiopathic Cases of Azoospermia and Oligozoospermia in Central Indian Population. Journal Clinical Diagnostic Research. 2014;8:88-91.
- Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions: State of the art. International Journal of Andrology. 2013;27:240–249.
CrossRef - Bor P, Hindkjær J, Ingerslev H.J, Kølvraa S. Multiplex Pcr For Screening Of Microdeletions On The Y Chromosome. Journal of Assisted Reproduction And Genetics. 2001;18(5):291-298.
CrossRef - Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocrinology Review. 2001;22:226-239.
- Zhang F, Lu C, Li Z, Xie P, Xia Y, Zhu X. Partial deletions are associated with an increased risk of complete deletion in AZFc: a new insight into the role of partial AZFc deletions in male infertility. Journal Medical Genetic. 2007;44:437–444.
CrossRef - Sachdeva K, Saxena R, Majumdar A, Chadda S, Verma I.C. Use of ethnicity-specific sequences tag site markers for Y chromosome microdeletion studies. Genetics Test & Molecular Biomarkers. 2011;15:451-459.
CrossRef - Lange J, Skaletsky H, Bell G.W, Page D.C. MSY Breakpoint Mapper, a database of sequence-tagged sites useful in defining naturally occurring deletions in the human Y chromosome. Nucleic Acids Research. 2008; 36:D809–D814.
CrossRef - Mitra A, Dada R, Kumar R, Gupta N.P, Kucheria K, Gupta S. K. Screening for Y-chromosome microdeletions in infertile Indian males: Utility of simplified multiplex PCR. Indian Journal of Medical Research. 2008;127: 127-132.
- Ambasudhan R, Singh K, Agarwal J.K, Singh S.K, Khanna A. Idiopathic cases of male infertility from a region in India show low incidence of Y-chromosome microdeletion. Journal of Biosciences. 2003;28:605–612.
CrossRef - Kim S.Y, Kim H. J, Lee B.Y, Park S. Y, Lee H.S, Seo J.T. Y Chromosome Microdeletions in Infertile Men with Non-obstructive Azoospermia and Severe Oligozoospermia. Journal of Reproductive Infertility. 2017;18(3):307-315.
- Raicu F, Popa L, Apostol P, Cimponeriu D, Dan L, Ilinca E, Dracea L.L, Marinescu B, Gavrila L. Screening for microdeletions in human Y chromosome – AZF candidate genes and male infertility. Journal of Cellular Molecular Medicine. 2003;7(1):43-48.
CrossRef - Tuttelmann F, Rajpert-De, Meyts E, Nieschlag E, Simoni M. Gene polymorphisms and male infertility–a meta-analysis and literature review. Reproduction Biomedicine Online. 2007;15:643-658.
CrossRef - Thangaraj K, Gupta N.J, Pavani K, Reddy A.G, Subramainan S, Singh L. Y Chromosome Deletions in Azoospermic Men in India. Journal of Andrology. 2003;24:588-597.
CrossRef - Dada R, Gupta N.P, Kucheria K. Yq microdeletions—azoospermia factor candidate genes and spermatogenic arrest. Journal Biomolecule Technique. 2004;15:176-183.
- Motovali-Bashi. M, Rezaei Z, Dehghanian F, Halimeh . Rezaei. Multiplex PCR based screening for micro/partial deletions in the AZF region of Y-chromosome in severe oligozoospermic and azoospermic infertile men in Iran. Iran Journal Reproduction Medicine. 2015;13(9):563-570.
- Asadi F, Sadighi Gilani M. A, Ghaheri A, Roodgar Saffari .J, Zamanian M. The prevalence of Y chromosome microdeletions in Iranian infertile men with azoospermia and severe oligospermia. Cell Journal. 2017;19(1): 27-33.
- Massart A, Lissens W, Tournaye H, Stouffs K. Genetic causes of spermatogenic failure. Asian Journal Andrology. 2012;14:40-48.
CrossRef - Skaletsky H, Kuroda-Kawaguchi T, Patrick J. M, Minx P.J,Cordum H.S, Hillier L. The male specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825-837.
CrossRef - Geoffroy-Siraudin C, Aknin-Seiffer I, Metzler-Guillemain C, Ghalamoun-Slaimi R, Bonzi M.F. Meiotic abnormalities in patients bearing complete AZFc deletion of Y chromosome. Human Reproduction. 2007;22: 1567-1572.
CrossRef - Singh K, Raman R. Male infertility: Y chromosome deletion and testicular aetiology in cases of azoo/oligospermia. Indian Journal of Experimental Biology. 2005;43:1088-1092.

This work is licensed under a Creative Commons Attribution 4.0 International License.





