How to Cite | Publication History | PlumX Article Matrix
Indu Verma1, 2 , Kriti Roopendra1
, Kriti Roopendra1 , Amaresh Chandra1
, Amaresh Chandra1 and Aisha Kamal2
and Aisha Kamal2
1Division of Plant Physiology and Biochemistry, ICAR- Indian Institute of Sugarcane Research, Lucknow-226002, India.
2Department of Biosciences, Integral University, Lucknow, 226021, India.
Corresponding Author E-mail: amaresh_chandra@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2667
ABSTRACT: Sugarcane being C4 crop exhibits distinct source-sink signaling pathway that helps in storing remarkably high amount of sucrose in its sink tissues that makes it a highly remunerable crop worldwide. In the present study sugar content was profiled in both source and sink tissues of early (CoJ64) and late (BO91) maturing sugarcane varieties. At early growth stage (i.e. at 210 DAP) sink tissues of both varieties exhibited higher reducing sugar and low sucrose content while in source tissues both sucrose and reducing sugar content was observed high, depicted lower sink demand for sucrose. With maturity, when sink demand for sucrose storage increased, rise in sucrose content was seen in sink tissues, whereas in source tissues gradual decrease in sucrose and reducing sugar content was observed. Accumulation of sucrose was found much higher in CoJ64 than those in BO91. In CoJ64 maximum sucrose content (64.2%) was seen at 330 DAP while in BO91 it was 41.8% at 390 DAP. At this stage, source tissues too exhibited higher sucrose and reducing sugar content. Thus sucrose synthesis in source tissues and its transportation to the sink tissues is primarily governed by the sink demand.
KEYWORDS: Sink Demand; Sink Strength; Source-Sink; Sucrose
Download this article as:| Copy the following to cite this article: Verma I, Roopendra K, Chandra A, Kamal A. Biochemical Profiling of Source and Sink Tissues at Different Growth Stages of Early and Late Maturing Varieties of Sugarcane (Saccharum spp. Hybrids). Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Verma I, Roopendra K, Chandra A, Kamal A. Biochemical Profiling of Source and Sink Tissues at Different Growth Stages of Early and Late Maturing Varieties of Sugarcane (Saccharum spp. Hybrids). Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=30638 |
Introduction
Sugarcane (Saccharum spp. hybrids) accumulates high concentration of sucrose in its stalk making one of the primary resources for world sucrose production.1,2 It shares around 70% of the global sugar production.1,3 In sugarcane, nearly 25% (on fresh weight basis) and 60% (on dry matter basis) of the crop biomass is comprised of sucrose. Modern sugarcane cultivars are multi species hybrid, differing in both sucrose storage capacity and accumulation mechanism during growth.4 Sucrose accumulation in sugarcane is controlled by many genes and regulatory sequences at different steps occurring in source (leaf) and sink (stalk) tissues. Several physiological processes viz. leaf photosynthetic rate and carbon partitioning to different metabolism, phloem loading occurring in leaf and unloading in the stalk/culm,5 culm metabolism6 and developmental constraints7 limit the extent of sucrose storage in the sink. Sink demand plays an additional limiting factor that largely affects crop yield. Thus sucrose content in the sink tissue is mainly regulated by the supply and demand relationship between source and sink tissues.8
Sugar accumulates in the storage tissues of sugarcane stalk against the concentration gradient using energy provided by respiration.9 Sucrose has been accounted to be there in the vacuoles as well as in symplastic and apoplastic spaces in similar concentration.10 Mature internodes of sugarcane accumulate higher sucrose than those in the young immature internodes. These differences in sucrose content between young and mature sink tissues are the consequence of varying rates of cycling of sucrose between vacuole, cytosol and apoplasm.11 Much research has focused on culm-specific processes,6 but the integration of source (photosynthetic) and sink (culm) processes in plants is still not fully understood.12 Earlier studies conducted on many plant species suggest that the photosynthetic activity of source and sink growth is closely coordinated and maintains a balance between source supply and sink demand.13 Evidences from expression studies conducted on photosynthetic gene strongly supports that sink demand strongly influence the photosynthesis – related enzyme activity.14 Studies conducted on Solanum tuberosum have demonstrated increase in photosynthetic rate15 and elevated photosynthate translocation16 when there was high sink demand in the form of rapidly growing tubers. Artificial removal of solanum tubers led to marked decrease in net photosynthetic activity17 resulted in accumulation of carbohydrate in source tissues.18 However, in sugarcane limited studies have been conducted to elucidate such kind of relationship in source and sink tissues.19
Sucrose synthesis, transportation and accumulation in sugarcane is complex process. Sucrose synthesized in the mesophyll cells of the source gets translocated through phloem of leaf sheath to the storage parenchyma in the culm/sink tissue, probably through symplastic and apoplastic routes20 and symplastically mainly in the mature internodes.21 Phloem loading efficiency of source decides the amount of photoassimilate available to the heterotrophic growth and storage sinks. Phloem loading of photoassimilates includes all the processes contributing to their short distance transport from the cellular site of sucrose synthesis to sieve element. Sucrose moves symplastically down the concentration gradient, from mesophyll cell to bundle sheath cell by diffusion, while it is retrieved apolastically from the vein by energy coupled transport mechanism which is mediated by sucrose/H+ symport.22 Phloem loading is largely regulated by the alterations in the sieve tube hydrostatic pressure which occur due to short term changes in sink demand.23 Photoassimilate from the leaves unloaded to the sink tissue, on the basis of which sink can be categorized into growth sink where sucrose is translocated to set the potential for sucrose biosynthesis and to enhance the storage volume and as culm storage sink, where sucrose finally accumulates.24 Thus, in the present study attempts were made to develop better understanding of this translocation process between source and sink, by estimating sugar content (sucrose and hexoses) at different growth stages in early maturing (CoJ64) and late maturing (BO91) varieties of sugarcane.
Materials and Methods
Plant material
Present study was conducted using early (CoJ64) and late maturing sugarcane variety (BO91). These were planted at the ICAR-Indian Institute of Sugarcane Research farm in the last week of February 2016, using three buds sett following normal agronomical practices- referred as spring planting. Cane maturation starts in October when minimum and maximum temperatures are 14-200C and 28-320C. Analysis was performed on three biological replications. Biological replicates were selected on the basis of plant height, determined from the bottom most internode to the LTM (last transverse mark) leaf. Sampling were conducted at 210 DAP (Days after planting) and then at every 30 days interval viz. 240 DAP, 270 DAP, 300 DAP and 330 DAP in CoJ64 and further more at 360 and 390 DAP in BO91. Tissues from the stalk were collected by dividing it into three equal portions depending on the internodes number, and then specific internodes was selected in each portion, marked as bottom middle and top internodes for further analysis. Sample from the leaves were collected from LTM (last transverse mark) leaf of both the varieties.
Extraction and Estimation of Total and Reducing Sugar
Sucrose % and reducing sugar % were determined from bottom, middle and top internodes and LTM leaf of the cane at different DAP viz. 210, 240, 270, 300, 330 in CoJ64 while in BO91 at 360 and 390 DAP. Fresh cane juice was extracted by hand cane crusher and diluted 100 times for the estimation of total sugar and reducing sugar from sink tissues. For estimation from source tissues, 500 mg LTM leaf was ground in 1.0 ml distilled water. Slurry was centrifuged at 10,000 RPM for 10 min and supernatant was collected in fresh Eppendorff tubes for further analysis of reducing sugar and total sugar. Reducing sugar content was determined following Nelson’s method (1944)25 taking D-glucose as standard. Non reducing sugar content was estimated by hydrolyzing the total sugar by adding 0.1N HCl solution and then boiling the sample for 15 min on water bath. After adding arseno-molybdate reagent, optical density of solution was estimated at 540 nm under UV-VIS spectrophotometer. Non reducing sugar% was determined by deducting the value of reducing sugar from total sugar. As in sugarcane, sucrose made the major portion of the non reducing sugar, thus the non reducing content was considered as direct depiction of sucrose content in the sample.
Statistical Analysis
Statistical analysis was done on the data derived from three biological replicates of both the varieties. Results were depicted as mean ± SE. Significant difference between two varieties were determined by performing 2 sided t-test using Microsoft excel 2007 software data analysis tool pack. These differences were considered statistically significant at P ≤ 0.05 (*), P ≤ 0.01(**), P ≤ 0.001 (***).
Results and Discussion
In both the varieties, highest sucrose% was found in bottom internode which gradually decline in the upper regions of the cane. Contrary to this reducing sugar content, mainly comprises of hexoses, was found decreasing from top to bottom. Sucrose % in bottom internode ranged from 32.62% to 50.3% in CoJ64 and from 12.56% to 41.8% in BO91. In middle internode sucrose% varied from 23.62% to 63.21% in CoJ64 whereas in BO91 it ranged from 12.1% to 41.17%; while in the top internode being immature demonstrated lower sucrose%, which ranged from 19.86% to 64.2% in case of CoJ64, on the other hand in BO91 it ranged between 6.86% to 33.23% (Fig. 1). This broad illustration of sucrose content in different parts of stalk reasserts that BO91 has a lower tendency to store sucrose in its stalk in comparison to CoJ64 and therefore it is referred as low sucrose accumulating variety while CoJ64 as high sucrose accumulating variety.
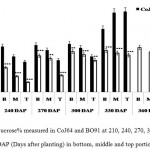 |
Figure 1: Sucrose% measured in CoJ64 and BO91 at 210, 240, 270, 300, 330, 360 and 390 DAP (Days after planting) in bottom, middle and top portions of cane.
|
Errors bars indicate ±SD from three independent experiments. Statistical differences between them were calculated by t-test. P ≤ 0.05 (*), P ≤ 0.01(**), P ≤ 0.001 (***)
Observations made at 210 DAP depicted highest reducing sugar (RS %) and lowest sucrose content in both the varieties. In CoJ64, RS % from bottom to top ranged between 3.78% and 8.62% whereas in BO91 it varied between 5.45% and 8.62%, indicated high reducing sugar content in the late maturing variety BO91 than those in early maturing variety like CoJ64. High reducing sugar content was seen in upper immature internodes of both the varieties. On the other hand at this stage sucrose % from top to bottom ranged 19.86% to 32.62% in CoJ64 and from 6.8% to 12.56% in BO91 (Fig. 1 and 2). In congruence with earlier works,26,27 all three portions of canes studied here the highest sucrose content was seen in bottom internode which gradually declined from middle to top internodes in both the varieties. Source tissues of both the varieties showed significantly high sucrose (2.56 % in CoJ64 and 1.4% in BO91) (Fig. 3) and reducing sugar content (1.14% in CoJ64 and 1.1% in BO91) (Fig. 4). This depicted that at 210 DAP, both the varieties were in elongation or active growth phase, and thus exhibited low tendency to store sucrose in the culm. This phase was marked by high sink strength of cane, and top internode being immature and actively elongating tissues showed highest sink strength, compared to middle and bottom internodes. Tejra et al. (2007)28 reported similar observations where growth activity of the sink contributed to sucrose yield by setting the potential for sucrose biosynthesis by creating photosynthetic leaf surface area for carbon capture and by generating potential culm volume for storage of leaf synthesized sucrose. Higher RS% and sucrose% in the source tissue illustrated that the reducing sugar synthesized during photosynthesis was not completely converted into sucrose, and the synthesized sucrose though translocated, was unavailable for storage in the sink tissue since it was utilized to generate biomass. Paul and Foyer (2001)29 have also described that the carbon demand of sink tissues influence the net photosynthetic rate and carbon status of the source organs.
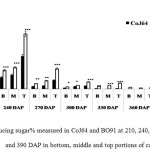 |
Figure 2: Reducing sugar% measured in CoJ64 and BO91 at 210, 240, 270, 300, 330, 360 and 390 DAP in bottom, middle and top portions of cane.
|
Errors bars indicate ±SD from three independent experiments. Statistical differences between them were calculated by t-test. P ≤ 0.05 (*), P ≤ 0.01(**), P ≤ 0.001 (***)
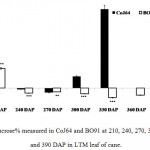 |
Figure 3: Sucrose% measured in CoJ64 and BO91 at 210, 240, 270, 300, 330, 360 and 390 DAP in LTM leaf of cane.
|
Errors bars indicate ±SD from three independent experiments. Statistical differences between them were calculated by t-test. P ≤ 0.05 (*), P ≤ 0.01(**), P ≤ 0.001 (***)
Conversely at 240, 270 and at 300 DAPs, there was gradual decrease in reducing sugar content while a progressive increase in sucrose content was seen in both the varieties. In bottom internode, sucrose content in CoJ64 ranged from 36.34% -38.37% while in BO91 it was found between 19.5%-32.28%. Reducing sugar content ranged between 0.13% to 1.39% in CoJ64 and 0.26% to 2.21% in BO91. In middle internode, sucrose% ranged from 28.37% to 35.25% and 12.7% to 29.85% whereas reducing sugar content 0.32 to 1.68% and 0.43% to 2.54% in CoJ64 and BO91 respectively. In top internode sucrose content ranged between 24.12% to 28.37% and from 7.84% to 25.32% while RS content varied from 0.37% to 3.88% and 0.83% to 6.48% in CoJ64 and BO91 respectively (Fig. 1 and 2). Observations as recorded at these growth stages displayed that BO91 still having higher RS content and lower sucrose content as compared to CoJ64 indicating that BO91 still had not acquired significant sucrose storage efficiency. While CoJ64 is being early maturing had achieved enough strength to store comparable higher sucrose than BO91. In leaf sample there was marked decrease in sucrose% at 210 DAP, which was found to decrease with due course of maturity, owing to increased sink demand in both the varieties, whereas in CoJ64 at 300 DAP there was slight increase (0.83%) in sucrose content was seen, this may be due to gradual saturation of sink tissue (Fig. 3). RS content was seen to gradually decline from 270 to 300 DAP in BO91 (0.86% to 0.68%) while in CoJ64 it was found to decline from 210 to 300 DAP (Fig. 4). This decline in reducing sugar content may be due to increased channelization of hexoses to the site of sucrose synthesis, which gets transported from source to the sink tissue. Decline in the sugar content of leaf (source tissues) demonstrated that the sucrose accumulation in the sink is restricted by source, similar view was suggested by the Wareing and Patrick (1976),30 when sink potential to accumulate/consume sugar surpassed the actual capacity of source output than carbon import to the sink is said to be limited by source.
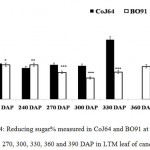 |
Figure 4: Reducing sugar% measured in CoJ64 and BO91 at 210, 240, 270, 300, 330, 360 and 390 DAP in LTM leaf of cane.
|
Errors bars indicate ±SD from three independent experiments. Statistical differences between them were calculated by t-test. P ≤ 0.05 (*), P ≤ 0.01(**), P ≤ 0.001 (***)
At 330 DAP, CoJ64 showed highest sucrose content and lowest reducing sugar content in all the internodes while BO91 showed comparatively higher sucrose content from the earlier growth phases. In CoJ64, invert sucrose pattern as observed showed highest sucrose content in upper internode (64.21%) than middle (63.21%) and bottom (50.3%) internodes, this invert pattern was due to post harvest deterioration of sucrose in CoJ64, as it is early maturing variety. Elevated sucrose (5.6%) and reducing sugar content (1.9%) was observed in LTM lead too, as sink tissue had attain saturated concentration of sucrose while elevated RS content indicating lesser conversion of reducing sugar to sucrose due to decreased sink demand. BO91 being late maturing variety, observations were further taken at 360 and 390 DAP. At this stage there was sudden decline in reducing sugar content and increase in sucrose content was seen comparable to earlier growth stages. Sucrose content in bottom internode was found 35.56% and 41.8% whereas reducing sugar content was 0.21% and 0.02% at 360 DAP and at 390 DAP respectively. While in middle internode, sucrose content observed was 32.06% and 41.17% while reducing sugar content was 0.12% and 0.07% whereas in top internode sucrose content was 29.98% and 33.23% and reducing sugar content was 0.17% and 0.05% at 360 and 390 DAP respectively. While in leaf tissues gradual increase in the sucrose and RS content was seen resembling sugar behavior of CoJ64 leaf at 330 DAP, this was due to attainment of decreased sink demand. Minchin et al. (2002)31 had also suggested that decreased sink demand slows the export, go along with an increase in mesophyll sucrose concentrations.32 Thus, the photosynthetic efficiency of the leaf in sugarcane shows a characteristic dependency on the sink tissue depicting sink limitation.25
Conclusion
A coupled relationship was seen between source and sink tissue that results in higher sucrose content in sugarcane in general. Phloem translocation of sucrose plays a vital role in determining the resource capturing efficiency and sink volume to store phloem –delivered sucrose. Sink limits the translocation of the photoassimilate, by sending feedback signals to source, when it attains saturated concentration of sucrose. Decline in the hexose content of source acted as positive signal for increased sink demand which also reduces the negative feedback regulation of photosynthesis.33 A negative correlation was established between leaf hexose content and its photosynthetic gas exchange,34 which probably denotes the inhibitory action of the increased hexose content. Therefore decreased hexose sugar in source is a clear indicative of the increase photosynthesis and sucrose synthesis activity against increased sink demand.35 Thus the present study provides the platform, for the future researches to enhance sugarcane sucrose yield by manipulating the signaling and metabolic pathway at source and sink levels.
Acknowledgements
Authors are thankful to Indian Institute of Sugarcane Research and Integral University for providing necessary facilities and guidance to carry out this work. IV is thankful to Integral University for providing manuscript ID (IU/R&D/2018-MCN000392).
Conflict of Interest
The authors declare that they have no conflict of interest.
Refrences
- Lunn J.E, Furbank R. T. Sucrose biosynthesis in C4. New Phytol. 1999;143:221–237.
CrossRef - Wu L, Birch R. G. Doubling sugar content in sugarcane plants modified to produce a sucrose isomer. Plant Biotech. J. 2007;5:109–117.
CrossRef - Lakshmanan P, Geijskes R.J, Aitken K.S, Grof C.P.L, Bonnet G. D, Smith G. R. Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev. Biol. Plant. 2005;41:345-363.
CrossRef - Lingle S. E. Seasonal internode development and sugar metabolism in sugarcane. Crop Sci. 1997;37:1222-1227.
CrossRef - Walsh K. B, Sky R.C, Brown S. M. The anatomy of the pathway of sucrose unloading within the sugarcane stalk. Plant Biol. 2005;32:367–374.
CrossRef - Uys , Botha F.C, Hofmeyr J. H.S, Rohwer J. M. Kinetic model of sucrose accumulation in maturing sugarcane culm tissue. Phytochemistry. 2007;68:2375–2392.
CrossRef - Moore P. H, Botha F. C, Furbank R.T, Grof C. P. L. Potential for overcoming physio-biochemical limits to sucrose accumulation. In: Intensive sugarcane production: meeting the challenges beyond 2000 (Keating B.A, Wilson J.R, eds). Wallingford: CAB International. 1997;141–155.
- McCormick A. J, Cramer M. D, Watt A. Culm sucrose accumulation promotes physiological decline of mature leaves in ripening sugarcane. Fields Crops Resh. 2008;108:250-258.
CrossRef - Bieleski R. L. The physiology of sugarcane III. Characteristics of sugar uptake in slices of mature and immature storage tissue. J. Biol. Sci. 1960;13:203-220.
- Welbaum G. E, Meinzer F. C. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol. 1990;93:1147–1153.|
CrossRef - Batta S.K, Singh R. Sucrose metabolism in sugarcane grown under varying climatic conditions: synthesis and storage of sucrose in relation to the activities of sucrose synthase, sucrose phosphate synthase and invertase. Phytochemistry. 1986;25:2431–2437.
CrossRef - Pego J.V, Kortstee A. J, Huijser C, Smeekens S.C. Photosynthesis, sugars and the regulation of gene expression. Exp. Bot. 2000;51:407-416.
CrossRef - Foyer C. H, Chaumont , Murchie E, Galtier N, Ferrario S. End-product modulation of carbon partitioning with a view to improved biomass production. In: Carbon partitioning and source–sinks interactions in plants (Madore M.A, Lucas W.J, eds). Rockville: American Society of Plant Physiologists. 1995;45–55.
- Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14:185–205.
CrossRef - Dwelle R. B, Kleinkopf G. E, Pavek J. J. Stomatal conductance and gross photosynthesis of potato (Solanum tuberosum ) as influenced by irradiance, temperature and growth stage. Potato Resh. 1981;24:49–59.
CrossRef - Moorby J. The physiology of growth and tuber yield. In: Potato crop: The scientific basis for improvement (Harris PM, ed). New York: Wiley. 1978;153–194.
CrossRef - Nosberger J, Humphries E. C. The influence of removing tubers on dry matter production and net assimilation rate of potato tubers. Bot. 1965;29:579–588.
CrossRef - Basu P. S, Sharma A, Garg I. D, Sukumaran N. P. Tuber sink modifies photosynthetic response in potato under water stress. Exp.Bot. 1999;42:25–29.
CrossRef - Marcelis L. F. M. Flower and fruit abortion in sweet pepper in relation to source and sink strength. Exp.Bot. 1996;55:2261–2268.
CrossRef - Rae A. L, Perroux J. M, Grof C. P. L. Sucrose partitioning between vascular bundles and storage parenchyma in the sugarcane stem: a potential role for the ShSUT1 sucrose transporter. Planta. 2005;220:817–825.
CrossRef - Patrick W. J, Botha F.C, Birch R. G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotech. J. 2013;11:142-156.
CrossRef - Lohaus G, Winter H, Riens B, Heldt H. W. Further Studies of the Phloem Loading Process in Leaves of Barley and Spinach. The Comparison of Metabolite.
- Concentrations in the Apoplastic Compartment with those in the Cytosolic Compartment and in the Sieve Tubes. Botanica Acta. 1995;108:270-275.
CrossRef - Smith J. A.C, Milburn J. A. Osmoregulation and the control of phloem sap composition in Ricinus communis L. Planta. 1980;148:28-34.
CrossRef - Waclawovsky A. J, Sato P. M, Lembke C.G, Moore P. H, Souza G. M. Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotech. J. 2010;8:263-276.
CrossRef - Nelson N. A photometric adaptation of Somogyi method for the determination of glucose. Biol. Chem. 1944;153:375–380.
- McCormick A. J, Cramer M. D, Watt D. A. Sink strength regulates photosynthesis in sugarcane. New Phytol. 2006;171:759–770.
CrossRef - Verma A. K, Upadhyaya S. K, Srivastava M. K, Verma P. C, Solomon S, Singh S. B. Transcript expression and soluble acid invertase during sucrose accumulation in sugarcane. Acta Physiol. 2011;33:1749–1757.
CrossRef - Tejera N. A, Rodés R, Ortega E, Campos R, Lluch C. Comparative analysis of physiological characteristics and yield components in sugarcane cultivars. Field Crops Resh. 2007;102:64-72.
CrossRef - Paul M. J, Foyer C. H. Sink regulation of photosynthesis. Exp. Bot. 2001;52:1381–1400.
CrossRef - Wareing P. F, Patrick J. Source-sink relations and partition of assimilates in the plant. In: Photosynthesis and Productivity in Different Environments (Cooper JP, ed). Cambridge: Cambridge University Press. 1976;481-499.
- Minchin P. E. H, Thorpe M. R, Farrar J. F, Koroleva O. A. Source–sink coupling in young barley plants and control of phloem loading. Exp. Bot. 2002;53:1671-1676.
CrossRef - Koroleva O. A, Tomos D. A, Farrar J, Pollock C. J. Changes in osmotic and turgor pressure in response to sugar accumulation in barley source leaves. Planta. 2002;215:210-219.
CrossRef - Foyer C. H. The basis for source-sink interaction in leaves. Plant Physiol. Biochem. 1987;25:649-657.
- McCormick A. J, Cramer M. D, Watt D. A. Changes in photosynthetic rates and gene expression of leaves during a source–sink perturbation in sugarcane. Bot. 2008;101:89-102.
CrossRef - Mirajkar S.J, Suprasanna P, Vaidya E. R. Spatial distribution and dynamics of sucrose metabolising enzymes in radiation induced mutants of sugarcane. Plant Physiol. Biochem. 2016;100:85-93.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





