How to Cite | Publication History | PlumX Article Matrix
Cytogenetic Analysis of A Hoverfly Eristalis Tenax (Diptera: Syrphidae)
Manvi Khajuria , Arshad Ayoub Bhatti
, Arshad Ayoub Bhatti  and N.K. Tripathi
and N.K. Tripathi
Department of Zoology, University of Jammu, Jammu and Kashmir-180006, India.
Corresponding Author E-mail: manvi.khajuria@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2672
ABSTRACT: Karyotypic, morphometric and meiotic details of a hoverfly, Eristalis tenax belonging to subfamily Eristalinae of family Syrphidae were studied during present investigation. This species is commonly known as drone fly due to its resemblance with the drones of honey bees. These are good pollinators and their larvae are called rat tailed maggots due to the presence of a long posterior tube for breathing. It showed diploid chromosome number 2n=12 (10+XY) in males. The sex mechanism is found to be XY, X is subtelocentric and Y is telocentric. Meiotic observations included leptotene, diplotene, metaphase-I and metaphase-II. Present studies will help to solve taxonomic problems with in the family Syrphidae and in general understanding of the course of evolution in order Diptera.
KEYWORDS: Chromosome; Diplotene; Eristalis; Syrphidae
Download this article as:| Copy the following to cite this article: Khajuria M, Bhatti A. A, Tripathi N. K. Cytogenetic Analysis of A Hoverfly Eristalis Tenax (Diptera: Syrphidae). Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Khajuria M, Bhatti A. A, Tripathi N. K. Cytogenetic Analysis of A Hoverfly Eristalis Tenax (Diptera: Syrphidae). Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31031 |
Introduction
The order Diptera (true flies) is one of the species rich orders and is ecologically and biologically important order constituting about 10–15% of total animal species. Dipterans are the important contributors to maintain plant diversity and form the main component of pollinating systems due to their participation in many pollination systems.1 Syrphid flies were noticed due to their important ecological services. These tiny flies play important role in pollination and serve good pollinator for many flowering plants.2 Subfamily Eristalinae of family Syrphidae includes species that mimic honeybee or wasps for example dronefly, Eristalis tenax mimic honeybees. Larvae of this fly are known as rat tailed maggots. These are ecologically important as they cause accidental myiasis in livestock and humans.3 Syrphid flies form good model system for genetic and cytogenetic studies. They are easy to recognize, capture and dissect. They also present various cytological advantages like mitotic and meiotic chromosomes of moderate size, variable in number and morphology and represent an intermediate stage in the evolution of Diptera.4 Cytogenetic and karyotypic work on the Syrphidae species is very limited and only chromosome number was reported. Moreover the relationship among some tribes of Syrphidae is undetermined. The present study was thus undertaken to study the karyotypic, morphometric and meiotic details of Eristalis tenax from the Jammu region of outer Himalayas.
Materials and Methods
Flies were collected from the botanical garden of University of Jammu during April-May 2015. Adult male flies (fig.2a) were used for the present studies and were identified from their holoptic eyes. Cytological preparations were made from testicular tissue. In hoverflies testis are minute brownish spherical bodies found in the last segment of abdomen. To dissect out the testis the flies were firstly anaesthetised with fumes of ethyl acetate. The anaesthetised flies were placed on a clean glass slide. The abdomen was cut from the tip and the last segments were squeezed gently so as to take out the testis. Slides were prepared by squash method5. Tissue was treated with 0.5% KCl for 45 minutes. Then the tissue was stained with 2% solution of lacto-aceto-orcein and the stained slides were covered with coverslip. The covered slides were squashed gently and the resulting temporary mounts were sealed with wax. Stained slides were scanned and results were photographed under Olympus CH20i BIMF microscope attached with Sony SSC-DC378P camera under 1000X magnification.
Results
The photokaryotype (fig.1b) prepared from the spermatogonial metaphase complement (fig.1a) showed 12 chromosomes having 5 autosomal pairs (4 pairs of metacentric, 1 pair of submetacentric) and one sex chromosome pair (X is subtelocentric and Y is telocentric). The diploid chromosomal formula has been worked out to be 8m+2sm+1st+1t. Histogram (fig.1c) was prepared on the basis of decreasing value of relative length percentage from chromosome pair no. 1 to pair no. 6 and the sex chromosome pair placed at the end showing relative length percentage corresponding to its value. Idiogram (fig.1d) of chromosomes was prepared to illustrate the chromosomes diagrammatically.
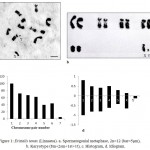 |
Figure 1: Eristalis tenax (Linnaeus). a. Spermatogonial metaphase, 2n=12 (bar=5μm), b. Karyotype (8m+2sm+1st+1t), c. Histogram, d. Idiogram.
|
The karyomorphometrical analysis (table 1) of the spermatogonial metaphase complement revealed the absolute length of autosomes to vary from 1.81μm to 0.72μm. The X chromosome being subtelocentric measured an absolute length of 0.80μm and 0.10μm while the telocentric Y chromosome measured 0.10μm. Meiotic stages included leptotene, diplotene, metaphase-I and metaphase-II (fig.2). In leptotene, chromosomes were observed in the form of a network, without visible free ends and the chromatin was showing beaded appearance (fig.2b). Diplotene was characterised by the presence of five autosomal bivalents. Most of the bivalents were V shaped in morphology and the largest bivalent showed the separation of chromosomes at a point that leads to the formation of a small ring like structure (fig.2c). Metaphase-I showed five autosomal bivalents. Sex bivalent was not visible. Bivalents were thick and condensed in morphology (fig.2d). Six chromosomes were clearly seen in metaphase-II stage (fig.2e). Both chromatids of a single chromosome were visible and most of the chromosomes were metacentric in morphology.
Table 1: Morphometric data of karyotype of male Eristalis tenax showing 2n=12 (6m+4sm+1st+1t).
| Chromosome pair number | Mean length of the short arm (p) in µm | Mean length of the long arm (q) in µm | Absolute length of the chromosome (p+q) in µm | Arm ratio (q/p) | Relative length percentage | Total complement length percentage | Centromeric index | Nomenclature |
| 1 | 0.81 | 1.0 | 1.81 | 1.23 | 100 | 25.52 | 45 | Metacentric |
| 2 | 0.43 | 0.85 | 1.28 | 1.97 | 70.71 | 18.13 | 34 | Submetacentric |
| 3 | 0.42 | 0.80 | 1.22 | 1.90 | 67.40 | 17.20 | 34 | Submetacentric |
| 4 | 0.51 | 0.62 | 1.13 | 1.21 | 62.43 | 15.93 | 45 | Metacentric |
| 5 | 0.30 | 0.42 | 0.72 | 1.40 | 39.78 | 10.15 | 42 | Metacentric |
| X | 0.15 | 0.65 | 0.80 | 4.33 | 44.20 | 11.28 | 19 | Subtelocentric |
| Y | 0 | 0.10 | 0.10 | – | 5.52 | 1.41 | – | Telocentric |
| Total | 2.62 | 4.44 | 7.06 |
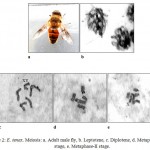 |
Figure 2: E. tenax. Meiosis: a. Adult male fly, b. Leptotene, c. Diplotene, d. Metaphase-I stage, e. Metaphase-II stage.
|
Discussion
Eristalis tenax showed diploid chromosome number 2n=12. Chromosome number in the family Syrphidae varies from n=3 to n=7.4,6,7,8,9 The present observation on this species is in conformity with the results given by Rozek et al.10 who also reported diploid chromosome number of 12 in this species. He also mentioned XY sex mechanism in males of E. tenax. The X chromosome was somewhat large in size and Y was telocentric in morphology. Present author also found somewhat large subtelocentric X chromosome and telocentric Y chromosome. The haploid chromosome number, n=6 in 14 species of the genus Eristalis has been reported4. The karyotype of Syrphidae is morphologically differentiated because its few chromosomes represent several types including metacentric, submetacentric, telocentric and subtelocentric chromosomes. Chromosome number decreases in the higher tribes by end to end fusions and reciprocal translocations. Considering the previous review and cytogenetic data of studied species it was suggested that the species with six pairs of chromosomes may be the more primitive ones.11
Conclusion
Eristalis tenax showed diploid chromosome number 2n=12 in males. The karyotype revealed 8 metacentric, 2 submetacentric, 1 subtelocentric and 1 telocentric chromosome. Sex mechanism was found to be XY in which X is subtelocentric and Y is small telocentric body. Meiosis represented by Leptotene, diplotene, metaphase-I and metaphase-II stages. This species might be among the ancestors of the species of tribe Syrphini due to moderate size chromosomes.
Acknowledgements
We would like to show our gratitude to Prof. Seema Langer, Head of the Department of Zoology, University of Jammu for providing necessary lab facilities and support.
Conflict of Interest
There is no conflict of interest.
Funding source
There is no particular funding source.
References
- Ssymank A., Kearns C. A., Pape T., Thompson F. C. Pollinating flies (Diptera). A major contribution to plant diversity and agricultural production. The Royal Society of Chemistry. 2008;9:86-89.
CrossRef - Larson B. M. H., Kevan P. G., Inouye D. W. Flies and flowers taxonomic diversity of anthophiles and pollinators. Canadian Entomologist. 2001;133(4):439-465.
CrossRef - Catts P. E., Gary M. Myias is (Muscoidea, Oestroidea). Medical and Veterinary Entomology. 2002;317-348.
- Boyes J. W., Brink V. J. M. Chromosomes of Syrphidae. Variations in karyo types. Chromosoma. 1964;15:579-590.
CrossRef - Boyes J. W., Brink V. J. M. Chromosomes of Syrphidae V. Micro chromosomes. Chromosoma. 1970;31:207-216.
CrossRef - Boyes J. W., Brink J. M. V. Chromosomes of Syrphidae III. Karyotypes of some species in the tribes Milesiini and Myoleptini. Chromosoma. 1967;22:417-455.
CrossRef - Boyes J. W., Brink V. J. M. Chromosomes of Syrphidae VI. The tribe Pipizini. Genetica. 1972;43:321-333.
CrossRef - Boyes J. W., Brink V. J. M., Mehta R. D. Chromosomes of Syrphidae IV. Karyo types of fourteen species in the tribe Chrysotoxini. 1968;24:233-242.
- Boyes J. W., Boyes B. C., Brink V. J. M., Vockeroth J. R. Cytotaxonomy of South American Syrphinae (Diptera Syrphidae). Genetica. 1973;44:368-415.
CrossRef - Rozek M., Garcia M. M. A., Lachowska D. C- banding patterns in chromosomes of four species of syrphid flies (Diptera, Syrphidae). Folia Biologia Krakow. 1995;43:107-109.
- Rozek M., Lachowska D., Marcos Garcia M. A. The C- banded karyotypes of Syrphus corolla Fabr and Sphaerophoria scripta (L.) (Diptera, Syrphidae). Folia Biologica Krakow. 1996;44:11-13.

This work is licensed under a Creative Commons Attribution 4.0 International License.





