Manuscript accepted on : 15-Sep-2018
Published online on: 25-09-2018
Plagiarism Check: Yes
S. Irudaya Monisha and J. Rosaline Vimala
and J. Rosaline Vimala
Research Department of Chemistry, Holy Cross College, Affiliated to Bharathidasan University, Tiruchirapalli, Tamil Nadu - 620 002, India.
Corresponding Author E-mail: irudaya.monisha@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2677
ABSTRACT: Plants are made up of chemicals of differing nature produced by metabolism. These phytochemicals show varied biological activity. The present work was aimed to identify such active principle present in the stem bark of Manilkara hexandra (Roxb.)Dubard. Proximate, fluorescence, histochemical, mineral analysis was carried out for the plant material. From the preliminary analysis, the flavonoid rich extract was identified which was then subjected to using chromatographic methods for compound separation. Characterization of the separated compound was done by UV, FT-IR, and GC-MS studies. 7,9- DI-TERT-BUTYL-1-OXASPIRO[4.5]DECA-6,9-DIENE-2,8-DIONE was identified by GC-MS analysis. Antibacterial activity was carried and it shows high zone of inhibition, when concentration increases from 30 to 150 μg/ml. The separated flavanoid in future could be used to study its wide pharmacological applications as a lead compound in drug delivery.
KEYWORDS: Fluorescence; Histochemical; Manilkara Hexandra; Mineral Analysis Proximate
Download this article as:| Copy the following to cite this article: Monisha S. I, Vimala J. R. Extraction, Identification and Pharmacological Evaluation of Phyto-Active Compound in Manilkara Hexandra (Roxb.) Dubard Stem Bark. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Monisha S. I, Vimala J. R. Extraction, Identification and Pharmacological Evaluation of Phyto-Active Compound in Manilkara Hexandra (Roxb.) Dubard Stem Bark. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31070 |
Introduction
Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. This study describes the structure of primary and secondary metabolic products found in plants such as leaves, vegetables and roots that have defense mechanism and protects from various diseases. The medicinal plants are useful for healing, curing of human disease because of the presence of phytochemical constituents.1 A plant containing beneficial phytochemicals are alkaloids, terpenoids, saponin, and phenolic compounds such as flavanoids, tannins and lignins,2 found in plants which act as anti- inflammatory, anti-bacterial,3 may supplement the needs of the human body by acting as natural antioxidant.4 These phytochemicals, which are part of a large and varied group of chemical compounds, also are responsible for the color, flavor, and odor of plant foods, such as blueberries dark hue, broccoli’s bitter taste, and garlic’s pungent odor. Thousands of phytochemicals have been identified, and researchers speculate that there are likely many more they haven’t yet discovered in the foods we eat. Though the broadest groups of phytochemicals, such as flavonoids, isoflavones, or anthocyanidins, often are referred to as a homogenous group, the individual compounds within each group have different chemical structures, are metabolized differently by the body, and may have different health effects.5 Research strongly suggests that consuming foods rich in phytochemicals provide health benefits, but not enough information exists to make specific recommendations for phytochemical intake.6 Proximate and nutrient analyses of edible plant and vegetables play a crucial role in assessing their nutritional significance.7 In general, 22 nutrients are present in each human body for their requirements such as Fe, Ca, Mg, K, and Zn.8 The antimicrobial activities have shown that the higher plants represent a potential source of novel antibiotic prototypes.9 This has forced scientist to search for new antimicrobial substances from various sources like the medicinal plants.10 The stem bark of Manilkara hexandra was used to cure gastro intestinal problem, burning sensation, febrifuge, odontopathy, hallucination, dyspepsia, anthelmintic, astringent and tonic.11
Thus the present work was aimed to find out phytochemicals present in Manilkara hexandra through, proximate, histochemical, fluorescence, mineral analysis, separation and identification by using the thin layer and column chromatography, characterizing the identified phytoconstituents using analytical techniques such as UV-Visible spectra, FT-IR and GC-MS and evaluation of their antimicrobial activity.
Material and Methods
Collection of the Plant Material
Manilkara hexandra (Roxb.) Dubard ( Authentification Number : 00I1) was collected on December to February (Winter) season from Jayakondam at Ariyalur District, Tamilnadu, India and authentified by Rapinet Herbarium, St.Joseph’s College (Autonomous), Trichy. The stem bark was washed with running water, shade dried, and grained by pestle mortar.
Preparation of the Plant Extract
All the chemicals (AR) were acquired from Sigma Aldrich. The powdered stem bark material was defatted with hexane, petroleum ether, and ethyl acetate by cold percolation method for 3 days. This solution was extracted by utilizing Whatman No.1.filter paper and stored in refrigerator at 4°C.
Phytochemical Screening of this Plant
The phytocompounds present in methanolic extract was analyzed by using standard procedure.12
Physical Characterizations
Physiochemical Parameters
The physiochemical factors were analyzed by using WHO guidelines and Indian Pharmacopeia for herbal ingredients.13 These include total ash content, acid insoluble ash, water soluble ash, moisture content, sulfated ash, dry matter and pH.
Total ash
3g of the sample was weighed accurately in a silica crucible. Then, it was incinerated by using muffle furnace at a temperature 400-500°C. The carbon particle was dried till it turns white in color. The total ash was cooled and weighed.
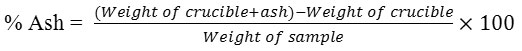
Moisture Content
3g of the sample was weighed in a crucible with lid. The sample was kept in hot air oven at 105°C and cooled by using desiccator. The successive results were obtained from the weight difference between before and after drying process.
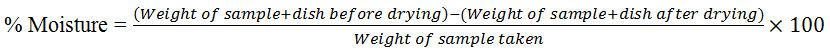
Acid insoluble ash
The above ash was boiled with 25 ml of dilute HCl and filtered by using Whatman No.1 filter paper. After filtration, this paper was placed and dried in a crucible to get an acid insoluble ash. Before and after, weight of crucible was calculated .
Water Soluble ash
The total ash (A) was boiled with 25 ml of distilled water in a crucible for 15 minutes at 450°C. This portion was filtered and the insoluble portion (B) was collected. Again, this insoluble part was dried and weighed. Calculate the water soluble ash from subtraction of A-B.
Sulfated ash
The total ash was ignited with Con. H2SO4 at 550°C to 650°C for 30 minutes and is then cooled using a desiccator until it reaches a constant weight.
Dry Matter
Dry matter of plant material consists of nutrition excluding the water content. It was obtained from percentage of moisture content.14
% Dry matter = 100 – % Moisture
pH
The stem bark powder was dissolved in distilled water and it was boiled for 25 minutes. Then, it was cooled and the filtrate was collected. Finally, the pH of the filtrate was noted by using a systronic pH meter.
Histochemical Analysis
The dried stem bark powder were kept on the slide and treated with 1-3 drops of newly prepared reagents such as phloroglucinal, concentrated HCl, dilute ammonia, sulfuric acid, mayer’s reagent (Mercuric chloride, potassium iodide, and water), ferric chloride solution, liberman (acetic anhydride and H2SO4 ), and toludine blue separately. The individual samples were prolonged and placed on stage in microscope to identify the phytoconstituents (Lignin, flavonoids, alkaloids, tannin, starch and steroids.15,16
Fluorescence Analysis
A pinch of stem bark powder was tested with hexane, chloroform, acetone, methanol, aqueous solvents and also added to chemical reagents such as sodium hydroxide in water, concentrated sulfuric acid, hydrochloric acid, nitric acid of one normality. The resultant specimens were examined under the presence of UV- Visible light of long and short wavelengths (365 – 254 nm).17
Mineral Analysis
Ash was examined precisely using Gravimetric method by ASTM.18 The stem bark of this plant was analyzed for organic content by the classical method of Walkey and Black.19 Total Nitrogen estimated by the method of Micro Kjeldhal which were still used extensively in agricultural research20. The Sulfur was measured using Gravimetric method.21 Total Phosphorous was determined by Pemberton (1927) method.22 For sodium, potassium, calcium and magnesium the flame intensity corresponding to the concentration of stock solution was noted using appropriate filters in Flame Photometry (Systronics mediflame 127) by the method of Stanford and English method.23 Total Zinc, Total Copper, Total Iron, Total Manganese, Total Boron, Total Molybdenum is analyzed by using Atomic Absorption Spectroscopy (Solar-AAS2-UK made). Perchloric-acid digestion method was used for elemental analysis.24
Separation Techniques
Column Chromatography
The column apparatus (15×4 cm) was washed with water and rinsed by using solvents. A small piece of cotton was inserted into the lower part of the column and is clamped to a stand. The silica gel (60-120 mesh) was activated in the oven at 120°C for 1 hour. The slurry of silica gel (100-150g) was prepared, packed into the column and digested with petroleum ether (60°C-80°C). The ethyl acetate extract was mixed with the above slurry of silica gel and the following eluents were successively run out starting from hexane, chloroform, ethyl acetate, methanol in their increasing order of polarity. Finally, flavonoids were isolated using an eluent mixture in the ratio 7:3 (MeOH: Water) and the fraction were collected in test tubes.25
Thin Layer Chromatography
The precoated TLC Silica gel 60 F254 on aluminium sheets (20×20 cm), were purchased from Merck. The isolated fractions were spotted by using a capillary tube on the cut plate (Stationary phase) (6.5×3.5). The spotted strip were dried and immersed into the developing eluents (Mobile phase) of system (n-Butanol, acetic acid and water (4:1:5)). Then, it was covered with a lid. Finally the four fraction plate was dried and retention factor of that chromatogram was noted using the following formula,26
![]()
Confirmatory Tests for Flavonoid
The presence of flavonoid in the fractions was confirmed by sprinkling NH3.
The presence of flavonoid was also confirmed by adding NaOH.
Characterization
UV-Visible Spectral Analysis
Lambda 35 UV-Visible spectrophotometer which is a PC controlled, double beam scanning spectrophotometer is a useful analytical technique to identify some functional groups in molecules thus aiding in structural elucidation in bio-molecules. It covers the wavelength range 200 nm to 800 nm with a setting accuracy of ± 1nm.
FT-IR Spectral Analysis
IR spectra of plant substance can be taken in an automatic recording IR spectrophotometer either in solution (in chloroform or carbon tetrachloride 1-5%) as a mull with nujol oil or in the solid state mixed with potassium bromide. In the latter case a thin disc is prepared under anhydrous condition from a powder containing about 1mg of material and 10 to 100mg KBr using mould and press. The range of measurement is from 4000 to 667cm-1 and the spectrum takes about three minutes to be recorded.
GC-MS Analysis
The GC-MS analysis was performed on a combined GC-MS instrument (ITQ 900 Model of Thermo Fisher Scientific make) using a HP-5 fused silica gel capillary column. The method to perform the analysis was designed for both GC and MS. 1 μL aliquot of the sample was injected into the column using a PTV (Programmed Temperature Vaporizing ) injector whose temperature was set at 275°C. The GC program was initiated by a column temperature set at 60°C for 5 min, increased to 300°C at a rate of 8 C/min, held for 10 min. Helium was used as the carrier gas (1.5 mL/min). The mass spectrometer was operated in EI(Electron ionization) mode with mass source set at 200°C. The chromatogram and spectrum of the peaks were visualized. The particular compounds present in the samples were identified by matching their mass spectral fragmentation patterns of the respective peaks in the chromatogram with those stored in the National Institute of Standards And Technology Mass Spectral database (NIST-MS, 1998) library.27
Antibacterial Assay
Collection of Test Organisms
To examine the antibacterial activity of methanol extract, two gram positive bacterial strains viz., Staphylococcus aureus (MTCC 25923) and Bacillus subtilis (MTCC 2451) respectively. One gram negative bacterial strain Escherichia coli (MTCC 25922) were prepared as test organisms. All the strains were procured from the Microbial Type Culture and Collection (MTCC) at Chandigarh, India. Bacterial strains were cultivated at 37°C and maintained on nutrient agar (Difco, USA) slant at for 4°C.
Screening of Antibacterial Activities
Antibacterial Activity of Methanol Extracts (Disc Diffusion Method)
Antibacterial activity of methanol: water (7:3) extract was determined using the disc diffusion method. The petri dishes (diameter 60 mm) were prepared with Muller Hinton Agar and inoculated with test organisms. Sterile disc of six millimeter width was impregnated with 10 μl of sample A at various concentrations of 30-150 μg/ml respectively. Prepared discs were placed onto the top layer of the agar plates and left for 30 minutes at room temperature for compound diffusion. Negative control was prepared using the respective solvent. The dishes were incubated for 24 h at 37°C and the zone of inhibition was recorded in millimetres.28
Result and Discussions
Physical Characterizations
Phytochemical Screening
The plant extract was subjected to phytochemical screening using standard procedures. The result reveals the presence of alkaloids, flavonoids, terpenoids, saponin, and steroids as shown in Table 1.
Table 1: Phytochemical Screening for stem bark methanolic extract of this plant.
| S.No | Phytocompounds | Observations |
| 1. | Alkaloids | ++ |
| 2. | Flavonoids | ++ |
| 3. | Terpenoids | + |
| 4. | Saponin | ++ |
| 5. | Steroids | ++ |
+ = Good ++ = Very Good
Physiochemical Parameters
Today essential chemical evaluation of physiochemical parameters such as total ash, moisture, acid insoluble ash, water soluble ash, sulfated ash, dry matter and pH is needed for the detection of adulterants and get a fix on drugs. The physiochemical parameters reported in the Table 2 shows the high presence of total ash (5.84 ± 0.15) in this plant indicates the presence of diverse minerals. Acid insoluble ash value (0.35±0.65), indicates the evidence for presence of silica and the sulfated ash(7.47±0.09) reveals that the presence inorganic substance in organic materials. The water soluble ash (3.76±0.12) determines the quantity of inorganic substances present in the stem bark of Manilkara hexandra. The pH of the sample is found to be 5.71.
Table 2: Physiochemical parameters of stem bark of Manilkara hexandra.
| S.No | Proximate Tests | Stem bark |
| 1 | Total ash | 5.89±0.15 |
| 2 | Moisture content | 6.25±0.07 |
| 3 | Acid insoluble ash | 0.35±0.65 |
| 4 | Water soluble ash | 3.76±0.12 |
| 5 | Sulphated ash | 7.47±0.09 |
| 6 | Dry Matter | 93.29±0.15 |
| 7 | pH | 5.71±0.19 |
Mean value ± standard deviation
Histochemical Analysis
By observing the variation of intensity in color viz; lignin (pink),flavonoids (yellow), alkaloid (reddish brown), steroid (green), tannins (black) and starch (blue) by histochemical analysis it is possible to categorize the quantitative presence of these metabolites in the stem bark as shown in Table 3 and Figure 1.
Table 3: Histochemical analysis for stem bark of Manilkara hexandra.
| S.No. | Test Reagents | Observation | Characterization | Stem bark |
| 1. | Phloroglucinol + concentrate HCl | Pink | Lignin | ++ |
| 2. | Dilute Ammonia + H2SO4 | Yellow | Flavonoids | ++ |
| 3. | Mayers’ Reagent | Reddish rown | Alkaloids | ++ |
| 4. | FeCl3 Solution | Dark Blue to Black | Tannin | ++ |
| 5. | Iodine | Blue | Starch grain | ++ |
| 6. | Liberman (5 drops of acetic anhydride + 5 drops of H2SO4 | Green | Steroids | + |
 |
Figure 1: Image of histochemical analysis.
|
Fluorescence Analysis of Stem Bark of Manilkara Hexandra
Fluorescence analysis is carried out to confirm the presence of secondary metabolites by examining under the presence of UV-Visible light of long and short wavelengths (365-254 nm). Their results are as mentioned in Table 4 and Figure 2.
Table 4: Observation of Fluorescence report for stem bark of Manilkara hexandra.
| S.No. | Chemicals | Visible Light | Short UV (254nm) | Long UV (365 nm) |
| 1 | Plant powder (PP) | Brown | Black | Black |
| 2 | Plant powder with water | Dark Green | Dark Green | Brown |
| 3 | PP with Hexane | Reddish Brown | Dark Brown | Dark Brown |
| 4 | PP with Chloroform | Black | Black | Black |
| 5 | PP with Methanol | Brown | Black | Black |
| 6 | PP with Acetone | Brown | Black | Black |
| 7 | PP with 1 N Sodium hydroxide in water | Brown | Green | Reddish Brown |
| 8 | PP with 1 N Hydrochloric acid | Light Green | Blackish Green | Dark Brown |
| 9 | PP with 1 NSulphuric acid | Dark Green | Blackish Green | Dark Brown |
| 10 | PP with1 N Nitric acid | Dark Green | Blackish Green | Dark Brown |
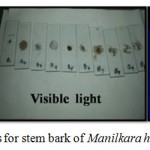 |
Figure 2: Fluorescence photos for stem bark of Manilkara hexandra.
|
Mineral Analysis
The impact of minerals plays an important role in our body. This report declares that the iron, calcium, magnesium, potassium, zinc, nitrogen were present in rich and other elements were present in trace amounts as shown in Table 5.
Table 5: Minerals consist in the stem bark of Manilkara hexandra.
| S.No | Name of the parameter | Obtained value | Benefits of the element |
| 1 | Organic Carbon (%) | 0.91 | To regulate the pH and acid balance29. |
| 2 | Total Sodium (%) | 0.16 | To control muscle contraction andinvolved in blood plasma, nervous system, digestive processes. |
| 3 | Total Potassium (%) | 4.54 | To maintain the body fluid and solve the renal problems30. |
| 4 | Total Phosphorus (%) | 0.32 | To filter the impurities of kidney, refresh of tissues and cells in the body. |
| 5 | Total Magnesium (%) | 4.31 | To use in dietary disorders. |
| 6 | Total Calcium (%) | 6.57 | To apply in teeth, bones, and nerves31. |
| 7 | Total Nitrogen (%) | 1.80 | To use in the digestion process |
| 8 | Total Sulfur (%) | 0.44 | To synthesized protein and involved in enzyme reactions32. |
| 9 | Total Copper (ppm) | 0.05 | It involves in a metabolic process and also maintains the strength blood vessels33. |
| 10 | Total Zinc (ppm) | 1.65 | It acts as a boosting agent of geriatric patients34. |
| 11 | Total Boron (ppm) | 0.042 | To improve bone and apply in wound healing, |
| 12 | Total olybdenum (ppm) | 0.02 | To retard the degradation of teeth enamel35. |
| 13 | Total Manganese (ppm) | 10.41 | To use in Dietary recommendations |
| 14 | Total Iron (ppm) | 129.51 | To increase the hemoglobin and develop the immune system36. |
Identification and Separation of Phytocomponents
Chromatographic Analysis
Column Chromatography
Column chromatography is commonly utilized for the purification of bioactive compounds. In this work, the methanolic stem bark extract of Manilkara hexandra was eluted with low polar to high polar eluents . The fractions eluted from petroleum ether, ethyl acetate, methanol and methanol: water (7:3) was collected as shown in Figure 3a,and b.
 |
Figure 3a: Collection of fractions (petroleum ether, ethyl acetate, methanol and methanol:water) b) Separation process in coloumn.
|
Rentention Factor (Rf)
The compounds identified from various solvent fractions (pet.ether, ethyl acetate, methanol, and methanol:water) spotted on TLC plate are shown in Table 6. The Rf values obtained were compared with literature data and it is inferred that most of them are flavonoids in nature.
Table 6: Rf value of flavonoids.
| S.No | Fractions | Rf | Compound |
| 1. | Petroleum ether | 0.62
0.82 |
Chalcones
Kaempferol |
| 2. | Ethyl acetate | 0.03
0.51 0.89 |
Unidentified
Apegenin Flavonones |
| 3. | Methanol | 0.03
0.68 |
Unidentified
Chalcones |
| 4. | Methanol: Water | 0.96 | Flavonoids (Quercetin derivatives) |
Thin Layer Chromatography
The methanol: water (7:3) fraction is spotted on a TLC plate to find out retention factor (Rf) using the solvent system, n- butanol:Acetic acid:Water (4:1:5). The methanol: water (7:3) gave single spot at (Rf = 0.96), which indicates that it might be due to the presence of the flavonoid. The presence of flavonoid is further confirmed qualitatively from the following analysis.
It gave yellow color on reaction with NaOH, which decolorized on the addition of HCl (Alkaline reagent test) Figure 4a, and b.
The compound turned from greenish yellow to deep yellow by spraying ammonia for above fractions. (Figure 4c,d and e).
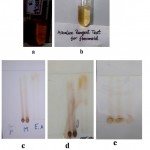 |
Figure 4: a) Addition of NaOH b) Addition of Hcl c) Before spraying ammonia d) After spraying ammonia e) Methanol: water (7:3).
|
Spectral Analysis
UV- Visible Spectroscopy
The UV – Visible spectra suggested that the absorbance of a compound at λ max= 318 nm, 284 nm and 231 nm is a flavonoid (quercetin), as shown in Figure 5.
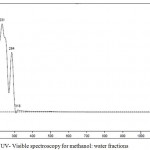 |
Figure 5: UV- Visible spectroscopy for methanol: water fractions.
|
FT-Infrared Spectroscopy
From the occurrence of peaks at 3319 cm-1, 2947-2835 cm-1, 1653 cm-1, 1452 cm-1, 1409 cm-1,1112 cm-1, 1030 cm-1 at 3319 cm-1suggested and further confirmed the presence of the flavonoid as shown in Figure 6.
 |
Figure 6: FT-IR spectrum of isolated flavonoid fraction on stem bark of Manilkara hexandra.
|
GC-MS Analysis
The GC-MS spectra reveals that the presence of flavonoid compound is 7,9- DI-TERT-BUTYL-1-OXASPIRO[4.5]DECA-6,9-DIENE-2,8-DIONE. The flavonoid compound appears at 14 minutes, (RT). Along with this, five major compounds were obtained and it was identified from NIST and WILEY as shown in Table 7 and Figure 7.
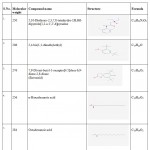 |
Table 7: GC-MS report of isolated compound from TLC.
|
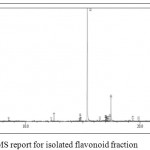 |
Figure 7: GC-MS report for isolated flavonoid fraction.
|
Antibacterial Activity
The results of the antibacterial activity of methanol extracts were tested against pathogens by disk diffusion method are shown in Table 8 and Figure 8. The sample showed growth inhibitory activity against Staphylococcus aureus (8 mm) at concentration 150 μg/ml and against Bacillus ubtilis (7 mm). At concentration 120 μg/ml, the sample exhibited the antibacterial activity against, Staphylococcus aureus (7 mm). However, the sample showed better inhibitory actions against pathogens at a concentration 90, 120 and 150 μg/ml than at lower concentration. As the concentration of extracts increased from 30-150 μg/ml, the inhibitory actions of the plant extracts increased towards all the strains used in this study.
Table 8: Antibacterial report for isolated flavonoid fraction.
| Plant extracts | Concentrations (µg/ml) | Organisms/Zone of inhibition (mm) | ||
| Methanolic extracts | ||||
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | ||
| Extracts | 30
50 90 120 150 |
0
0 0 7 8 |
0
0 0 6 7 |
0
0 0 6 6 |
| Methanol | 10 µl/disc | 0 | 0 | 0 |
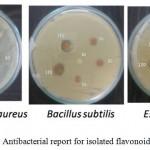 |
Figure 8: Antibacterial report for isolated flavonoid fraction.
|
Conclusion
From the present investigation the following conclusion can be drawn. Biologically active phytochemicals such as alkaloids, flavanoids, saponins and steroids are present in the methanolic extract of Manilkara hexandra by carrying out phytochemical analysis. Histochemical and Fluorescent analysis further confirms their presence. Separation and identification of the isolated compound done by using chromatographic techniques such as Column chromatography and Thin layer chromatography using the subsequent eluting method made ease to separate active flavanoids (quercetin derivative) from the methanol:water fraction confirmed by GC-MS analysis also. This was further confirmed using spectral tools such as UV-Visible spectroscopy and FT-IR. Good antimicrobial activity was also found in the separated compound with increase in the concentrations. Thus, this study is only a preliminary study pertaining to the occurrence of certain properties of Manilkara hexandra. An in-depth study will provide a concrete base for all the phytochemical functions exhibited by this plant used in traditional medicine.
References
- Nostro A., Germanò M. P., D’Angelo V., Marino A., Cannatelli M. A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Appl. Microbiol. 2000;30:379-384.
CrossRef - Krishnaiah D., Sarbatly R., Bono A. Phytochemical antioxidants for health and medicine A move towards nature. Mol. Biol. Rev. 2007;1(4):97-104.
- Mahato S. B., Sen S. Advances in Triter penoid research 1990-1994. Phytochemistry. 1997;44(7):1185-1236.
CrossRef - Kappers I. F., Aharoni A., Herpen T. V. W., Luckerhoff L. L., Dicke M., Bouwmeester H. J. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsi. Science. 2005;309:2070-2072.
CrossRef - Erdman J. W.,Balentine D., Arab L., Beecher G., Dwyer J. T., Folts J., Harnly J., Hollman P., Keen C. L., Mazza G., Messina M., Scalbert A., Vita J., Williamson G., Burrowes J. Flavonoids and heart health: Proceedings of the ILSI North America Flavonoids Workshop. Nutr. 2007;137:718-737.
CrossRef - Webb D. Phytochemicals role in good health. Today dietitian. 2013;15:70.
- Pandey M., Abidi A. B., Singh S., Singh R. P. Nutritional Evaluation of Leafy Vegetable Paratha . Hum. Ecol. 2006;19(2):155-156.
CrossRef - Welch R. M., Graham R. D. Breeding for micro nutrients in staple food crops from a human nutrition perspective. Exp. Bot.2004;55(396):353-64.
CrossRef - Afolayan A. J. Extracts from the shoots of Arctotis artotoides inhibit the growth of bacteria and fungi. Biol. 2003;41:22-25.
- Wu.M. W., Duncan A. R., Okunji C. O. J. ASHA Press Alexandra V.A. 1999;457-462.
- Mishra N., Pareek A. Traditional Uses Phytochemistry and Pharmacology of Mimu sops hexandra Roxb. Adv. Pharm. Ethnomed. 2014;2(2):32–35.
CrossRef - Monisha S. I., Dayana G., Immaculate A. A., Vimala R. J. Comparative studies on yield and the phytochemical appraisal (Quality and Quantity) of Manilkara hexandra (Roxb) Dubard using leaf stem and bark. JPP. 2017; 6(4):2052-2058.
- I. T. B. S. Publishers Quality Control Methods for Medicinal Plant Materials, Anauthorized publication of WHO. Geneva. 2002.
- Nancy J. T., Erem T. V. Determination of Water (Moisture) and Dry Matter in Animal Feed, Grain, and Forage (Plant Tissue) by Karl Fischer Titration: Collaborative Study. AOAC Int. 2002;85(2):318-327.
- Savithramma N., Rao L. M., Venkateswarlu P. Histo chemical Studies of Boswellia ovalifoliolata Bal. & Henry-An Endemic Endangered and Threatened Medicinal Plant of Seshachalam Hill Range of Eastern Ghats of India. IJPPR. 2014;6(1):1-6.
- Asokan J. Botanical micro technique principles and practice. Sky Graphics. 2006;14-20.
- Selvam A. B. D., Bandyopadhyay S. Fluorescence Analysis on the Roots of Rauvolfia Serpentina (L.). Benth. Ex Kurz under UV Radiation. Ancient Science of Life. 2005;24(4):164-167.
- ASTM C. 138. Standard test Method for unit weight yield and air content ( Gravimetric) of concrete. Annual book of ASTM standards. 1988;4(02):80-82.
- Walkley A., Black I. A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29-37.
CrossRef - Bremner J. M. Total Nitrogen. In: Methods of soil analysis (Black CA, ed). Part 2: Chemical and microbial properties. Number 9 in series Agronomy. American Society of Agronomy Inc. Publisher, Madison, USA. 1965;1049-1178.
- Ehyaei A. M. The soil chemical analysis methods, Publication no. 1024, Research Institute of Soil and water. 1997.
- Pemberton H. The determination of phosphoric acid by the titration of the yellow precipitate with standard alkali. Amer. Chem. Soc. 1893;15:382-395.
CrossRef - Stanford S., English L. Use of flame photometer in rapid soil tests for K and Ca. J. 1949; 4:446.
- Allen S. E., Grimshaw H. M., Parkinson J. A., Quarmby C. Chemical analysis of ecological materials. Oxford: Blackwell Scientific. 1974.
- Harbone J. B. Phytochemical methods a guide to modern techniques of plant analysis 2 nd edition, London newyork.
- Intekhab J., Aslam M. Isolation of a flavonoid from the root of citirus sinensis, malassiyan J. Pharm. Sci. 2009;7(1):1-8.
- Rani P. V., Moorthy P. B. B., Priya K. S., Nancy A. A., Kumari M. Phytochemical Screening and GC-MS Analysis in Wild Variety of Coccinia indica – An Future Promising Therapeutic Source. AJPCT. 2016;4(04):098-105.
- Kowti R., Harsha R., Gulzar M. A., Hareesh A. R., Gowda T. S. S., Dinesha R., Kumar S. B. P., Ali I. M. Antimicrobial activity of ethanol extract of leaf and flower of spathodea campanulatap. Beauv. RJPBCS. 2010;1:691-698.
- Wattoo F. H., Tirmizi S. A., Wattoo M. H. S., Anjum A., Iqbal J., Ghanghro A. B. Analytical investigation of selected inorganic nutrients of desert growing aloes. Chem. Soc. Pak. 2008;30(3):394-9.
- Haas E. M.: Role of potassium in maintaining health. Period Paral Inter [Online] Available from https://hkpp.org/patients/potassium-health. 2011.
- Charles P. Calcium absorption and calcium bioavailability. Int. Med. 1992;231(2):161-5.
CrossRef - Chouhan F., Wattoo M. H. S., Tirmizi S. A., Menon F. Z., Rahman A., Tufail M. Analytical investigation of inorganic nutritive elements of Capparis decidua grown in Cholistan desert. 2002;39(3–4):95-9.
- Stern B. R. Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. Toxicol. Environ . Health A. 2010;73(2):114-27.
CrossRef - Haase H., Mocchegiani E., Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–8.
CrossRef - Curzon M. E. J., Kubota J., Bibby B. G. Environmental Effects of Molybdenum on Caries. Dent. Res. 1971;50(1):74–77.
CrossRef - Ullah R., Khader J. A., Hussain I., Abd-Elsalam N. M., Talha M., Khan N. Investigation of macro and micro-nutrients in selected medicinal plants. J. Pharm Pharmacol. 2012;6(25):1829-32.

This work is licensed under a Creative Commons Attribution 4.0 International License.





