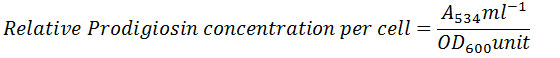How to Cite | Publication History | PlumX Article Matrix
Influence of Linoleic acid on Quorum Sensing in Proteus Mirabilis and Serratia Marcescens
Kirti Marathe , Sunita Bundale, Nandita Nashikkar and Avinash Upadhyay
, Sunita Bundale, Nandita Nashikkar and Avinash Upadhyay
Hislop school of biotechnology, Hislop College, Temple Road, Civil Lines Nagpur, 440001, India.
Corresponding Author E-mail: kirtidubli@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2674
ABSTRACT: Quorum sensing (QS) is a bacterial cell density dependent mode of communication involved in regulation of virulence in pathogens including biofilm formation. Accordingly, curbing QS might prove to be an anti-virulence approach of controlling nosocomial infections caused by multi drug resistant bacteria. The report presented here documents the QS inhibitory properties of linoleic acid against Proteus mirabilis and Serratia marcescens known to cause nosocomial infections. Urease assay, prodigiosin assay, protease assay, biofilm formation assay and growth curve analysis were performed to investigate the effectiveness of linoleic acid in controlling virulence of P. mirabilis and S. marcescens. 2.5mM linoleic acid reduced the urease activity and biofilm formation to 42.11% and 11.11% respectively in P. mirabilis; and prodigiosin synthesis, protease activity and biofilm formation to 0%, 65.91% and 33.33% correspondingly in S. marcescens. Therefore, analysis of QS inhibitory behaviour of linoleic acid substantiates its use as a plausible drug for anti-virulence therapy without subjecting the bacteria to discerning force of antibiotics.
KEYWORDS: Biofilm; Linoleic Acid; P. Mirabilis; Marcescens; Quorum Sensing (QS); S.Virulence
Download this article as:| Copy the following to cite this article: Marathe K, Bundale S, Nashikkar N. Upadhyay A. Influence of Linoleic acid on Quorum Sensing in Proteus Mirabilis and Serratia Marcescens. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Marathe K, Bundale S, Nashikkar N. Upadhyay A. Influence of Linoleic acid on Quorum Sensing in Proteus Mirabilis and Serratia Marcescens. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=30891 |
Introduction
Communication among cells is a quintessential process synchronising assorted functions of higher organisms. Similar interaction also occurs amongst bacteria and is dependent on bacterial cell density. It is referred to as quorum sensing (QS). QS accounts for orchestrating bacterial functions and gene expression1 and is mediated by small diffusible molecules, autoinducers. The nature of these autoinducers is oligopeptides in gram-positive bacteria and N-Acyl Homoserine Lactones (AHLs) in gram- negative bacteria.2 Each bacterium produces autoinducers in small amounts. As the bacterial cell number increases so does the concentration of these autoinducers, thus reaching a threshold value. Subsequently, autoinducers bind to receptors on bacterial cell and trigger expression of certain genes while repressing other genes.3 This behaviour helps in biofilm formation, horizontal gene transfer, protease and exoenzyme antibiotic synthesis.
Proteus mirabilis and Serratia marcescens are gram-negative opportunistic pathogens causing nosocomial infections. P. mirabilis is primarily found associated with urinary tract infections, characterised by swarm migration.4 P. mirabilis forms biofilm in the host organism besides synthesising urease, hemolysin and other virulence factors.5 S. marcescens frequently appears as nosocomial pathogen causing infections of wound, respiratory tract and urinary tract. This bacterium produces virulence factors like protease and prodigiosin and forms biofilm. Besides, this bacterium exhibits swimming and swarming motility.6 Secretion of virulence factors and biofilm formation, under the control of QS facilitates bacteria to successfully establish infection in the host. Present technique of dealing with bacterial infection involves use of antibiotics that are lethal to bacteria, generating selective pressure that results in emergence of drug resistant strains.7 Thus, impeding QS would be a favourable method of dealing with bacterial infections caused by multi-drug resistant (MDR) bacteria without causing selective pressure on them.
Fatty acids (saturated and unsaturated) and their derivatives have been reported to possess antimicrobial properties; specifically fatty acids containing two double bonds, like linoleic acid, is more potent bacteriostatic compound.8 Moreover, conjugated linoleic acid has also been described to inhibit bacterial growth through lipid peroxidation in the membranes.9 Recent studies made on the effect of conjugated linoleic acid on colon, has indicated its ability to offset the development of inflammatory lesions.10 Linoleic acid and fatty acids like oleic acid, palmitic acid and stearic acid are capable of inhibiting autoinducer -2 (AI-2) activity consecutively affecting QS.11 Considerable work has been done on antimicrobial nature of linoleic acid but very little work has been accomplished to scrutinize its QS inhibitory activity. Hence, analysis made in this report deals with QS inhibitory effect of linoleic acid on S. marcescens and P. mirabilis.
Materials and Methods
Linoleic acid, free acid (C18H32O2, CAS no. 60-33-3) was purchased from Himedia Laboratories, India. 357mM Stock solution was prepared by dissolving linoleic acid in DMSO and stored at 4⁰C. Different concentrations of linoleic acid were tested against P. mirabilis and S. marcescens based on earlier made reports.11,12 Concentrations of 1.0mM to 2.5mM of linoleic acid were found to be non – inhibitory and hence were used for further assays.
Bacterial Strains and Growth Conditions
S. marcescens was obtained from Vishakha Labs Pvt. Ltd.,Nagpur and P. mirabilis was obtained from Institute of Microbial Technology (IMTech), Chandigarh. The bacteria were grown and maintained on nutrient agar (peptone 5g/l, sodium chloride 5g/l, beef extract 1.5g/l, yeast extract 1.5g/l, agar 15g/l and pH 7.4±0.2) slants and stored at 4⁰C till further use.
Urease Assay (for P. mirabilis)
Effect of linoleic acid on urease activity of P. mirabilis was ascertained by quantifying the amount of hydrolysed urea in urea – Luria Bertani (LB) broth.13 Sterile LB broth (casein enzyme hydrolysate 10g/l, yeast extract 5g/l, sodium chloride 10g/l and pH 7.5±0.2) tubes containing urea were prepared. Different concentrations of linoleic acid (1mM, 1.5mM, 2mM and 2.5mM) were added to these tubes. 1% overnight broth culture was inoculated in these tubes and incubated for 48 hours at 37⁰C. After incubation, amount of hydrolysed urea was determined by comparing with a suitable control.
Prodigiosin Assay (for S. Marcescens)
Overnight culture of S. marcescens was used for development of assay. 1% of broth culture was inoculated in sterile nutrient broth tubes containing different concentrations of linoleic acid (1.0mM – 2.5mM) and incubated overnight. After incubation the cells were pelleted by centrifugation at 10,000 rpm for 10 minutes and resuspended in acidified ethanol solution (96ml of ethanol containing 4% of 1M HCl) for extraction of prodigiosin. The prodigiosin extracted was determined by reading absorbance at 534nm.14,15 The relative prodigiosin concentration was determined using the following equation:
Protease assay (for S. Marcescens)
Effect of linoleic acid on protease synthesis was analysed using skimmed milk agar assay.16 S. marcescens was inoculated in sterile broth tubes containing different concentrations of linoleic acid (1.0mM – 2.5mM) and incubated overnight. After incubation, cells were pelleted by centrifugation at 10,000rpm for 15 minutes. The supernatant obtained was loaded into wells of skimmed milk agar (skimmed milk powder 28g/l, casein enzyme hydrolysate 5g/l, yeast extract 2.5g/l, dextrose 1g/l, agar 15g/l and pH 7.0±0.2) plates. Plates were incubated overnight at 37⁰C and observed for zone of clearance using suitable control.
Biofilm Formation Assay
Influence of linoleic acid on biofilm formation ability of P. mirabilis and S. marcescens was investigated using crystal violet assay.15 1% of overnight broth culture (OD600 = 0.4) was inoculated in LB broth and 1 ml of freshly inoculated broth was then transferred into wells of microtiter plate. Different concentrations of linoleic acid (1.0mM – 2.5mM) were then added to these wells and incubated overnight. After incubation, planktonic cells were removed from the wells by washing twice with sterile water and biofilm was stained with 0.4% crystal violet. Excess of stain was removed by washing with sterile water. Crystal violet bound cells were solubilised using 1ml of ethanol and absorbance was read at 570nm.
Microscopic Analysis
Microscopic analysis of biofilm formation by P. mirabilis and S. marcescens in presence of linoleic acid was studied using air-liquid interface assay as described by Merritt et. al.17 0.1% inoculum was added to sterile LB broth and 250μl of this freshly inoculated broth was transferred to wells of microtiter plate placed at an angle of 30 – 50⁰. 50μl of different concentrations of linoleic acid (1.0mM – 2.5mM) were then added to these wells and incubated overnight in the angled position. Then the spent medium was removed carefully followed by washing twice with sterile medium. 200μl of sterile medium was then added to each of the wells and plate was gradually lowered on the flat surface of inverted microscope and cells of biofilm were visualised.
Growth Curve Assay
Effect of linoleic acid on quorum sensing was studied using growth curve assay described by Hall et. al. with few modifications.18 Sterile nutrient broth tubes containing different concentrations of linoleic acid were inoculated with 0.1% overnight culture of P. mirabilis and S. marcescens. 100ml of this freshly inoculated broth of each tube was transferred to different wells of microtiter plate. The plates were covered and immediately the absorbance of the plate was read at 600nm using plate reader. The plates were then transferred to shaker incubator and OD600 was read at 2, 4, 6, 8 and 24 hours and compared with control.
Statistical Analysis
All the experiments were performed in triplicates and the results are expressed in the form of mean ± standard deviation (SD). Statistical significance was determined by Student’s t-test using Microsoft Excel (Version 16.4.1) and p value < 0.05 was considered to be significant.
Results and Discussion
QS is a bacterial communication synchronizing biofilm formation and virulence factor synthesis.19 Work presented here documents the influence of linoleic acid on formation of biofilm and production of virulence factors in P. mirabilis and S. marcescens.
Urease activity of P. mirabilis decreased with increasing concentration of linoleic acid. While, urease activity was marginally affected at 1.0mM of linoleic acid, highest inhibition was seen at 2.5mM linoleic acid, where the activity of urease was reduced to 40.21% with respect to control. Urease activity and its percentage inhibition in presence of linoleic acid are presented in Table 1. The effect of linoleic acid on urease activity is graphically represented in Fig 1. Previous studies of napthoquinones from plant Diospyrus lotus,20 allicin from garlic,21 curcumin22 and fluoroquinolones23 have reported inhibition of urease activity in P. mirabilis. Urease enzyme containing nickel ions, a major virulence factor produced by P. mirabilis, hydrolyses urea to yield ammonia.24 The products of urease action increase pH of urine and eventually initiate formation of struvites or apatites in bladder or kidney. Moreover, this increased pH is also toxic to host cells.25 Thus, interfering with or reducing urease activity could possibly decrease virulence of pathogen in turn suggesting a possible role for linoleic acid in clinical applications.
Table 1: Effect of Linoleic acid on Urease Activity of Proteus Mirabilis.
| Concentration of Linoleic acid (mM) | Urea remaining in reaction mixture after incubation (10-3 μmoles)
Mean ± SD |
Percentage of Urease Activity |
| Control | 7.6±2.82 | 100.00 |
| 1.0 | 8.4±0.14 | 90.48 |
| 1.5 | 9.7±1.05 | 78.35 |
| 2.0 | 11.2±0.14 | 67.86 |
| 2.5 | 18.9±0.28* | 40.21 |
Experiments were performed in triplicates and *p<0.02.
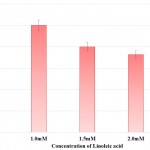 |
Figure 1: Effect of Linoleic acid on Urease activity of P. mirabilis. |
Experiments were performed in triplicates and *p<0.02 with respect to control.
Prodigiosin synthesis in S. marcescens was drastically reduced in presence of linoleic acid. Synthesis of prodigiosin was observed to be nil at 2.0mM and 2.5mM of linoleic acid as perceived from Table 2 and Fig 2. 1.0mM and 1.5mM of linoleic acid decreased prodigiosin synthesis to 67.98% and 21.91% in S. marcescens. Earlier studies made using petroselinic acid26 and ambroxol6 have demonstrated significant reduction of prodigiosin synthesis in S. marcescens. However, linoleic acid used in the present study was successfully able to inhibit prodigiosin synthesis. Synthesis of prodigiosin in S. marcescens is a QS controlled function and restraining its synthesis completely by linoleic acid highlights the effectivity of molecule in disrupting QS system of bacterium.
Table 2: Effect of Linoleic acid on Prodigiosin Synthesis and Protease Synthesis of S. Marcescens.
| Concentration of Linoleic acid (mM) | Prodigiosin Synthesis | Protease Synthesis | ||
| Relative Prodigiosin Synthesis
Mean ± SD |
Percentage of Prodigiosin Synthesis | Zone of Clearance (cm)
Mean ± SD |
Percentage of Protease Activity | |
| Control | 0.09±0.03 | 100.00 | 2.20±0.10 | 100.00 |
| 1.0 | 0.06±0.02 | 67.98 | 1.70±0.07# | 77.27 |
| 1.5 | 0.02±0.02* | 21.91 | 1.58±0.04 | 71.82 |
| 2.0 | 0.00±0.00^ | 0.00 | 1.50±0.10$ | 68.18 |
| 2.5 | 0.00±0.00^ | 0.00 | 1.45±0.05& | 65.91 |
Experiments were performed in triplicate and *p<0.04, ^p<0.01, #p<0.005, $p<0.003 and &p<0.001
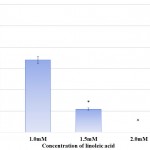 |
Figure 2: Effect of Linoleic acid on Prodigiosin synthesis of S. marcescens. |
Error bars indicate that experiments were performed in triplicates; *p<0.04 and ^p<0.01.
Protease synthesis in S. marcescens was affected in a dose dependent manner in presence of linoleic acid. The protease synthesis was gradually reduced from 77.27% to 65.91% in presence of 1.0mM and 2.5mM linoleic acid respectively. Effect of linoleic acid on protease activity of S. marcescens is depicted in Table 2 and Fig 3. Inhibition of protease activity in S. marcescens, an important virulence factor regulated by QS, has also been described in previous analysis carried out using phenol, 2,4-bis(1,1-dimethylethyl),27 phytol from Piper betle,28 α-Bisabolol from Padina gymnospora29 and vanillic acid from Actinidia deliciosa.30 Proteases secreted by S. marcescens influence its pathogenicity by degrading various secreted proteins of host like immunoglobulins. Thus, protease synthesis benefits the bacterium by evading defence mechanism and hence establishing infection.31 Consequently, decreased protease synthesis by S. marcescens in presence of linoleic acid might serve as a useful tool for controlling pathogenicity and curbing the infection.
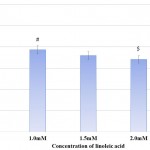 |
Figure 3: Effect of Linoleic acid on Protease activity of S. marcescens.
|
Error bars indicate that experiments were performed in triplicates; #p<0.005, $p<0.003 and &p<0.0001.
Bacterial biofilms are exemplary structures of group behaviour formed under the regulation of QS 32. Biofilm formation imparts bacteria with advantages like improved resistance to antibiotics and increased tolerance to environmental stress.33 Linoleic acid affected biofilm formation in both P. mirabilis and S. marcescens. Biofilm formation was also reduced in studies carried out using eucalyptus oil 34 and Capparis spinosa.35 The effect of linoleic acid on biofilm formation of both bacteria has been depicted in Table 3 and illustrated in Fig 4. Biofilm formation was 11.11% and 33.33% in P. mirabilis and S. marcescens respectively at 2.5mM linoleic acid. Thus, biofilm inhibiting potential of linoleic acid was higher against P. mirabilis than S. marcescens. This apparent difference of biofilm inhibition by linoleic acid might be a result of type of biofilms formed by these bacteria. P. mirabilis forms crystalline biofilms which gradually flatten out, followed by swarming of cells for spreading the infection to new sites. Crystalline biofilms result from urease activity exhibited by the bacterium.36 Conversely, S. marcescens biofilm are highly organised, porous and filamentous structures with chains and clusters of cells.37 Accordingly, P. mirabilis biofilm formation was inhibited more than S. marcescens because of reduced urease activity.
Table 3: Effect of Linoleic acid on Biofilm Formation in P. Mirabilis and S. Marcescens.
| Concentration of Linoleic acid (mM) | P. mirabilis | S. marcescens | ||
| O. D. 570 Mean ± S. D. | Percentage of Biofilm Formation | O. D. 570 Mean ± SD | Percentage of Biofilm Formation | |
| Control | 0.009±0.0039 | 100.00 | 0.012±0.0043 | 100.00 |
| 1.0 | 0.008±0.0000 | 88.89 | 0.010±0.0023& | 83.33 |
| 1.5 | 0.008±0.0012# | 88.89 | 0.008±0.0041 | 66.67 |
| 2.0 | 0.005±0.0026$ | 55.56 | 0.006±0.0013* | 50.00 |
| 2.5 | 0.001±0.0056^ | 11.11 | 0.004±0.0017* | 33.33 |
Experiments were performed in triplicate and #p<0.02, $p<0.03, ^p<0.01, &p<0.006 and *p<0.001 and O.D. 570 – Optical Density at 570nm
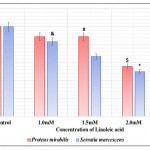 |
Figure 4: Effect of Linoleic acid on Biofilm formation of P. mirabilis and S. marcescens. |
Error bars indicate that experiments were performed in triplicates; #p<0.02, $p<0.03, ^p<0.01, &p<0.006 and *p<0.001.
Microscopic analysis of biofilm formation by P. mirabilis and S. marcescens in presence of 2.5mM linoleic acid indicated diminished biofilm formed when compared to control as seen from Fig 5. Previous work done with palmitoleic acid and myristoleic acid also depicted similar inhibitory effects.38 Hence, this observation fortifies biofilm inhibitory potential of linoleic acid.
 |
Figure 5: Microscopic analysis of Biofilm (a) Biofilm of P. mirabilis in presence of Control (b) Biofilm of P. mirabilis in presence of 2.5mM linoleic acid (c) Biofilm of S. marcescens in presence of Control (d) Biofilm of S. marcescens in presence of 2.5mM linoleic acid. |
 |
Figure 6: Growth curve analysis of linoleic acid against (a) P. mirabilis and (b) S. marcescen. |
Growth curve assay of P. mirabilis and S. marcescens was accomplished to evaluate whether the changes seen in QS regulated properties are result of quorum inhibitory activity of linoleic acid and not its anti-bacterial activity. Analysis confirmed that there was no significant change observed in cell densities of P. mirabilis and S. marcescens at 24 hours when compared to control as seen from Fig 6. Similar results were perceived in prior investigation performed using cinnamon oil16 and proanthocyanidins derived from cranberry.39 Therefore, linoleic acid was able to efficaciously reduce virulence and quench quorum of P. mirabilis and S. marcescens without inflicting selective pressure.
Conclusion
Fatty acids including linoleic acid are known to be antimicrobial in nature.8 But, the results of present study explained virulence inhibitory properties of linoleic acid and subsequently its QS inhibitory potential. Linoleic acid effectively reduced urease synthesis and biofilm formation in P. mirabilis. Moreover, it decreased QS controlled protease and prodigiosin synthesis in S. marcescens besides diminishing formation of biofilm.
Though, the exact route of this QS inhibition by linoleic acid is not known, this work provides an alternative approach of anti-virulence therapy from nutritional source for combatting bacterial infections in a milieu of increasing antibiotic resistance.
Acknowledgement
The authors would like to express our gratitude towards CSIR for providing funding in the form fellowship [File no. 08/611(0001)2013-EMR-I].
Conflict of Interest
There is no conflict of interest.
References
- Zhang L. H., Dong Y. H. Quorum sensing and signal interference: Diverse implications. Mol Microbiol. 2004;53(6):1563-1571. doi:10.1111/j.1365-2958.2004.04234.x.
CrossRef - Choo J. H., Rukayadi Y., Hwang J. K. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637-641. doi:10.1111/j.1472-765X.2006.01928.x
CrossRef - Antunes L. C. M., Ferreira R. B. R., Buckner M. M. C., Finlay B. B. Quorum sensing in bacterial virulence. Microbiology. 2010;156(8):2271-2282. doi:10.1099/mic.0.038794-0.
CrossRef - Wang W. B., Lai H. C., Hsueh P. R., Chiou R. Y. Y., Lin S. B., Liaw S. J. Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J Med Microbiol. 2006;55(10):1313-1321. doi:10.1099/jmm.0.46661-0.
CrossRef - Baldo C., Paulo S., Rocha D., Rocha S. P. D. Virulence Factors Of Uropathogenic Proteus mirabilis – A Mini Review. Int J Sci Technol Res. 2014;3(11):1-4.
- Abbas H. A., Hegazy W. A. H. Targeting the Virulence Factors of Serratia marcescens by Ambroxol. Roum Arch Microbiol Immunol. 2017;76(2):27-32. https://www.researchgate.net/publication/320858588.
- Galloway W. R. J. D., Hodgkinson J. T., Bowden S. D., Welch M., Spring D. R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111(1):28-67. doi:10.1021/cr100109t.
CrossRef - Kabara J. J., Swieczkowski D. M., Conley A. J., Truant J. P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2(1):23-28. doi:10.1128/AAC.2.1.23.
CrossRef - Byeon J. I. l., Song H. S., O. h. T. W., et al. Growth inhibition of foodborne and pathogenic bacteria by conjugated linoleic acid. J Agric Food Chem. 2009;57(8):3164-3172. doi:10.1021/jf8031167.
CrossRef - Hontecillas R., Wannemeulher M. J., Zimmerman D. R., et al. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr. 2002;132(7):2019-2027.
CrossRef - Soni K. A., Jesudhasan P., Cepeda M., et al. Identification of ground beef derived fatty acid inhibitors of auto-inducer-2 based cell signaling. J Food Prot. 2008;71(1):134-138.
CrossRef - Dilika F., Bremner P. D., Meyer J. J. M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia. 2000;71(4):450-452. doi:10.1016/S0367-326X(00)00150-7.
CrossRef - Nashikkar N., Begde D., Bundale S., Pise M., Rudra J., Upadhyay a. Inhibition of swarming motility, biofilm formation and virulence factor expression of urinary pathogens by Euphorbia trigona latex extracts. Int J Pharm Res. 2011;2(3):558-566.
- Morohoshi T., Shiono T., Takidouchi K., et al. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl Environ Microbiol. 2007;73(20):6339-6344. doi:10.1128/AEM.00593-07.
CrossRef - Salini R., Pandian S. K. Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. FEMS Pathog Dis. 2015;73:1-8. doi:10.1093/femspd/ftv038.
CrossRef - Kalia M., Yadav V. K., Singh P. K., et al. Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. PLoS One. 2015;10(8):1-18. doi:10.1371/journal.pone.0135495.
CrossRef - Merritt J. H., Kadouri D. E.,Toole O. G. A. Growing and Analyzing Static Biofilms. Curr Protoc Microbiol. 2005;3(3):1-29. doi:10.1128/microbiolspec. MB-0011-2014.Bacterial.
- Hall B. G., Acar H., Nandipati A., Barlow M. Growth rates made easy. Mol Biol Evol. 2014;31(1):232-238. doi:10.1093/molbev/mst187.
CrossRef - Rémy B., Mion S., Plener L., Elias M., Chabrière E., Daudé D. Interference in Bacterial Quorum Sensing : A Biopharmaceutical Perspective. Front Pharmacol. 2018;9(March):1-17. doi:10.3389/fphar.2018.00203.
CrossRef - Rauf A., Uddin G., Siddiqui B. S., et al. Bioassay-guided isolation of novel and selective urease inhibitors from Diospyros lotus. Chin J Nat Med. 2017;15(112):865-870. doi:10.3724/SP.J.1009.2017.00865.
- Ranjbar-Omid M., Arzanlou M., Amani M., Shokri Al-Hashem S. K., Mozafari N. A., Doghaheh H. P. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol Lett. 2015;362(9):1-9. doi:10.1093/femsle/fnv049.
CrossRef - Prywer J., Torzewska A. Effect of curcumin against Proteus mirabilis during crystallization of struvite from artificial urine. Evidence-based Complement Altern Med. 2012;(3):1-7. doi:10.1155/2012/862794.
CrossRef - Abdullah M. A. A., El-baky R. M. A., Hassan H. A., Abdelhafez A. S. M. N., Abduo-Rahma G. E. D. A. Fluoroquinolones as Urease Inhibitors : Anti- Proteus mirabilis Activity and Molecular Docking Studies. Am J Microbiol Res. 2016;4(3):81-84. doi:10.12691/ajmr-4-3-3.
- Konieczna I., Zarnowiec P., Kwinkowski M., et al. Bacterial Urease and its Role in Long-Lasting Human Diseases. Curr Protein Pept Sci. 2012;13(8):789-806. doi:10.2174/138920312804871094.
CrossRef - Jones B. D., Lockatell C . V., Johnson D. E., Warren J. W., Mobley H. L. T. Construction of a urease-negative mutant of Proteus mirabilis: Analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990;58(4):1120-1123.
- Ramanathan S., Ravindran D., Arunachalam K., Arumugam V. R. Inhibition of quorum sensing-dependent biofilm and virulence genes expression in environmental pathogen Serratia marcescens by petroselinic acid. Antonie Van Leeuwenhoek. 2017;November:1-15. doi:10.1007/s10482-017-0971-y.
CrossRef - Padmavathi A. R., Abinaya B., Pandian S. K. Phenol, 2,4-bis(1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen. Serratia marcescens. Biofouling. 2014;30(9):1111-1122. doi:10.1080/08927014.2014.972386.
CrossRef - Srinivasan R., Devi K. R., Kannappan A., Pandian S. K., Ravi A. V. Piper betle and its bioactive metabolite phytol mitigates quorum sensing mediated virulence factors and biofilm of nosocomial pathogen Serratia marcescens in vitro . J Ethnopharmacol. 2016;193:592-603. doi:10.1016/j.jep.2016.10.017.
CrossRef - Sethupathy S., Shanmuganathan B., Kasi P. D., Pandian K. S. Alpha-bisabolol from brown macroalga Padina gymnospora mitigates biofilm formation and quorum sensing controlled virulence factor production in Serratia marcescens. J Appl Phycol. 2016;28(3):1987-1996. doi:10.1007/s10811-015-0717-z.
CrossRef - Sethupathy S., Ananthi S., Selvaraj A., et al. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci Rep. 2017;7(1):16328. doi:10.1038/s41598-017-16507-x.
CrossRef - Aravindraja C., Valliammai A., Viszwapriya D., Pandian S. K. Quorum sensing mediated virulence inhibition of an opportunistic human pathogen Serratia marcescens from unexplored marine sediment of Palk Bay through function driven metagenomic approach. Indian J Exp Biol. 2017;55(July):448-452.
- Costerton J. W., Stewart P. S., Greenberg E. P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science (80- ). 1999;284(5418):1318-1322. doi:10.1126/science.284.5418.1318.
CrossRef - Guttenplan S. B., Kearns D. B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013;37(6):849-871. doi:10.1111/1574-6976.12018.
CrossRef - Mathur S., Udgire M., Khambhapati A. Effect of Essential oils on Biofilm formation by Proteus mirabilis. Int J Pharma Bio Sci. 2013;4(4):1282-1289.
- Abraham S. V. I., Palani A., Ramaswamy B. R., Shunmugaiah K. P., Armugam V. R. Antiquorum Sensing and Antibiofilm Potential of Capparis spinosa. Arch Med Res. 2017;42(8):658-668.
CrossRef - Jacobsen S. M., Shirtliff M. E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence. 2011;2(5):460-465. doi:10.4161/viru.2.5.17783.
CrossRef - Rice S. A., Koh K. S., Queck S. Y., et al. Biofilm Formation and Sloughing in. Serratia marcescens. Are Controlled by Quorum Sensing and Nutrient Cues. 2005;187(10):3477-3485. doi:10.1128/JB.187.10.3477.
- Nicol M., Luizet J., Skogman M., Jouenne T., Salcedo S. P., Emmanuelle D. Unsaturated Fatty Acids Affect Quorum Sensing Communication System and Inhibit Motility and Biofilm Formation of Acinetobacter baumannii. Int J Mol Sci. 2018;19(214):1-10. doi:10.3390/ijms19010214.
CrossRef - Maisuria V. B., Santos Y. L. L., Tufenkji N., Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci Rep. 2016;6:1-12. doi:10.1038/srep30169.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.