How to Cite | Publication History | PlumX Article Matrix
Phytochemical Analysis, Antipyretic and Antifungal Activities of Cyrtomium Caryotideum
Shakir Ullah1 , Gul Jan1, Farzana Gul Jan1, Siraj Khan2, Maria Khattak2, Hamida Bibi3 and Mohsin Ihsan2
, Gul Jan1, Farzana Gul Jan1, Siraj Khan2, Maria Khattak2, Hamida Bibi3 and Mohsin Ihsan2
1,2Department of Botany Garden Campus, Abdul Wali Khan University, Mardan, Pakistan.
3Department of Botany, Hameeda bibi Hazara University, Mansehra, Pakistan.
Corresponding author E-mail: shakirawkum321@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2664
ABSTRACT: In the present research work the phytochemical investigation of methanolic, ethanolic and chloroform extracts of Cyrtomium caryotideum, Anti-pyretic and antifungal activities in methanolic, ethanolic and chloroform extracts was carried out. The phytochemicals analysis showing the presence of carbohydrates, flavonoids, phlobatannins, alkaloids, saponins, tannins, phenols, terpenoids, cardiac glycosides was present in methanolic and ethanolic extracts, while alkaloids, phlobatannins, glycosides and protein were absent and quantative phytochemistry showed the flavonoids in chloroform extract as (14.20±0.15mg/ml), Alkaloids (12.10±0.15mg/ml), phenolics (10.45± 0.10mg/ml), Saponins (06.22±0.14mg/ml) and Tannins (04.60±0.65 mg/ml). The pharmacological activities such as, Anti-pyretic was carried out by brewer yeast induced pyrexia. The dose of 600 mg/kg of extract showed remarkable anti-pyretic activity (59.43%) when compared with positive control paracetamol (37.24oC) inhibition (73.23%).In antifungal activity the most active among the extracts was with (17.00±0.48 mm) zone of inhibition at the concentration of18 mg/µl against Verticellium. Fallowed by Pythium (16.27±0.93mm), Acremonium (16.20±1.89mm) and Trichoderma (16.11± 0.82) with concentration of 12 mg/µl.
KEYWORDS: Antipyretic; Antifungal Activities; Cyrtomium Caryotideum; Khyber Pakhtoon Khwa Pakistan; Phytochemical Analysis
Download this article as:| Copy the following to cite this article: Ullah S, Jan G, Jan F. G, Khan S, Khattak M, Bibi H, Ihsan M. Phytochemical Analysis, Antipyretic and Antifungal Activities of Cyrtomium Caryotideum. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Ullah S, Jan G, Jan F. G, Khan S, Khattak M, Bibi H, Ihsan M. Phytochemical Analysis, Antipyretic and Antifungal Activities of Cyrtomium Caryotideum. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31152 |
Introduction
Phytochemicals are naturally occurring chemical, biologically active compounds found in plants, which be responsible for health benefits for humans further these recognized to micronutrients and macronutrients (Hasler &Blumberg, 1999). They protect plants from damage and disease and contribute to the plant’s color, flavor and aroma. In common, the plant chemicals that defend plant cells from environmental threats such as stress, drought, pollution, pathogenic attack and UV exposure are called as phytochemicals (Gibson et al, 1998).Plants are contain various phytochemical constituents such as tannins, flavonoids terpenoids, phenolic acids, vitamins, lignins, quinines, stilbenes, amines, coumarins, betalains, alkaloids, and other phytochemicals, which have the potential of antioxidant activity (Zheng and wang, 2001). Research have showed that several of these antioxidant compounds have ant atherosclerotic, anti-inflammatory, anti-mutagenic, antitumor, antibacterial, antiviral and anti-carcinogenic activities (Ganesan et al., 2017). In modern research, plant phytochemicals, formerly with unknown and biological activities, have been widely studied as a basis of medicinal agents (Krishnaraju et al., 2015). Therefore, it is estimated that plants phytochemicals with sufficient antibacterial ability will be used for the cure of bacterial infections (Gracelin et al., 2013). Fever or pyretic is defined as the elevation of core body temperature above normal; in normal adults, the average oral temperature is 36.98C (98.58F). In oncology practice, a single temperature of more than 38.3°C (101°F) or three readings (at least 1 hour apart) of more than 38°C (100.4°F) are considered significant. Lower temperature elevations in the very young or old and in patients receiving steroids or other immune suppressants are considered abnormal (Mackowiak et al., 1997). Ferns and their allies are in a major division of the Plant Kingdom called Pteridophyta and they have been around for millions of years. There are over 250 different genera and 12,000 species of ferns reported all over the world (Chang et al., 2011). It has been observed that pteridophytes are not infected by microbial pathogens which may be one of the important factors for the evolutionary success of pteridophytes and the fact that they survived for more than 350 million years (Sharma and Vyas 1985). Cyrtomium caryotideum is a member of family Dryopteridaceae. It is a most common terrestrial herb growing in semi-shaded localities Ghats at lower altitudes and completely dry soils in plains areas. Plants 30-60 cm tall. Rhizome erect, densely covered with lanceolate blackish brown scales. Stipe stramineous, 16-32 cm, 2-3 mm in diam. at base, lower portion densely scaly; scales blackish brown or brown with a central blackish brown stripe, ovate or lanceolate, margins ciliate. Lamina oblong or oblong-lanceolate. Whole plant used is anti-bacterial and anthelminthic (Naqshi, 2016).
Material and Methods
Collection of Plant Materials and Botanical Identification
In the present study, Cyrtomium caryotideum whole plant was collected in November, 2016 from Mansehra Hazara division of Khyber Pakhtoon khwa Province Pakistan. With the help of Flora of Pakistan plant were taxonomically identified and placed in the Herbarium of Abdul Wali Khan University Mardan Garden campus.
Solvents
For the crude extract preparation of the Cyrtomium caryotideum methanol, ethanol and chloroform was used.
Crashing and Filtration of the Plant
The dried plant was powdered with the help of electric grinder. The powder were kept in air tight plastic bottles for further phytochemical, nutritional analysis, pharmacological and antifungal activities. 15 gm of plant powdered was retained in distinct conical flask and 90 ml of solvent i.e. (methanol, ethanol and chloroform) was added to the powdered separately. With the help of aluminum foil the flask were covered and retained in shaker for 72 hours for the shaking purposes. After 72 hours the extracts were filtered with the help of Whatman filter paper and then through filtration process plant extracts were removed (Pirzada et al., 2010).
Phytochemical Analysis
Qualitative Study
The plant extract i.e. methanol, ethanol and chloroform were tasted for the absence or presence of phytochemical constituents’ like alkaloids, tannins ,Phlobatannins, flavonoids, carbohydrates, phenols, saponin, cardiac glycosides, proteins, glycosides and terpenoids (Soni et al., 2011).
Tests for Alkaloids
For detection of alkaloids, a few drops of Wagner’s reagent (Potassium iodine) are add to 2 ml of all three methanol, ethanol and chloroform extracts. The presence of alkaloids was confirmed by the formation of reddish brown precipitate (Khandewal et al., 2015).
Tests for Tannins
For the detection of tannins Ferric chloride test was done. Ferric chloride (FeCl3) solution was mixed with all three extracts separately. Formation of blue green coloration indicated the presence of tannins. (Kokate et al., 2008).
Tests for Phlobatannins
In test tubes 0.5 ml of all the three extracts was taken separately, added 3ml distilled water and shaken for a few minutes then 1% aqueous hydro chloride (HCl) was added and boiled on water both. The presence of phlobatannins is indicated by the formation of red color (Wadood et al., 2013).
Tests for Flavonoids
For flavonoids detection, sodium hydroxide (NaOH) solution was added to all the three extracts of the plant. Red precipitation formation of indicate the presence of flavonoids (Kokate et al., 2008).
Tests for Carbohydrates
For detection of carbohydrates, 0.5 ml of all three extracts were treated with 0.5 ml of Benedict’s regent. Solution were heated for 2 minutes on a water bath. By the formation of reddish brown precipitate the presence of carbohydrate was confirmed (Bussau, et al., 2002).
Tests for Phenols
For phenol detection, 2 ml of ferric chloride (FeCl3) solution was added to 2 ml of all the three extracts separately in a test tube. Deep bluish green coloration formations indicated the presence of phenol (Dahiru et al., 2006).
Tests for Saponins
For the detection of saponin, in test tube 5 ml of all three extracts were shaken vigorously. the presence of saponins was confirmed by froth formation (Rajesh et al., 2016).
Tests for (Cardiac) Glycosides
For cardiac glycosides detection, 2 ml of all three extracts solution were shaken with 2 ml of glacial acetic acid than added few drops of concentrated sulphuric acid (H2SO4) and iron tri chloride (FeCl3). Brown ring formation indicated the presence of Cardiac glycosides (Soni et al., 2011).
Tests for proteins
Xanthoproteic Test
For the detection of protein, 1 ml from of all three extracts were treated with 1ml of concentrated nitric acid (HNO3) solution. The presence of proteins indicated by the formation of yellow color (Rajesh et al., 2016).
Tests for terpenoids
Salkowski Test
One ml of plant extracts (methanol, ethanol and chloroform) was added with 2 ml of chloroform and carefully added concentrated sulphuric acid (H2SO4) along the sides of tube to form a layer. The reddish brown coloration formation indicated the presence of terpenoids (Dahiru et al., 2006).
Tests for Glycosides
For the detection of glycosides, 5% of Ferric chloride solution and 1 ml glacial acetic acid were added to 5 ml of all three extracts and then further addition of few drops of concentered sulphuric acid (H2SO4). The presence of glycosides was conformed through the formation of greenish blue color (Rajesh et al., 2016).
Quantitative Phytochemicals Analysis
Determination of total Flavonoids Constituents
Ethanol, methanol and chloroform extracts were used for the detection of total flavonoids contents. Total flavonoids quantification was done by taking 0.5 g of plant extracts. Than the sample were mixed with 4.3 ml methanol and then more addition of 0.1 ml of aluminum tri chloride from 10% prepared solutions of aluminum tri chloride laterally. Potassium acetate (0.1 ml) was added the volume was reached to 5 ml. The mixtures were shaken by vortex to make uniform solution and then these mixture were placed at room temperature for 30 minutes for the purpose of incubation. After the completion of incubation process, the absorption was checked at 415 nm in spectrum. The Quercetin was used as a standard (Daffodil et al ., 2013).
Determination of Total Phenolic Constituents
Total phenolic quantification was done by the addition of 0.5g plant extract to 1 ml of 80% ethanol. Then the mixture were centrifuged for 15 minutes at 12,000 rpm. After that the supernatant were kept in test tube and these process were repeated 6 times. After collecting the supernatant were placed in water bath for drying. The distilled water was added to the supernatant until its volume reached to 3 ml. 2 ml (Na2CO3) of 20% were added in this solution. To this 0.5 ml Folin -ciocalteau regent was added and after 5 minutes more addition of 2 ml (Na2Co3) from 20% Na2Co3 solutions. The solution were mixed homogenously and then the test tube were brought in to the water bath in boiling water. At 650 nm their absorbance were checked. The Catechol was used as a standard (Gracelin et al., 2013).
Quantification of Total Alkaloids Constituents
5 gm of the all the three extracts was weighed in a 250 ml beaker and 200 ml of 10% acetic acid in ethanol was added and covered than allowed for 4 hours to stand. Extracts was filtered and was concentrated on a water bath to one-quarter of the original volume. Until the precipitation was complete Drop wise to the extract concentrated ammonium hydroxide was added. The solution was allowed to settle and collected the precipitated and washed with dilute ammonium hydroxide and then filtered. The residue was the alkaloid, was dried and weighed (Gracelin et al., 2013).
Determination of total tannins constituents
500 mg of the sample was weighed in a 50 ml plastic bottle. Added 50 ml of distilled water and shacked for 1 hours in a mechanical shaker. In a 50 ml volumetric flask was filtered and made up to the mark. Into a test tube, 5 ml of the filtered was pipetted out and mixed with 2 ml of 0.1 M FeCl3 in 0.I N HCl and 0.008 M potassium Ferro cyanide. At 120 nm their absorbance was cheeked out (Gracelin et al., 2013).
Determination of Total Saponins Constituents
Into a conical flask 20 gm of each extracts were put and 100 cm3 of 20% ethanol, aqueous were added. Over a hot water bath for 4 hours the samples were heated with continuous stirring at about 55°C. The residue re-extracted with another 200 ml 20% ethanol, and mixture was filtered. At about 90°C through water bath the combined extracts were reduced to 40 ml. Into a 250 ml separatory funnel the concentrate was transferred and 20 ml of diethyl ether was added and vigorously shaken. 60 ml of n-butanol was added. With 10 ml of 5% aqueous sodium chloride the combined n-butanol extracts were washed twice. In a water bath the remaining solution was heated. The samples were dried in the oven to a constant weight after evaporation; the saponin content was calculated (Gracelin et al., 2013).
Pharmacological Activities
Pharmacological activities was carried out in methanolic extracts of rhizome of Cyrtomium caryotideum plant.
Experimental Animals
Both sexes of the albino mice of weight about 25 – 30 gm were brought from the animal house of National Institute of Health (NIH), Islamabad. The animals were supplied with adlibitum water and standard pellet diet.
Drugs used and Chemicals Used
Aspirin (Bayer, Germany), Paracetamol (Glaxo Smith Kline, U.K), were used as standard drugs in the experiment for selected activities. Methanol 95% (Merck, Germany), Normal saline (Immunasol NS, A.Z. Pharmaceuticals Co.pak), Brewer’s yeast.
Evaluation of Antipyretic activity by brewer’s yeast induced pyrexia
For the evaluation of antipyretic activity five groups of albino mice were taken. The initial temperature of all mice were checked. For antipyretic activity, the brewer yeast solution was prepared by dissolving 7% brewer yeast in 100 ml of water. The paracetamol solution was used as a standard. Paracetamol solution was injected to the mice of group 2nd. To the group 3rd, 4th and 5th methanolic extract at the dose of 200, 400 and 600mg/Kg was injected. After the injection of standard drug (paracetamol) and methanolic extract, the temperature of all the mice were checked at interval of one hour up to four hours.
The results of methanolic extract was compared with the standard drug (paracetamol) for low or high antipyretic potential. % reduction in temperature are calculated by using the following formula (Janaranjani et al., 2014).
% reduction = B – Cn/B – A × 100
B= represents temperature after pyrexia induction;
Cn= temperature after 1, 2, 3, 4 and 5 hours, A= normal body temperature.
Anti-fungal Activity
Antifungal activity was carried out in ethanolic, methanolic and chloroform extracts.
Media Preparation for Fungal Growth
Dissolve 39 gm of Potato dextrose agar (PDA) in 1litre of distilled water, sterilized by autoclaved at 15psi (121oC) for 15 minutes. Cool to room temperature and pour into sterilized Petri plates to solidify. Kept at room temperature to solidify for 30 minutes.
Agar Well Diffusion Method
The micropipette using, in sterile distilled water (SDW) was placed of 100μl of different fungal cultures over the surface of an agar plate and with the help of a sterile inoculation loop it was spread, hole were made in each of the culture plates using a sterile cork borer. 75μl of crude extract of selected plants were added. The culture plates were then incubated at 37°C, and after 24 hours the results were observed depending on the fungal growth. Around each well the clear zone was measured in mm, the clear zone formation showed the antifungal activity of all extract against the each fungus. All activity were for formed in triplicated and then calculated the standard deviation. Agar well diffusion method was followed as described by Samie et al., (2010).
Statistical Analysis
All the tests were performed as individual triplicate experiment. All the data are shown as mean ± standard error of mean (S.E.M., n = number of Experiments). The statistical analyses were obtained by the one way analysis of variance (ANOVA), followed by the Dennett’s test where necessary. P<0.05 was considered Significant.
Result and Discussion
Phytochemical Analysis
In the present research work the phytochemical investigation of methanolic, ethanolic and chloroform extracts of Cyrtomium caryotideum and Pharmacological activities of methanolic extracts Anti-pyretic and antifungal activities in ethanolic ,methanolic and chloroform extracts was carried out.
Phytochemical detection in the leaves and rhizome of Cyrtomium caryotideum
Qualitative analysis of Cyrtomium caryotideum was carried out for the detection of alkaloid, flavonoids, carbohydrate, phlobatannins, glycosides, saponins, phenol, terpenoids, tannins, cardiac glycosides and proteins. The results showed that alkaloids, flavonoids, carbohydrates, phlobatannins, saponins, phenols, terpenoids, tannins, cardiac glycosides was found in methanolic and ethanolic extracts, while alkaloids, phlobatannins, glycosides and protein were found absent in the chloroform extracts. Flavonoids, carbohydrates, saponins, phenols and terpenoids were found present in in the rhizome methanolic and ethanolic extracts. In these results +++ indicate that the secondary metabolites present in highest amount, the ++ indicated that the moderate level of phytochemicals’ are present and the + indicated that low level of phytochemicals are present and – indicated that the phytochemicals are absent in all these three extracts plants (Table 1, 2).
Table 1: Qualitative phytochemicals detection in the rhizome of Cyrtomium caryotideum in methanolic, ethanolic and chloroform extracts.
| S.NO | Phytochemical test | Methanolic | Ethanolic | chloroform |
| 1 | Alkaloid | +++ | ++ | _ |
| 2 | Flavonoids | + | +++ | + |
| 3 | Carbohydrate | +++ | + | + |
| 4 | Phlobatannins | +++ | ++ | _ |
| 5 | Glycosides | + | +++ | + |
| 6 | Saponins | + | ++ | + |
| 7 | Phenol | +++ | ++ | + |
| 8 | Terpenoids | ++ | +++ | _ |
| 9 | Tannins | +++ | ++ | + |
| 10 | Cardiac glycosides | ++ | + | _ |
| 11 | Proteins | ++ | + | _ |
Key: +++: present highest level, ++ showed moderate level, + showed low level – absent
Table 2: Phytochemicals detection in the leaves of Cyrtomium caryotideum in methanolic, ethanolic and chloroform extracts.
| S.NO | Phytochemical test | Methanolic | Ethanolic | chloroform |
| 1 | Alkaloid | ++ | + | _ |
| 2 | Flavonoids | + | +++ | + |
| 3 | Carbohydrate | +++ | ++ | + |
| 4 | Phlobatannins | +++ | ++ | _ |
| 5 | Glycosides | ++ | +++ | _ |
| 6 | Saponins | +++ | +++ | + |
| 7 | Phenol | +++ | + | + |
| 8 | Terpenoids | ++ | +++ | + |
| 9 | Tannins | +++ | ++ | + |
| 10 | Cardiac glycosides | ++ | +++ | _ |
| 11 | Proteins | ++ | ++ | _ |
Key: +++: present highest level, ++ showed moderate level, + showed low level – absent
Total Phenolic, Flavonoids, Tannins, Saponins and Alkaloids Contents in chloroform, methanol and Ethanol
Highest amount of flavonoids was found in the chloroform extract as (14.20 ± 0.15mg/ml) followed by Alkaloids (10.14±0.12mg/ml), phenolics (10.45 ± 0.10mg/ml), Saponins (06.22 ± 0.14mg/ml) and lowest amount of Tannins was found in (04.60 ± 0.65 mg/ml). The flavonoids was found in highest amount in methanolic as (17.55 ± 0.10mg/ml), followed by phenols (13.25 ± 0.50mg/ml), Tannins (11.55 ± 0.30mg/ml), Alkaloids (10.05 ± 0.10mg/ml) and Saponins was found in lowest amount (08.40 ± 0.45 mg/ml). The flavonoids was found in highest amount in methanolic as (13.25 ± 0.50 mg/ml), followed by phenols (12.64±0.14mg/ml), Alkaloids (09.50 ± 0.15 mg/ml), Tannins (06.25 ± 0.40 mg/ml) and Saponins was found in lowest amount (05.40 ± 0.25 mg/ml). The data is showed in (Table 3, 4 and 5).
Table 3: Quantitative Phytochemicals detection of Cyrtomium caryotideum in chloroform extracts.
| S.No | Phytochemicals name | Concentration mg/ml |
| 1 | Total phenol | 10.45 ± 0.10 |
| 2 | Total flavonoids | 14.20 ± 0.15 |
| 4 | Total Tannins | 04.60 ± 0.65 |
| 5 | Total Saponins | 06.22 ± 0.14 |
| 6 | Total Alkaloids | 10.14±0.12 |
Table 4: Quantitative Phytochemicals detection of Cyrtomium caryotideum in methanolic extracts.
| S.No | Phytochemicals name | Concentration mg/ml |
| 1 | Total phenol | 13.25 ± 0.50 |
| 2 | Total flavonoids | 17.55 ± 0.10 |
| 4 | Total Tannins | 11.55 ± 0.30 |
| 5 | Total Saponins | 08.40 ± 0.45 |
| 6 | Total Alkaloids | 10.05 ± 0.10 |
Table 5: Quantitative Phytochemicals detection of Cyrtomium caryotideum in ethanolic extracts.
| S.No | Phytochemicals name | Concentration mg/ml |
| 1 | Total phenol | 12.64 ± 0.14 |
| 2 | Total flavonoids | 13.25 ± 0.50 |
| 4 | Total Tannins | 06.25 ± 0.40 |
| 5 | Total Saponins | 05.40 ± 0.25 |
| 6 | Total Alkaloids | 09.50 ± 0.15 |
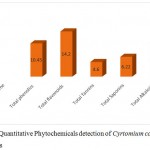 |
Figure 1: Showed Quantitative Phytochemicals detection of Cyrtomium caryotideum in chloroform extracts.
|
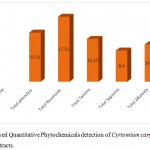 |
Figure 2: showed Quantitative Phytochemicals detection of Cyrtomium caryotideum in methanolic extracts.
|
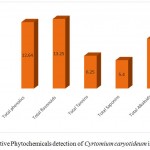 |
Figure 3: Quantitative Phytochemicals detection of Cyrtomium caryotideum in ethanolic extracts.
|
Pharmacological activities
For pharmacological activities methanolic extracts of rhizome was used.
Anti-pyretic Activity
Anti-pyretic activity of Cyrtomium caryotideum whole plant was performed using brewer yeast induced pyrexia test. In experimental mice subcutaneous administration injection of yeast suspension markedly elevate the rectal temperature after 24 hours. Treatment with the Cyrtomium caryotideum extract at the doses of 200, 400 and 600 mg/kg decreased the rectal temperature at 3hours were 37.10±0.9°C, 37.09±1.43°C and 37.24±1.12°C respectively. There was a dose dependent responses were observed in experimental mice. The antipyretic effect started as from the first hour and the effect was maintained for 3 hours, after administration of the extract. The dose of 600 mg/kg of extract showed remarkable anti-pyretic activity (46.12%) when compared with positive control paracetamol inhibition (61.53%). The data was showed in table (6).
Table 6: Anti-pyretic activity of Cyrtomium caryotideum rhizome methanolic extract.
| Rectal temperature (°C) | |||||||
|
Drug |
Dose |
Before Yeast injection (mean ± SEM) | After Yeast injection (mean ± SEM) | 1 hour
(mean ± SEM) |
2 hours
(mean ± SEM) |
3 hours
(mean ± SEM) |
% Anti-pyretic Inhibition |
| Control | N/S | 37.64±0.26 | 38.93±0.01 | 38.19±0.25 | 37.87±0.23 | 37.59±0.15 | …………….. |
| Paracetamol | 150mg/Kg | 37.10± 0.25 | 38.94±0.19 | 38.72±0.10 | 37.67±0.15 | 37.74±.03 | 73.23% |
| Methanolic extract | 200mg/Kg | 37.07±0.21 | 38.66±1.0 | 38.41±0.26 | 37.61±0.22 | 37.10±0.9 | 27.34% |
| Methanolic extract | 400mg/Kg | 37.01±0.15 | 38.52±0.10 | 38.06±0.31 | 37.33±1.20 | 37.09±1.43 | 42.18% |
| Methanolic extract | 600mg/Kg | 37.07±0.15 | 38.63±0.72 | 38.68±0.18 | 37.53±0.86 | 37.24±1.12 | 59.43% |
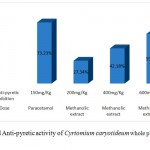 |
Figure 4: showed Anti-pyretic activity of Cyrtomium caryotideum whole plant in methanolic extracts
|
Antifungal activity of methanolic, ethanolic and chloroform extracts of Cyrtomium caryotideum against selected fungal strains
The results of antifungal activity showed that Crude methanolic Extract were active against all fungal species and showed different range of zone of inhibition. The most active among the extracts was with (17.00± 0.48 mm) zone of inhibition at the concentration of18 mg/µl against Verticellium. Fallowed by Pythium (16.27±0.93mm), Acremonium (16.20± 1.89mm) and Trichoderma (16.11± 0.82) with concentration of 12 mg/µl. And the ethanolic extracts showed maximum zone of inhibition (16.06± 0.97 mm) at the concentration 12 mg/µl against Trichoderma fallowed by Acremonium ( 15.33± 1.12 mm) at the concentration of 6 mg/µl and lowest amount of inhibition was showed against Verticellium (11.13±1.65mm) and Trichoderma (10.062±1.32mm). The chloroform extracts showed maximum inhibition against Verticellium (16.63± 0.65mm) at the concentration of12 mg/µl, followed by Trichoderma (16.18± 1.43mm) at the concentration of 6 mg/µl, Alternaria (14.40± 1.34) at the concentration of 12 mg/µl and lowest amount of inhibition was showed Acremonium (10.23± 0.88mm), Pythium (10.00± 3.60mm) and Alternaria (8.56± 1.75mm) at the concentration of 18 mg/µl and 6 mg/µl. The data are shown in table (7, 8 and 9).
Table 7: Antifungal activity of chloroform extracts of Cyrtomium caryotideum against selected fungal strains
|
Extracts concentration |
Alternaria | Acremonium | Verticellium | Pythium | Trichoderma |
| 6 mg/µl | 8.56± 1.75
|
13.37± 1.52 | 9.83± 0.46 | 12.33± 0.76 | 16.18± 1.43 |
| 12 mg/µl | 14.40± 1.34
|
10.47± 0.67 | 16.63± 0.65 | 13.47± 1.32 | 14.09± 0.87 |
| 18 mg/µl
|
11.00± 1.20 | 10.23± 0.88 | 12.37± 1.86 | 10.00± 3.60 | 12.00± 2.08 |
Table 8: Antifungal activity of ethanolic extracts of Cyrtomium caryotideum against selected fungal strains
|
Extracts concentration |
Alternaria | Acremonium | Verticellium | Pythium | Trichoderma |
| 6 mg/µl | 9.76± 1.65
|
15.33± 1.12 | 7.93± 0.66 | 10.23± 0.96 | 13.12± 1.03 |
| 12 mg/µl | 14.50± 1.25
|
13.57± 0.87 | 12.83± 0.95 | 14.27± 1.22 | 16.06± 0.97 |
| 18 mg/µl
|
6.32± 1.00 | 12.13± 0.78 | 11.13±1.65 | 13.16±0.57 | 10.062±1.32 |
Table 9: Antifungal activity of methanolic extracts of Cyrtomium caryotideum against selected fungal strains
| Extracts concentration
|
Alternaria | Acremonium | Verticellium | Pythium | Trichoderma |
| 6 mg/µl | 6.89± 0.11 | 16.20± 1.89
|
13.44± 0.53 | 10.67± 1.30 | 11.65± 1.75 |
| 12 mg/µl | 10.33± 0.88 | 14.12± 0.192
|
17.00± 0.48 | 16.27± 0.93 | 16.11± 0.82 |
| 18 mg/µl
|
10.80± 0.58 | 9.89± 0.63 | 13.62± 0.87 | 9.17± 1.01 | 14.77± 1.13 |
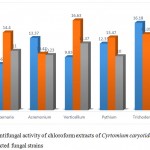 |
Figure 5: Antifungal activity of chloroform extracts of Cyrtomium caryotideum against selected fungal strains
|
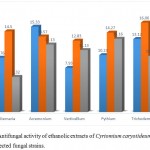 |
Figure 6: Antifungal activity of ethanolic extracts of Cyrtomium caryotideum against selected fungal strains
|
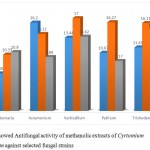 |
Figure 7: showed Antifungal activity of methanolic extracts of Cyrtomium caryotideum against selected fungal strains
|
Discussion
In the present research work the phytochemical investigation of methanolic, ethanolic and chloroform extracts of Cyrtomium caryotideum Anti-pyretic and antifungal activities in methanolic, ethanolic and chloroform extracts was carried out. Qualitative analysis of Cyrtomium caryotideum was carried out for the detection of alkaloid, flavonoids, carbohydrate, phlobatannins, glycosides, saponins, phenol, terpenoids, tannins, cardiac glycosides and proteins. The results showed that alkaloids, flavonoids, carbohydrates, phlobatannins, saponins, phenols, terpenoids, tannins, cardiac glycosides was found in methanolic and ethanolic extracts, while alkaloids, phlobatannins, glycosides and protein were found absent in the aqueous extracts. Flavonoids, carbohydrates, saponins, phenols and terpenoids were found present in in the rhizome methanolic and ethanolic extracts. Highest amount of flavonoids was found in the chloroform extract as (14.20 ± 0.15mg/ml) followed by Alkaloids (10.14±0.12mg/ml), phenolics (10.45 ± 0.10mg/ml), Saponins (06.22 ± 0.14mg/ml) and lowest amount of Tannins was found in (04.60 ± 0.65 mg/ml). The flavonoids was found in highest amount in methanolic as (17.55 ± 0.10mg/ml), followed by phenols (13.25 ± 0.50mg/ml), Tannins (11.55 ± 0.30mg/ml), Alkaloids (10.05 ± 0.10mg/ml) and Saponins was found in lowest amount (08.40 ± 0.45 mg/ml).Cyrtomium caryotideum extract at the doses of 200, 400 and 600 mg/kg decreased the rectal temperature at 3hours were 37.10±0.9ᵒC, 37.09±1.43ᵒC and 37.24±1.12ᵒC respectively. The dose of 600 mg/kg of extract showed remarkable anti-pyretic activity (59.43%) when compared with positive control paracetamol (37.24oC) inhibition (73.23%). Extract of Cyrtomium caryotideum were active against all fungal species and showed different range of zone of inhibition. The most active among the extracts was with (17.00± 0.48 mm) zone of inhibition at the concentration of18 mg/µl against Verticellium. Fallowed by Pythium (16.27±0.93mm), Acremonium (16.20± 1.89mm) and Trichoderma (16.11± 0.82) with concentration of 12 mg/µl. And the ethanolic extracts showed maximum zone of inhibition (16.06± 0.97 mm) at the concentration 12 mg/µl against Trichoderma fallowed by Acremonium ( 15.33± 1.12 mm) at the concentration of 6 mg/µl and lowest amount of inhibition was showed against Verticellium (11.13±1.65mm) and Trichoderma (10.062±1.32mm). The chloroform extracts showed maximum inhibition against Verticellium (16.63± 0.65mm) at the concentration of12 mg/µl, followed by Trichoderma (16.18± 1.43mm) at the concentration of 6 mg/µl, Alternaria (14.40± 1.34) at the concentration of 12 mg/µl. Phytochemical ingredients which are present in plant samples are known to be biologically active compounds and they are responsible for diverse activities such as antioxidant, antimicrobial, anticancer, antifungal, and antidiabetic (Hossain & Nagooru, 2011). Wide variety of pharmacological activities showed by different phytochemicals, which may help in protection against chronic diseases. Tannins, flavonoids, saponins, glycosides, and amino acids have anti-inflammatory and hypoglycemic activities. Steroids and terpenoids shows central nervous system (CNS) activities and analgesic properties. Because of their antimicrobial activity saponins are involved in plant defense system (Ayoola et al., 2008). These phytochemicals showed antimicrobial activity through different mechanisms. With proline-rich protein tannins have been found to form irreversible complexes (Shimada, 2006) resulting in the inhibition of cell protein synthesis. (Parekh and Chanda, 2007) reported that tannins are known to react with proteins to deliver the typical tanning effect which is essential for the treatment of ulcerated or inflamed tissues. Herbs that have tannins as their key components are astringent in nature and are used for treating intestinal disorders such as dysentery and diarrhea (Dharmananda, 2003). Tannins and their derivatives are phenolic compounds considered to be primary antioxidants or free radical scavengers (Barile et al., 2007). These observations therefore support the use of Cyrtomium caryotideum in herbal cure remedies, thus suggesting that Cyrtomium caryotideum has potential as a source of important bioactive molecules for the treatment and prevention of cancer. The presence of tannins in Cyrtomium caryotideum supports the traditional medicinal use of this plant in the treatment of different ailments. Alkaloid was another phytochemicals constituent’s observed in the extract of Pteris quadriaurita. One of the best common biological properties of alkaloids is their toxicity against cells of foreign organisms. These activities have been widely studied for their potential use in the reduction and elimination of human cancer cell lines (Nobori, et al., 1994). One of the largest groups of phytochemicals as alkaloids in plants which have amazing effects on humans and this has led to the development of powerful pain killer medications (Kam and Liew, 2002). (Just et al., 1998) shown the inhibitory effect of saponins on inflamed cells. Saponin was found to be present in Cyrtomium caryotideum extracts and has supported the usefulness of this plant in managing inflammation. Flavonoids, another phytochemicals shows a varied range of pharmacological activities like anti-inflammatory, antimicrobial, analgesic, anti-angionic, cytostatic, antioxidant and anti-allergic properties (Hodek et al., 2002). Several reports are presented on flavonoid groups which showing high potential biological activities such as anti-inflammatory, antioxidant, antiallergic reactions (Thitilertdecha et al., 2008). In the crude extracts the bioactive compounds such as flavonoids and tannins phytochemicals were present. Yet, these phytochemicals compounds were inducing the antimicrobial and antioxidants activities. By fractionation the amount of active constituents in the crude extracts might be dilute or improved their concentrations (Anyasor et al., 2010).
Conclusion
The above results confirmed that Cyrtomium caryotideum has better anti-inflammatory analgesic and antipyretic activity. The pharmacological activity of the Cyrtomium caryotideum may be due to the presence of phytochemical constituents. Some of these compounds possess analgesic, anti-inflammatory, antipyretic and antifungal activities. Further studies involving the purification of the chemical constituents of the plant and investigation in the biochemical pathway may results in the development of a potent analgesic, anti-inflammatory, anti-pyretic and antifungal agent with low toxicity and better therapeutic index.
Acknowledgement
I am thankful my supervisor Dr.Gul Jan, Department of Botany, Abdul Wali Khan University Mardan Garden Campus Pakistan for providing necessary facilities and cooperation during this research work.
References
- Anyasor, G.N., Ogunwenmo, O., Oyelana, O.A. and Akpofunure, B.E., 2010. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl (Costaceae). African Journal of Biotechnology, 9(31), pp.4880-4884.
- Ayoola, G.A., Lawore, F.M., Adelowotan, T., Aibinu, I.E., Adenipekun, E., Coker, H.A.B. and Odugbemi, T.O., 2008. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). African Journal of Microbiology Research, 2(7), pp.162-166.
- Barile, L., Messina, E., Giacomello, A. and Marbán, E., 2007. Endogenous cardiac stem cells. Progress in cardiovascular diseases, 50(1), pp.31-48.
- Bussau, V.A., Fairchild, T.J., Rao, A., Steele, P. and Fournier, P.A., 2002. Carbohydrate loading in human muscle: an improved 1 day protocol. European journal of applied physiology, 87(3), pp.290-295.
- Chang, H.C., Gupta, S.K. and Tsay, H.S., 2011. Studies on Folk Medicinal Fern: An Example of “Gu-Sui-Bu”. In Working with Ferns(pp. 285-304). Springer New York.
- Daffodil, E.D., Raja Lakshmi, K. and Mohan, V.R., 2013. Antioxidant activity, total phenolics and flavonoids of Salicornia brachiata Roxb. Leaf extracts (Chenopodiaceae). World J. of Pharmacy and Pharmaceutical Sciences, 2(1), pp.352-366.
- Dahiru, D., Onubiyi, J.A. and Umaru, H.A., 2006. Phytochemical screening and antiulcerogenic effect of Moringa oleifera aqueous leaf extract. African Journal of Traditional, Complementary and Alternative medicines (AJTCAM), 3(3), pp.70-75.
- Dharmananda, S., 2003. Gallnuts and the uses of Tannins in Chinese Medicine. ITM.
- Ganesan, K. and Xu, B., 2017. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Science and Human Wellness.
- Gibson, L.F., 1998. Bacteriocin activity and probiotic activity of Aeromonas media. Journal of applied microbiology, 85(S1).
- Gracelin, D.H.S., Britto, A. and Kumar, B.J.R.P., 2013. Qualitative and quantitative analysis of phytochemicals in five Pteris species. Int J Pharm Pharma Sci, 5, pp.105-7.
- Gracelin, D.H.S., Britto, A. and Kumar, B.J.R.P., 2013. Qualitative and quantitative analysis of phytochemicals in five Pteris species. Int J Pharm Pharma Sci, 5, pp.105-7.
- Hasler, C.M. and Blumberg, J.B., 1999. Introduction. The Journal of nutrition, 129(3), pp.756S-757S.
- Hodek, P., Trefil, P. and Stiborová, M., 2002. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chemico-biological interactions, 139(1), pp.1-21.
- Hossain, M.A. and Nagooru, M.R., 2011. Biochemical profiling and total flavonoids contents of leaves crude extract of endemic medicinal plant Corydyline terminalis L. Kunth. Pharmacognosy Journal, 3(24), pp.25-30.
- Huq, S., 2015. Comparative phytochemical evaluation and biological activity screening of Murdannia nudiflora and Tradescantia pallida.
- Just, M.J., Recio, M.C., Giner, R.M., Cuéllar, M.J., Máñez, S., Bilia, A.R. and Ríos, J.L., 1998. Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta medica, 64(05), pp.404-407.
- Kam, P.C.A. and Liew, S., 2002. Traditional Chinese herbal medicine and anaesthesia. Anaesthesia, 57(11), pp.1083-1089.
- Khandelwal, N., Kross, E.K., Engelberg, R.A., Coe, N.B., Long, A.C. and Curtis, J.R., 2015. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Critical care medicine, 43(5), p.1102.
- Kokate, A., Li, X. and Jasti, B., 2008. HPLC detection of marker compounds during buccal permeation enhancement studies. Journal of pharmaceutical and biomedical analysis, 47(1), pp.190-194.
- Lee‐Thorp, J.A., 2008. On isotopes and old bones. Archaeometry, 50(6), pp.925-950.
- Mackowiak, P.A., Borden, E.C., Goldblum, S.E., Has day, J.D., Munford, R.S., Nasr away, S.A., Stolley, P.D. and Woodward, T.E., 1997. Concepts of fever: recent advances and lingering dogma. Clinical Infectious Diseases, 25(1), pp.119-138.
- Naqshi, A.R., 2016. Medicinal ferns of Kashmir, India. International Journal of Bioassays, 5(7), pp.1-9.
- Nobori, T., Miura, K., Wu, D.J., Lois, A., Takabayashi, K. and Carson, D.A., 1994. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature, 368(6473), p.753.
- Parekh, J. and Chanda, S., 2007. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. African Journal of Biomedical Research, 10(2).
- Pirzada, Z., Personick, M., Biba, M., Gong, X., Zhou, L., Schafer, W., Roussel, C. and Welch, C.J., 2010. Systematic evaluation of new chiral stationary phases for supercritical fluid chromatography using a standard racemate library. Journal of Chromatography A, 1217(7), pp.1134-1138.
- Rajesh, K.D., Vasantha, S., Panneerselvam, A., Rajesh, N.V. and Jeyathilakan, N., 2016. Phytochemical analysis, in vitro antioxidant potential and gas chromatography mass spectrometry studies of Dicranopteris linearis. Asian J Pharm Clin Res, 9(2), pp.1-6.
- Robert, C., Thomas, L., Bondarenko, I., O’day, S., Weber, J., Garbe, C., Lebbe, C., Baurain, J.F., Testori, A., Grob, J.J. and Davidson, N., 2011. Ipilimumab plus decarbonize for previously untreated metastatic melanoma. New England Journal of Medicine, 364(26), pp.2517-2526.
- Samie, A., Tambani, T., Harshfield, E., Green, E., Ramalivhana, J.N. and Bessong, P.O., 2010. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. African Journal of Biotechnology, 9(20).
- Sharma, B.D. and Vyas, M.S., 1985. Ethnobotanical studies on the ferns and fern allies of Rajasthan. Nelumbo, 27(1-4), pp.90-91.
- Shimada, T., 2006. Salivary proteins as a defense against dietary tannins. Journal of chemical ecology, 32(6), pp.1149-1163.
- Soni, J., Ansari, U., Sharma, D. and Soni, S., 2011. Predictive data mining for medical diagnosis: An overview of heart disease prediction. International Journal of Computer Applications, 17(8), pp.43-48.
- Wadood, A., Ghufran, M., Jamal, S.B., Naeem, M., Khan, A. and Ghaffar, R., 2013. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem
- Zheng, W. and Wang, S.Y., 2001. Antioxidant activity and phenolic compounds in selected herbs. Journal of Agricultural and Food chemistry, 49(11), pp.5165-5170.

This work is licensed under a Creative Commons Attribution 4.0 International License.





