How to Cite | Publication History | PlumX Article Matrix
Rabbul Ibne A. Ahad , Balakyntiewshisha L. Kynshi and Mayashree B. Syiem
, Balakyntiewshisha L. Kynshi and Mayashree B. Syiem
Department of Biochemistry, North-Eastern Hill University, Shillong – 793022, India.
Corresponding Author E-mail: mayashreesyiem@yahoo.co.in
DOI : http://dx.doi.org/10.13005/bbra/2665
ABSTRACT: Ca2+ has been reported to play a protective role in many cyanobacteria against toxic effects of various metals. However there are very few reports of Ca2+ mediated protection in Cu2+ treated cyanobacterial cells. An initial study conducted to assess the influence of Ca2+ over Cu2+ induced effects on morphology, ultra-structure, photosynthetic pigments and total protein content of cyanobacterial Nostoc muscorum Meg 1 revealed that as little as 3 ppm Cu2+ can induce reduction in all these parameters by 50-80%. However when 10 ppm Ca2+ was present along with 3 ppm Cu2+, the Cu2+ induced toxic effects were lessened by 55-85% within 7 days. Bright field and scanning electron microscopic study showed that morphological changes including broken filaments; rupture, elongation and shrivelling of cells were lessened upon inclusion of Ca2+. Ultra-structural studies conducted using transmission electron microscopy showed detachment of cell membrane from cell wall, shrinkage of cellular matter; compromised thylakoid membranes and increased number of polyphosphate bodies in the Cu2+ treated cells whereas these effects were convincingly less in presence of Ca2+. Similarly decrease in protein concentration under the influence of Cu2+ was also positively modulated by the presence of Ca2+.
KEYWORDS: Ca2+ Mediated Protection; Cu2+ Toxicity; Morphology; Nostoc Muscorum Meg 1; Photo-Pigments; Ultra-Structure
Download this article as:| Copy the following to cite this article: Ahad R. I. A, Kynshi B. L, Syiem M. B. Protective Role of Ca2+ Towards Cu2+ Induced Toxicity on Photosynthetic Pigments, Morphology and Ultra-Structures of the Cyanobacterium Nostoc Muscorum Meg 1. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Ahad R. I. A, Kynshi B. L, Syiem M. B. Protective Role of Ca2+ Towards Cu2+ Induced Toxicity on Photosynthetic Pigments, Morphology and Ultra-Structures of the Cyanobacterium Nostoc Muscorum Meg 1. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31217 |
Introduction
Although Cu2+ is an essential metal ion for cyanobacteria it becomes toxic when present in excess amount. The toxicity is mediated via binding to different enzymes and functional proteins leading to inhibition of their crucial functions.1,2 Cu exists in three different oxidation states; however, Cu2+ is more toxic than other forms and is carcinogenic in nature.3 Cu2+ is released in the environment by various industrial activities, such as petroleum refining, smelting, metal finishing, paints and pigments production, coal mining and electroplating.4 The permissible limit of Cu2+ is 1.3 mg/L and 1.5 mg/L in water as per the World Health Organization (WHO) and United State Environment Protection Agency (US EPA) respectively.5,6 The issue of Cu2+ contamination and its toxic effects on any organism present in the Cu2+ polluted sites has drawn considerable attention from environmentalists.7 Cu2+ in high concentration has been shown to restrain photosynthesis and respiration, arrest cell division and initiate cell death and in turn leads to generation of reactive oxygen species (ROS) in animals, plants and microbes (algae, bacteria including cyanobacteria).8-10
Among microbes, cyanobacteria are one of the diverse groups of photosynthetic prokaryotes that are distributed widely in many habitats including fresh water, marine water, rocks and soil, deserts, hot spring and in many metal contaminated sites.11 They require minimal nutrients for their growth; have large surface area, considerable mucilage volume and high metal binding abilities, all of which make them superior than other organisms for various biotechnological applications.12 They can fix atmospheric carbon dioxide and nitrogen into carbohydrate and ammonia respectively which make them nutritionally self sufficient.13 Cyanobacteria are beneficial microorganisms to mankind as they act as natural bio-fertilizer, improve soil quality and texture by releasing extracellular polymeric substances (EPS), can biosorb heavy metals and degrade polycyclic aromatic hydrocarbons for energy production.14-16 In tropical areas, their presence can be seen in waterlogged rice fields as they can avail optimum light, temperature and nutrients conditions in rice field environment.17,18 Apart from that, many cyanobacteria are also found growing in metal contaminated sites where they are continuously exposed to multiple metal contaminants.19 The cell surfaces of cyanobacteria contains negatively charged hydroxyl, sulfhydryl, carbonyl and carboxyl groups etc. that are available to bind positively charged metal ions leading to accumulation of metals in the exposed cells.20 However, continuous existence in such contaminated environment has led to their developing many cellular strategies to combat the adverse effects of metal ions. Production of polyphosphate bodies for metal ion sequestration and synthesis of metallothionein for metal binding and metal homeostasis by influx-efflux processes are some of the means that they adopt to survive in metal contaminated areas. Presence of common cations such as Na+, K+ and Ca2+ in the surrounding also help in reducing internal accumulation of metals.21,22 Cation uptake has been extensively studied in cyanobacteria with reference to Zn2+ 23, Cd2+ 24, Ni2+ 25, Cu2+ 26, Hg2+ 27 and Ca2+.28 In 1994, Hogstrand et al.29 have stated that various metal ions including Cu2+ have been shown to inhibit Ca2+ uptake in fish. Other reports have shown that in presence of high concentrations of competing ions (Na+, Ca2+, etc.), Cu2+ uptake and expression of its toxic effects on the Daphnia magna cells were minimized.30,31 Ca2+ binding to various Ca2+ binding site(s)32 can be affected significantly when Cu2+ is present in high concentrations and in contrast Ca2+ binding can be maximized by increasing Ca2+ concentrations in relation to Cu2+. 33 All these reports confirmed that both Cu2+ and Ca2+ offer competition to each other for binding and uptake into the living cells when present together.
Ca2+ is one of the essential regulatory ions for wide range of organisms.34 Regulation of biological functions by Ca2+ in eukaryotic cells is well established whereas a very little information is known for prokaryotes.35 Ca2+ plays an important role in photosystem II (PS II) activity in plants, algae and cyanobacteria.36 Ca2+ is required for heterocyst differentiation, nitrogen fixation37 and phosphate uptake38 in cyanobacteria. Besides the role of Ca2+ in O2 evolution in PS II activity, it has been shown to regulate H+ transfer through the water channel of O2 evolving complex in Thermosynechococcus elongatus.31 Earlier investigations have established that Ca2+ transport in bacterial cells occurs via diffusion, import and export.39 Ca2+ import occurred via a uniporter or a leak driven by the membrane potential often against concentration gradient. Export of Ca2+ occurs across the membrane by diffusion and often against concentration gradient using ATP dependent transporters.
In this study, the cyanobacterium Nostoc muscorum Meg 1 was exposed to both Cu2+ and Ca2+ simultaneously in order to assess the influence of Ca2+ over Cu2+ induced toxicity on the organism. The overall cellular morphology and ultra-structure as well as various photosynthetic pigments responsible for light capturing for photosynthesis were evaluated in presence of Cu2+ in medium supplemented with and without Ca2+. The effect on heterocyst frequency crucial for nitrogen fixation and the end product of nitrogen fixation i.e. protein concentration were also analysed.
Materials and Methods
Growth and maintenance of the Cyanobacterium Nostoc Muscorum Meg 1
The cyanobacterium Nostoc muscorum Meg 1 isolated from rice field in Sohra, Meghalaya, India that receives contaminated water from nearby coal mining. The cyanobacterium was grown and maintained in BG-110 medium in aseptic conditions inside a culture room at pH of 7.5, temperature of 25 ± 2°C and under continuous light at a photon fluence rate of 50 μmol/m2s1. For all experiments, ten days old mid log phase cultures were taken at a concentration of 3 µg/mL chlorophyll a initial inoculums size which was standardized earlier in our lab.40
Metal (Cu2+ and Ca2+) Treatment
The sources of Cu2+ and Ca2+ for all the experiments were CuSO4 · 5H2O and CaCl2 · 2H2O. 100 ppm of Cu2+ and Ca2+ stock solutions were prepared. Experimental Cu2+ (3 ppm) and Ca2+ (5, 10 and 15 ppm) solutions were prepared by diluting the stock appropriately with BG-110 medium. An incubation period of seven days was allowed to study the effect of Ca2+ on Cu2+ induced toxicity in the cyanobacterium in order to allow sufficient time for the metal ions to generate their effects.
Determination of Chlorophyll a
For chlorophyll a measurement, 3 mL of each cyanobacterial cultures were centrifuged and the supernatant was discarded; to the pellet 3 mL methanol was added. The solution was kept in the refrigerator at 4°C overnight for extraction of chlorophyll a. Following this, the methanolic solution was first vortexed and then centrifuged and the absorbance of the supernatant was read at 663 nm using UV-Vis spectrophotometer (Smart Spec Plus; Bio-Rad, USA).41
Determination of total protein
Protein estimation was done by taking 3 mL cyanobacterial culture, centrifuged at 2500 rpm for 3 min and the pellet was re-suspended in milliQ water. The cells were disrupted by ultra-sonication (Sonics Vibra cell sonicator, VC 505, USA) and 0.5 mL of extract was taken and volume was raised to 1 mL by adding milliQ water. To this, 5 mL of solution C (Solution A, 98 mL: solution B, 2 mL) was added and incubated for 20 min. Once the incubation periods over, 1 N of 1 mL Folin-Ciocalteu’s phenol reagent was added and the mixture was allowed to stand for 10 min to develop the blue colour. The absorption was read at 750 nm against blank using UV-Vis spectrophotometer. Protein concentration was calculated from a standard curve using BSA.42
Determination of phycobiliproteins
For phycobiliproteins estimation, 5 mL of cyanobacterial cultures were taken, centrifuged at 2500 rpm for 3 min and to the pellet 5 mL of phosphate buffer saline, PBS (pH 7.0) was added. The cultures were ultra-sonicated followed by centrifugation at 13000 rpm at 4°C for 45 min. The absorbance of the supernatant was read at 615, 652 and 562 nm taking PBS as blank. The amounts of phycocyanin (PC), allophycocyanin (APC) and phycoerythrin (PE) were calculated based on the formulae developed by Bennett and Bogorad (1973).43
Bright field microscopy
Cyanobacterial culture (1 mL) of each sample was taken from different experimental sets in an Eppendorf tube and centrifuged at 3000 rpm for 3 min. The pellets were washed thrice by adding 1 mL of PBS, pH 7.0. Subsequently, the samples were mounted on glass slides, covered and viewed in 100X magnification under fluorescence microscope using EMP TL-BF filter (Leica Microsystems, SFL 4000).
Percent heterocyst frequency
A total of 500 cells were counted under Olympus BX-53 light microscope (Tokyo, Japan) for each heterocyst frequency count and their percentage was calculated in total number of cells.44
Scanning electron microscopic (SEM) analysis
Morphological studies of control and treated cells were performed using SEM (JEOL-JSM-6360; JEOL, Tokyo, Japan). Control and treated samples were pre-treated in 4% glutaryldehyde and kept at 4°C for 24 h followed by washing in 0.1 M sodium cocodylate buffer thrice at an interval of 15 min. After washing, samples were dehydrated in 30%, 50%, 70%, 80%, 90%, 95% and 100% acetone with two changes. Dehydrated samples were mounted on brass stubs, coated with gold and viewed under the SEM.
Transmission electron microscope (TEM) study
Ultra-structural studies were performed using TEM (JEM-2100, JEOL, Tokyo, Japan). The samples were pre-fixed in 4% glutaraldehyde at 4 °C for 24 h. Following this, cells were washed thrice in sodium cacodylate buffer (0.1 M concentration) for 15 min each change. Post-fixation was carried out in 2% OsO4 prepared in 0.2 M sodium cacodylate buffer for 1 h. The samples were rinsed in sodium cacodylate buffer with 3 changes for 15 min each. Samples were then gradually dehydrated in 10–90% acetone at 4 °C and further treated with propylene oxide for 30 min. After this samples were infiltrated in 4 steps using propylene oxide as well as embedding medium (Araldite CY212) and finally transferred to embedding molds (BEEM Inc., West Chester, Pennsylvania, USA) and oriented properly. Pure embedding medium was poured into the molds and incubated in oven at 50 °C for 24 h which was further incubated at 60 °C for 48 h for polymerization. Ultra-microtome MTX (Boeckeler Instruments, Tucson, Arizona, USA) was used to section the samples into 60-90 nm and the sections were stained with uranyl acetate and lead citrate before viewing under TEM.
Results
Determination of Chlorophyll a Content
Estimation of chlorophyll a pigment is a standard measure of growth in cyanobacteria. For calculation, chlorophyll a concentration of control culture was taken as 100%. There was nominal improvement in chlorophyll a (106.7%, 108.2% and 105.7%) in presence of 5, 10 and 15 ppm of Ca2+ in cultures without any added Cu2+. However in presence of 3 ppm Cu2+ chlorophyll a concentration was reduced to 58.7%. Simultaneous addition of 5, 10 and 15 ppm Ca2+ in the medium containing 3 ppm Cu2+ showed reduction in Cu2+ induced toxicity on chlorophyll a content to 65.2%, 70.4% and 63.5%, respectively (Fig. 1). Highest protection conferred towards chlorophyll a (70.4%) content was found on addition of 10 ppm Ca2+ in the culture medium indicating that beyond this concentration even Ca2+ tends to become adverse to the organism.
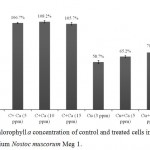 |
Figure 1: Chlorophyll a concentration of control and treated cells in the cyanobacterium Nostoc muscorum Meg 1.
|
After seven days of treatment. C = control culture; C+Ca = control culture treated with Ca2+, Cu = Cu2+ treated cultures and Cu+Ca = culture treated with 3 ppm Cu2+ and 5/10/15 ppm Ca2+. Taking control cultures as 100%. All the values are expressed as Mean ± SD (n = 3).
Thus, 10 ppm Ca2+ was chosen for the rest of the experiments. At this concentration, growth (measured as increase in chlorophyll concentration) of the cyanobacterium was improved by 11.7% compared to Cu2+ treated culture.
Photosynthetic Accessory Pigments
Photosynthetic accessory pigments such as phycocyanin, allophycocyanin, phycoerythrin, a, β-carotene and zeaxanthin assist in absorbing light energy at different wavelengths for photosynthesis.45 The contents of phycobiliproteins in the Nostoc muscorum Meg 1 cultures were severely reduced in presence of 3 ppm Cu2+ whereas the toxicity was considerably lowered when Ca2+ was included in the medium containing Cu2+ (Fig. 2). In presence of Cu2+, phycocyanin, allophycocyanin and phycoerythrin contents were decreased to 58%, 54% and 50%, however simultaneous supplementation of 10 ppm Ca2+ and 3 ppm Cu2+ in the culture medium led to improvement in pigment contents 15%, 31% and 6%, respectively (Fig. 3A; B; C). Among the pigments, phycoerythrin was more sensitive to Cu2+. The highest improvement of photosynthetic pigment content in presence of Ca2+ was found in allophycocyanin (31%).
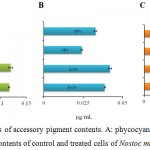 |
Figure 2: Measurements of accessory pigment contents. A: phycocyanin; B: allophycocyanin and C: phycoerythrin contents of control and treated cells of Nostoc muscorum Meg 1.
|
Duration of the treatment: seven days. C = control culture; C+Ca = control culture treated 10 ppm Ca2+, Cu = 3 ppm Cu2+ treated culture, Cu+Ca = culture treated with 3 ppm Cu2+ and 10 ppm Ca2+. Taking control cultures as 100%. All the values are expressed as Mean ± SD (n = 3).
Bright Field Microscopic Study
Morphology of the control and treated cells were studied under fluorescence microscope (Leica Microsystems, SFL 4000) at the end of seven days (Fig. 3). In control cells, healthy cells intact in long filaments were seen (Fig. 3A). Similar observation was also found in the culture treated with 10 ppm Ca2+ indicating Ca2+ supplementation on its own had no adverse effect on the morphology of the cyanobacterium (Fig 3B). Filament breakage, disintegration of cells from the filaments and distinctive cell damages were observed in the cells treated with 3 ppm Cu2+ (Fig. 3C). However, simultaneous supplementation of Cu2+ and 10 ppm Ca2+ in the culture medium showed reduction in such damages caused by Cu2+. The less filament breakage and cell damages observed in the cyanobacterium in presence of Ca2+ indicated towards its protective role against Cu2+ induced damages (Fig. 3D).
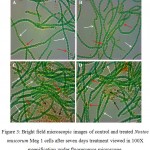 |
Figure 3: Bright field microscopic images of control and treated Nostoc muscorum Meg 1 cells after seven days treatment viewed in 100X magnification under fluorescence microscope.
|
Fig. 3A – control cells; B – control cells treated with 10 ppm Ca2+; C – cells treated with 3 ppm Cu2+ and D – cells treated with 3 ppm Cu2+ along with 10 ppm Ca2+. White coloured arrows indicate vegetative cells, red coloured arrows indicate heterocyst cells and black coloured arrows indicated damage and disintegrated cells.
Scanning Electron Microscopic Analysis
Morphological changes were observed in detail in control and treated cyanobacterial cells at the end of seven day period of incubation after treatment under scanning electron microscope (Fig. 4). Healthy cells in long filaments were recorded in control cultures (Fig. 4A). There were no morphological changes observed in cells treated with 10 ppm Ca2+ (Fig. 4B). The cells were stretched, elongated, distorted and distinctly shriveled upon Cu2+ (3 ppm) treatment (Fig. 4C) and except for a slight stretch between the cells no other form of abnormalities appeared in the cells treated with both Cu2+ (3 ppm) and Ca2+ (10 ppm) (Fig. 4D). There was formation of EPS surrounding the cells that were treated with both Cu2+ and Ca2+ indicating that there was restriction of access of the metal ions to the cells. This probably is the reason for expression of reduced Cu2+ induced toxicity in presence of Ca2+.
 |
Figure 4: Scanning electron microscopic images of control and treated cells at the end of seven day exposure.
|
The images were recorded in 3000X magnification. A: control cells; B: cells treated with 10 ppm Ca2+; C: cells treated with 3 ppm Cu2+ and D: cells treated with 3 ppm Ca2+ and 10 ppm Ca2+. Arrows indicate healthy cells in long filaments of control cells (Fig. 4A); long filaments with intact cells in the cells treated with 10 ppm Ca2+ (Fig. 4B). Arrows indicate cell stretching, elongation, shrivelling, and filaments breakage in the Cu2+ (3 ppm) treated cells (Fig. 4C) and cells were almost similar to control cultures except for few random stretching in the cultures treated with both Cu2+ (3 ppm) and Ca2+ (10 ppm) (Fig. 4D). Note the appearance of EPS (indicated by blue coloured arrow) in the cultures treated with both Cu2+ (3 ppm) and Ca2+ (10 ppm) (Fig. 4D).
Ultra-structural studies using transmission electron microscope
The ultra-structural study under TEM revealed that in control cells, cell wall and cell membranes were intact, thylakoids and phycobilisomes appeared in a regular pattern (Fig. 5A). There were detachment of cell membrane from the cell wall, shrinkage of cellular components, aggregation of dissolving phycobilisomes and appearance of polyphosphate bodies, air vacuoles and gaps within the thylakoid membranes and disappearance of thylakoids in the Cu2+ treated cells (Fig. 5B). The effects on the ultra-structure in the cyanobacterium treated with Cu2+ and Ca2+ was moderate compared to only Cu2+ treated cells (Fig. 5C).
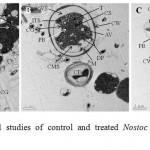 |
Figure 5: Ultra-structural studies of control and treated Nostoc muscorum Meg 1 cells exposed for seven days.
|
(A) Control; (B) Cu2+ (3 ppm) treated cells and (C) cells treated with both Cu2+ (3 ppm) and Ca2+ (10 ppm). Cells were viewed in 2000X magnification under TEM. CG: cyanophycin granule; CM: cytoplasmic membrane; CW: cell wall; CWS: space between cell wall and cell membrane; T: thylakoids; ITS: intra-thylakoid space; CS: carboxysome; PB: polyphosphate body; TD: thylakoid membrane disintegration; AV: air vacuoles; MS: membrane shrinkage; DP: aggregates of dissolving phycobilisome; CMS: cell matter shrinkage; CD: cell death.
Heterocyst Frequency
Heterocysts are specialized cyanobacterial cells, host of nitrogenase enzyme where the atmospheric N2 is fixed to NH4+. 46 Presence of Ca2+ in the growth medium improved heterocyst frequency by ~2-3% within seven days of exposure (Fig. 6). Although, percent heterocyst frequency in the Cu2+ treated cultures declined to 66.5% within the similar period of exposure, inclusion of 10 ppm Ca2+ in Cu2+ supplemented medium saw reduction in heterocyst frequency to only ~77%. Reduction of the Cu2+ toxicity on heterocyst frequency by ~11% upon addition of Ca2+ further indicated that Ca2+ had a positive impact against the toxic effect of Cu2+ in the cyanobacterium.
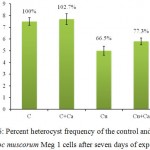 |
Figure 6: Percent heterocyst frequency of the control and treated Nostoc muscorum Meg 1 cells after seven days of exposure.
|
C = control culture; C+Ca = control culture treated 10 ppm Ca2+, Cu = 3 ppm Cu2+ treated culture, Cu+Ca = culture treated with 3 ppm Cu2+ and 10 ppm Ca2+. Taking control cultures as 100%. All the values are expressed as Mean ± SD (n = 3).
Total Protein Content
Measurement of total protein content of control and treated cells is depicted in figure 7. There was marginal increase of ~2% in the total protein content when Ca2+ (10 ppm) included in the experiment. The total protein content of the Cu2+ treated cells was reduced to 68.3% whereas supplementation of 10 ppm Ca2+ in the Cu2+ treated cultures led to decrease in total protein content to only 83.4% in seven days indicating that Ca2+ addition had reduced the Cu2+ induced effect on the total protein content by 15%.
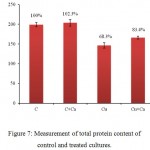 |
Figure 7: Measurement of total protein content of control and treated cultures.
|
Duration of treatment: seven days. C = control culture; C+Ca = control culture treated 10 ppm Ca2+, Cu = 3 ppm Cu2+ treated culture, Cu+Ca = culture treated with 3 ppm Cu2+ and 10 ppm Ca2+. Taking control cultures as 100%. All the values are expressed as Mean ± SD (n = 3).
Discussion
Both Ca2+ and Cu2+ are essential cations for living organisms, Ca2+ facilitates heterocyst differentiation, nitrogen fixation, PS II activity and phosphate uptake in cyanobacteria35,47,48 while Cu2+ plays an important role in cyanobacterial photosynthesis and respiration. Cu2+ is a structural constituent of plastocyanin: a component of photosynthetic electron transfer chain and cytochrome c oxidase.49 Apart from its role in photosynthesis, Cu2+ is an essential cofactor of many enzymes, e.g. superoxide dismutase50. Hattab et al. (2009)51, Surosz and Palinska (2004)8 and Nongrum and Syiem (2012)52 reported that high Cu2+ concentration hampers photosynthesis and rate of respiration, inhibits cell division leading to cell death in primary producers of the food webs such as plants, algae and bacteria including cyanobacteria. Exposure to excess amount of Cu2+ in cyanobacteria is harmful as it generates reactive oxygen species (ROS) causing breakdown of proteins and membrane lipids.53 Reduced glutathione and other metalloprotein functions are also critically compromised.54 In order to combat such compromised cell’s functions, cyanobacteria develop many protective and regulatory mechanisms.22 One such protective measure is the sequestration of metal ions in extracellular polymeric substances (EPS).55 However, there are other cellular mechanisms that maintain homeostasis by exporting-importing metal ions using P-type ATPases. cop1/cop2 and ctaA transporters are involved in Cu2+ transport into the cells of Synechocystis sp.56 whereas in the Synechocystis sp. 7942, efflux of Cu2+ is reported to be done using pacS transporter.57,58 High ion concentrations inside the cells are regulated by binding into metallothioneins, metallochaperones and phytochelatins and sequestration in polyphosphate bodies.59 Other than these strategies, cell adopts enzymatic mechanisms to reduce the toxic effects of metal ions, e.g. Superoxide dismutase (SOD) converts superoxide anion into hydrogen peroxide and catalase which breaks hydrogen peroxide into water60. Pandey et al. (1996)28; Checchetto et al. (2013)61 and Sulaymon et al. (2013)62 and had reported that toxicity of Cu2+ in animals, plants and microorganisms (algae and cyanobacteria) could be reduced or abolished in presence of various essential cations, i.e. Mg2+, Na+, K+, Ca2+, etc. Wheatly and Ayers (1995)63 and Tellis et al. (2014)10 has reported that Na+/Ca2+ uptake is a concentration dependent process in Daphnia pulex juveniles and Cu2+ competes with Na+/Ca2+ uptake in a concentration dependent manner. Thus, the report can be taken as an evidence of sharing transport system of Na+/Ca2+ by Cu2+. In low Cu2+ and high Ca2+ concentrations, survivability of Daphnia pulex juveniles is more than high Cu2+ and low Ca2+ concentrations33 indicating that at higher concentration of Ca2+, the competition severely restricts Cu2+ entry into the cells and thus reduces the intracellular accumulation of Cu2+.
The cyanobacterium Nostoc muscorum Meg 1 was isolated from a rice field in Sohra, Meghalaya where there is extensive coal and lime mining. Effluents from the mining sites are continuously draining to these rice fields due to the heavy rain that occurs in the region. Since the effluents contain metal ions such as Cd2+, Cu2+, Zn2+, Fe2+, Cr3+, Mn2+, Ca2+, Na+; over time, such metal ions get deposited in increasing concentration in these low laying rice fields.40 The presence of cyanobacteria in these rice fields are intriguing as it indicates that the organisms have developed successful strategies to combat the adverse effects of high and chronic metal exposure. Additionally, presence of competing ions such as Ca2+ could also be aiding their survival strategies by reducing entry of toxic ions into the cells. That the Ca2+ from limestone mining also reaching the rice fields in this area raises the possibility of presence of Ca2+ in increasing concentration in the surrounding along with other ions. This is what prompted us to look into impact in increased concentration of Ca2+ on the adverse effect brought about by chronic presence of Cu2+ in the vicinity of a cyanobacterium. Indeed we found that when both Cu2+ and Ca2+ are present, the harmful effect of Cu2+ on photosynthetic pigments, heterocyst frequency, protein synthesis as well as on overall morphology and ultra-structures are significantly lowered suggesting that Ca2+ played a protective role towards Cu2+ induced toxicity on these parameters which in turn allowed the organism to grow and proliferate better in the Cu2+ containing surroundings in presence of Ca2+. Addition of Ca2+ in Cu2+ supplemented medium led to an improvement of 11.7% in chlorophyll a concentration from the one registered under 3 ppm Cu2+ supplementation (Fig. 1). Similarly improvement in phycocyanin, allophycocyanin and phycoerythrin were 15%, 31% and 6% indicating that Ca2+ was definitely able to reduce the harmful effect of Cu2+ exposure in the cyanobacterium (Fig. 2). Lesser toxic effects both on morphology and ultra-structure upon Ca2+ addition in Cu2+ supplemented medium further reiterated the protective role played by Ca2+ in Cu2+ exposed cells (Fig. 3-5). Thus, cyanobacteria growing in Cu2+ polluted region where Ca2+ is also present may experience less toxicity and thereby able to survive and thrive under these adverse conditions.
Conclusion
Simultaneous presence of Cu2+ and Ca2+ in the culture medium reduced the overall toxic effects of Cu2+ in the cyanobacterium Nostoc muscorum Meg 1. This indicated that Ca2+ played a protective role in shielding the cyanobacterial cells from Cu2+ toxicity. This may be the reason of growth and proliferation of cyanobacteria seen in the coal and lime mining areas. Hence, Ca2+ supplementation in rice fields contaminated with metal ions may be beneficial to these useful organisms.
Acknowledgement
The authors thank to Prof. S. R. Joshi, Department of Biotechnology & Bioinformatics, North-Eastern Hill University, Shillong – 793022 for providing bright field microscopic facility. The authors also extended thank to Sophisticated Analytical and Instrumentation Facility (SAIF), NEHU for SEM and TEM facilities. The first author thank to the Department of Science and Technology, New Delhi for providing INSPIRE Fellowship.
References
- Macomber L., Imlay J. A. The iron-sulfur clusters of de-hydratases are primary intra cellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA. 2009;106(20):8344-8349. https://doi.org/10.1073/pnas.0812808106.
CrossRef - Tottey S., Patterson C. J., Banci L., Bertini I., Felli I. C., Pavelkova A., Dainty S. J., Pernil R., Waldron K. J., Foster A. W., Robinson A. J. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proc. Natl. Acad. Sci. USA. 2012;109(1):95-100.
CrossRef - Yan H., Pan G. Toxicity and bio accumulation of copper in three green micro algal species. Chemosphere. 2002;49(5):471-6. http://dx.doi.org/10.1016/ S0045-6535(02)00285-0.
- Akbari M., Hallajisani A., Keshtkar A. R, Shahbeig H., Ghorbanian S. A. Equilibrium and kinetic study and modeling of Cu (II) and Co (II) synergistic biosorption from Cu (II)-Co (II) single and binary mixtures on brown algae C. indica. J. Environ. Chem. Eng. 2015;3(1):140-149. http://dx.doi.org/10.1016/j.jece. 2014.11.004.
- James D., Cook M. D. Determinants of non-heme iron adsorption in man. Food Technol. 1983;124-6.
- Veglio F., Beolchini F. Removal of metals by biosorption a review. Hydrometal. 1997;44:301-16. https://doi.org/10.1016/S0304-386X(96)00059-X.
CrossRef - Ebbs S. D., Kochian L. V. Toxicity of Zinc and Copper to Brassica Species: Implications for Phytore mediation. J. Environ. Qual. 1997;26:776-781.
CrossRef - Surosz W., Palinska K. A. Effect of heavy metal stress on cyanobacterium Anabaena flos–aquae. Arch. Environ. Contam. Toxicol. 2004;48(1):40-48.
CrossRef - Latifi A., Ruiz M., Zhang C. C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009;33:258-278. doi: 10.1111/j.1574-6976.2008.00134.x.
CrossRef - Tellis M. S., Lauer M. M., Nadella S., Bianchini A., Wood C. M. The effects of copper and nickel on the embryonic life stages of the purple sea urchin (Strongylocentrotus purpuratus). Arch. Environ. Contam. Toxicol. 2014;679(3):453-464.
CrossRef - Hazarika J., Pakshirajan K., Sinharoy A., Syiem M. B. Bioremoval of Cu (II), Zn (II), Pb (II) and Cd (II) by Nostoc muscorum isolated from a coal mining site. J. Appl. Phycol. 2015;27(4):1525-1534. http://dx.doi.org/10.1007/s10811-014-0475-3.
CrossRef - Gupta V. K, Rastogi A. Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp. A comparative study. Colloids Surf B. 2008;4:170-178. doi: 10.1016/j.colsurfb. 2008.01.019.
- Prasanna R., Jaiswal P., Singh Y., Singh P. Influence of bio fertilizers and organic amendments on nitrogenase activity and photo trophic biomass of soil under wheat. Acta Agronomica Hung. 2008;56(2):149-159. doi:10.1556/AAgr.56.2008.2.4.
CrossRef - Rani N., Kaushik A., Kaushik C. K. Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma. 2007;138(1-2):49-56. doi: 10.1016/j.geoderma. 2006.10.007.
- Philippis D. R., Colica G., Micheletti E . Exopolysaccharide-producing cyano bacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011;92:697-708.
CrossRef - Bulgariu D., Bulgariu L. Sorption of Pb(II) onto a mixture of algae waste biomass and anion exchanger resin in a packed-bed column. Bioresour. Technol. 2013;129:374-380.
CrossRef - Jha M. N., Prasad A. N., Sharma S. G., Bharati R. C. Effects of fertilization rate and crop rotation on diazotrophic cyanobacteria in paddy field. World J. Microbiol. Biotechnol. 2001;17(5):463-468.
CrossRef - Prasanna R., Sood A., Rath S. K., Singh P. K. Cyano bacteria as a green option for sustainable agriculture. In: Cyanobacteria an economic perspective. 2014;145-166. (Sharma N. K., Rai A. K., Stal L. J., ed.). London Wiley.
- De Philippis R., Colica G., Micheletti E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011;92:697-708.
CrossRef - Ahad R. I. A., Syiem M. B. Copper-induced morphological, physiological and biochemical responses in the cyanobacterium Nostoc muscorum Meg 1. Nat. Environ. Pollut. Technol. 2018;17:4 (In press).
- Fernandez‐Piňas F., Mateo P., Bonilla I. Cadmium toxicity in Nostoc UAM 208 protection by calcium. New Phytol. 1995;131:403-407. doi: 10.1111/j.1469-8137. 1995.tb03077.x.
- Ahad R. I. A., Syiem M. B. Ameliorating potential of Ca2+ on Cd2+ induced toxicity on carbon assimilation in the cyanobacterium Nostoc muscorum Meg 1. Res. J. Life Sci. Bioinform. Pharma. Chem. Sci. 2018;4(2):322-337.
- Shehata F. H. A., Whitton B. A. Zinc tolerance in strains of blue green alga Anacystis nidulans. Br. Phycol. J. 1982;17(1):5-12.
CrossRef - Singh S. P., Yadav V. Cadmium uptake in Anacystis nidulans, effect of modifying factor. J. Gen. Appl. Microbiol. 1985;31(1):39-48.
CrossRef - Campbell P. M., Smith G. D. Transport and accumulation of nickel ions in the cyanobacterium Anabaena cylindrica. Arch. Biochem. Biophys. 1986;244(2):470-477. https://doi.org/10.1016/0003-9861(86)90615-6.
CrossRef - Verma S. K., Singh S. P. Factors regulating copper uptake in a cyanobacterium. Curr. Microbiol. 1990;21(1):33-37. https://doi.org/10.1007/BF02090097.
CrossRef - Pandey P. K., Singh S. P. Hg2+ uptake in a cyanobacterium. Curr. Microbiol. 1993;26(3):155-159. https://doi.org/10.1007/BF01577371.
CrossRef - Pandey P. K., Singh B. B., Mishra R., Bisen P. S. Ca2+ uptake and its regulation in the cyano bacterium Nostoc MAC. Curr. Microbiol. 1996;32(6):332–335.
CrossRef - Hogstrand C., Wilson R. W., Polgar D., Wood C. M. Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J. Exp. Biol. 1994;186:55-73.
- Tan Q. G, Wang W. X. Two-Compartment Toxico kinetic-Toxico dynamic Model to Predict Metal Toxicity in Daphnia magna. Environ. Sci. Technol. 2012;46(17):9709-9715.
CrossRef - Kruk J., Burda K., Jemiola-Rzeminska M., Strzalka K. The 33 kDa protein of photosystem II is a low-affinity calcium- and lanthanide-binding protein. Biochem. 2003;42:14862-14867. Doi: 10.1021/bi0351413.
CrossRef - Murray J. W., Barber J. Identification of a calcium binding site in the PsbO protein of photosystem II. Biochem. 2006;45(13):4128-4130. doi: 10.1021/bi052503t.
CrossRef - Liorti A., Crease T., Heyland A. Interactive effects of copper and calcium in Daphnia pulex. J. Limnol. 2017;76:281-291. doi: 10.4081/jlimnol.2016.1498.
CrossRef - Dominguez D. C. Calcium signalling in bacteria. Mol. Microbiol. 2004;54:291-297. doi:10.1111/j.1365-2958.2004.04276.x.
CrossRef - Chen T. H., Huang T. C., Chow T. J. Calcium requirement in nitrogen fixation in the cyanobacterium Synechococcus RF-1. Planta. 1988;173:253-256. doi: 10.1007/BF00403017.
CrossRef - McEvoy J. P., Brudvig G. W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006;106:4455-4483. doi: 10.1021/cr0204294.
CrossRef - Smith R. J., Hobson S., Ellis I. Evidence for calcium-mediated regulation of heterocyst frequency and nitrogenase activity in Nostoc 6720. New Phytol. 1987;105(4):531-541.
CrossRef - Keurson G. W., Miernyk J. A., Budd K. Evidence for the occurrence of, and possible physiological role for, cyano bacterial calmodulin. Plant Physiol. 1984;75:222-224.
CrossRef - Rosen B. P. Calcium transport in microorganisms In: Academic Press. (Carafoli E., ed.) Membrane transport of calcium. London. 1982;187-216.
- Ahad R. I. A., Goswami S., Syiem M. B. Biosorption and equilibrium isotherms study of cadmium removal by Nostoc muscorum Meg 1: morphological physiological and biochemical alterations. 3 Biotech. 2017;7:104. doi: 10.1007/s13205-017-0730-9.
CrossRef - Mackinney G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941;140:315-322.
- Lowry O. H., Roserbough N. J., Farr A. L., Randall R. J. The folin by oliver. J. Biol. Chem. 1951;193:265-275. doi: 10.1016/0304-3894(92)87011-4.
CrossRef - Bennett A., Bogorad L. Complementary chromatic adaption in a filamentous blue-green alga. J. Cell. Biol. 1973;58:419-435. doi: 10.1083/jcb.58.2.419.
CrossRef - Wolk C. P. Control of sporulation in a blue-green alga. Dev. Biol. 1965;12(1):15-35. https://doi.org/10.1016/0012-1606(65)90018-7.
CrossRef - Mishra S. K., Shrivastav A., Maurya R. R., Patidar S. K., Haldar S., Mishra S. Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. Protein Expr. Purif. 2012;81(1):5-10.
CrossRef - Meeks J. C. Physiological Adaptations in Nitrogen-fixing Nostoc–Plant Symbiotic Associations. In Prokaryotic Symbionts in Plants. Microbiol. Monographs. (Pawlowski K., ed.). Berlin Heidelberg. Springer. 2007;8.
CrossRef - Piccioni R. G., Mauzerall D. C. Calcium and photosynthetic oxygen evolution in cyanobacteria. Biochim. Biophys. Acta. 1978;504(3):384-97.
CrossRef - Becker D. W., Brand J. J. Anacystis nidulans demonstrates a photo system II cation requirement satisfied only by Ca2+ or Na+. Plant Physiol. 1985;79:552-558.
CrossRef - Vermaas W. F. J. (ed) Photosynthesis and respiration in cyanobacteria. Encyclopedia of life sciences. John Wiley & Sons. 2001. https://doi.org/10.1038/npg.els.0001670.
CrossRef - Yruela I., Alfonso M., Baron M., Picorel R. Copper effect on protein composition of photosystem II. Physiol. Planta. 2000;110:551-557.
CrossRef - Hattab S., Dridi B., Chouba L., Ben Kheder M., Bousetta H. Photosynthesis and growth responses of pea Pisum sativum L. under heavy metals stress. J. Environ. Sci. 2009;21:1552-1556.
CrossRef - Nongrum N. A., Syiem M. B. Effects of Copper ion (Cu2+) on the physiological and biochemical activities of the cyano bacterium Nostoc ANTH. Environ. Eng. Res. 2012;17(S1):S63–S67.
CrossRef - Chen L. M., Lin C. C. Kao C. H. Copper toxicity in rice seedlings: Changes in anti oxidative enzyme activities, H2O2 level and cell wall peroxidise activity in roots. Bot. Bullet. Acad. Sin. 2000;41:99-103.
- Imlay J. A. The Mismetallation of enzymes during oxidative stress.J. Biol. Chem. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814.
CrossRef - Khataee A. R., Zarei M., Pourhassan M. Bioremediation of Malachite green from contaminated water by three microalgae: Neural network modeling. Clean – Soil Air Water. 2010;38:96-103. https://doi.org/10.1002/clen.200900233.
CrossRef - Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6802. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109-136.
CrossRef - Kanamura K., Kashiwagi S., Mizuno T. The cyano bacterium, Synechococcus sp. PCC7942 possess two distinct genes encoding cation-transporting P-type ATPases. FEBS Lett. 1993;330:99-104.
CrossRef - Nies D. H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003;27(2-3):313-339.
CrossRef - Baptista M. S., Vasconcelos M. T. Cyano bacteria metal interactions requirements, toxicity and ecological implications. Crit. Rev. Microbiol. 2006;32(3):127-137.
CrossRef - Narainsamy K., Marteyn B., Sakr S., Cassier-Chauvat C., Chauvat F. Genomics of the pleïotropic glutathione system in cyanobacteria. In: Advances in Botanical Resrarch (Chauvat F., Cassier-Chauvat C., editors). Amsterdam, The Netherlands. Elsevier. 2013;157-188.
- Checchetto V., Formentin E., Carraretto L., et al. Functional characterization and determination of the physiological role of a calcium-dependent potassium channel from cyanobacteria. Plant Physiol. 2013;162:953-64. doi: 10.1104/pp.113.215129.
CrossRef - Sulaymon A. H., Mohammed A. A., Al-musawi T. J. Competitive biosorption of lead cadmium copper and arsenic ions using algae. Environ. Sci. Poll. Res. 2013;20(5):3011-3023. https://doi.org/10.1007/s11356-012-1208-2.
CrossRef - Wheatly M. G., Zanotto F. P., Hubbard M. G. Calcium homeostasis in crustaceans: subcellular Ca dynamics. Comparative biochemistry and physiology. Biochem. Mol. Biol. 2002;132(1):163-178. doi: 10.1016/S1096-4959(01)00520-6.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





