How to Cite | Publication History | PlumX Article Matrix
Sodium Nitroprusside Improves Performance of Barley (Hordeum Vulgare L.) Under Salt Stress
Zahid Khorshid Abbas
Department of Biology, Faculty of Science, University of Tabuk, Tabuk 71491, Saudi Arabia.
Corresponding Author E-mail: znourabbas@ut.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2666
ABSTRACT: Soil salinity creates osmotic and ionic stress in plants that result in the suppressed water and nutrients status in plants leading to reduced growth and yield of crop plants. Although, plants activate their defense system to counter various stresses but this defense system has limitations. Therefore, it is highly desirable to manipulate the plant’s cellular system to counter the detrimental effects of stresses efficiently. Nitric oxide (NO) has been shown to act as an important signaling molecule which plays vital role in growth and development of plants and plays important role in the responses of plants to biotic and abiotic stresses. Keeping in view the vital roles of NO in plants, the present experiment was performed to study the impact of NO donor sodium nitroprusside (SNP) on growth, physiological and biochemical parameters of barley (Hordeum vulgare L. cv. Sahrawi) plants grown under 200 mM NaCl. The results showed that salt-stressed plants accumulated higher levels of proline (Pro) and glycine betaine (GB) and showed enhanced activities of antioxidant enzymes viz. superoxide dismutase (SOD), peroxidase (POX) and catalase (CAT) as compared with the control plants. But increase in osmolytes and enzyme activities could not protect the plants from NaCl-induced damage and exhibited enhanced H2O2 and O2−· content, TBARS and electrolyte leakage. All these alterations negatively affected growth (fresh and dry weight of shoot and root), and physiological and biochemical parameters [leaf chlorophyll (Chl) content, carbonic anhydrase (CA) activity and leaf relative water content (LRWC)] of stressed plants. However, NaCl-stressed plants treated with the NO donor, SNP, exhibited enhanced synthesis of Pro and GB content and activities of antioxidant enzymes that resulted in reduced H2O2 and O2−· content, TBARS and electrolyte leakage and enhanced CA activity, leaf Chl content and LRWC. Application of SNP to salt-stressed plants also improved growth characteristics. On the contrary, application of NO scavenger cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide] along with SNP and NaCl suppressed the effect of NO and resulted in poor defense against salinity.
KEYWORDS: Antioxidant Enzymes; Glycine Betaine; Nitric Oxide; Proline; Salinity
Download this article as:| Copy the following to cite this article: Abbas Z. K. Sodium Nitroprusside Improves Performance of Barley (Hordeum Vulgare L.) Under Salt Stress. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Abbas Z. K. Sodium Nitroprusside Improves Performance of Barley (Hordeum Vulgare L.) Under Salt Stress. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=30572 |
Introduction
Plants are constantly exposed to various abiotic stresses. Of these, soil salinity is considered as one of the menaces to the growth and productivity of crop plants across the globe. Soil salinity results due to poor quality of irrigation water, and also due to increasing temperature which directly sets plants under heat stress and also affects plants indirectly through accelerating evaporation of water from soil leaving behind higher content of salts. Onset of ionic and osmotic stress are one of the prime effects of over accumulated salts, chiefly NaCl, in the soil that diminishes the water and mineral uptake capacity of plants.1,2 Excessive accumulation of salts in the soil also enters the cytosol of the plant cells that affect metabolic activities and causes membrane disorganization and leakage of electrolytes.3,4 Salinity also induces the generation of reactive oxygen species (ROS) that causes an imbalance between generation and scavenging of ROS.5 Excessive generation of ROS creates oxidative stress that causes oxidation of biomolecules6 that results in early senescence of leaves and deprived photosynthetic efficiency that causes reduced dry matter assimilation and reduced harvest. However, to endure oxidative stress plants have antioxidant system of various enzymes like superoxide dismutase (SOD), peroxidase (POX), catalase (CAT) etc. SOD converts superoxide radicals to H2O2, while CAT and POX transform H2O2 into water and oxygen. However, to cope with osmotic stress plants accumulate compatible solutes such as proline (Pro) and glycine betaine (GB). Moreover, plants also adopt the strategy of stress avoidance through reduced uptake of toxic ions or by diverting the absorbed ions to the apoplast or vacuole.7,8 However, only the presence of various defense system at cellular and sub-cellular levels in plants is not sufficient to protect the plants against the stress. But timely and accurate activation of these defense systems prior to the onset of damage is vital for the survival of plants under stressful conditions which is carried out by a network of signaling molecules.
Among various signaling molecules, nitric oxide (NO) has gained much attention in plant biology research. NO has been shown to play significant role in plants starting from seed germination to flowering and senescence.9-14 It also facilitates the responses of plants to various biotic and abiotic stresses.4,15–18 However, meager or elusive information is available on the role of NO in the salt tolerance mechanism in barley (Hordeum vulgare L.). Therefore, keeping in view the importance of NO in plants, the present study was planned to unravel the effect of salt stress on crop plants and NO donor SNP at growth, physiological and biochemical characteristics of stressed plants.
Materials and Methods
Plant Culture and Treatments
A sand culture pot experiment was performed to test the hypothesis. Surface sterilized healthy seeds of barley (Hordeum vulgare L. cv. Sahrawi) were sown in 20 cm diameter plastic pots. In each pot ten seeds were sown and later on, five healthy plants were maintained in each pot. 30 days after sowing (DAS) plants were treated with: (i) DDW (Control), (ii) 200 mM NaCl, (iii) 0.2 mM SNP, (iv) 0.2 mM SNP+200 mM NaCl, and (v) 0.2 mM SNP+200 mM NaCl+0.2 mM cPTIO. The experimental set up was maintained under natural illuminated conditions. Nutrient requirement of growing plants was fulfilled by adding 50 mL of Raukura’s nutrient solution19 to each pot every day. Along with nutrient solution, 200 mM NaCl was also applied. The concentration of NaCl solution was increased by 25 mM every two days until the desired concentration of 200 mM was achieved, so that osmotic shock could be avoided. Sodium nitroprusside (SNP) was used as NO donor, NaCl as salt stress inducer and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) was used as NO scavenger. Each pot was considered as one replicate and each treatment was repeated three times.
Response of plants to salt stress and SNP was analyzed at 60 DAS in terms of growth parameters viz. fresh weight (FW) and dry weight (DW) of shoot and root; and physiological and biochemical parameters viz. leaf chlorophyll content (Chl), carbonic anhydrase (CA) activity, hydrogen peroxide (H2O2) and superoxide (O2−·) content, lipid peroxidation, leaf relative water content (LRWC), electrolyte leakage, and proline (Pro) and glycine betaine (GB) content and activities of antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX)].
Measurement of Growth, Physiological and Biochemical Parameters
Effect of SNP and NaCl on plant growth was evaluated by measuring fresh weight (FW) of shoot and root, and dry weight of shoot and root. For the measurement of shoot and root FW, plants were uprooted. Surface adhered particles were washed out and water was removed by blotting paper. Cleaned shoot and root were weighed using electronic balance and the data for each treatment was recorded. Dry weight of shoot and root was recorded by drying the plants at 80˚C for 24 h.
Leaf Chl content was estimated by the method of Lichtenthaler and Buschmann.20 Method of Dwivedi and Randhawa21 was adopted to measure CA activity, while H2O2 and O2−· content were estimated by the method of Velikova et al22 and Elstner and Heupel,23 respectively. Peroxidation of membrane lipid was assessed by determining the level of thiobarbituric acid reactive substances (TBARS) using the method of Cakmak and Horst.24 The method of Yamasaki and Dillenburg25 was adopted to measure LRWC (%). Membrane permeability was assessed in term of electrolyte leakage (%) by the method of Lutts et al.26 Content of Pro and GB was estimated by the methods of Bates et al27 and Grieve and Grattan,28 respectively.
Assay of antioxidant enzymes was performed by homogenizing leaf tissues at 15,000 g for 20 min at 4˚C with three volumes (w/v) of an ice-cold extraction buffer (50 mM Tris-HCl, pH 7.8, 1 mM EDTA, 1 mM MgCl2 and 1.5% (w/w) polyvinyl pyrrolidone). The crude extract from the supernatant was used for the assay of enzyme activities. Activity of SOD, POX and CAT was determined by the method of Beauchamp and Fridovich,29 Upadhyaya et al30 and Cakmak and Marschner,31 respectively.
Statistical Analysis
Statistical analysis was done using SPSS-11 statistical software (SPSS Inc., Chicago, IL, USA). Each treatment was considered as one replicates and all the treatments were replicated three times. Mean was statistically compared by Duncan’s Multiple Range Test (DMRT) at P< 0.05% level.
Results and Discussion
Effect of SNP and NaCl on Growth Attributes
Effect of SNP and NaCl on growth characteristics was assessed by estimating fresh and dry weight of shoot and root. The data showed that salt stress reduced shoot and root fresh and dry weight by 69.1% and 46.9%, and 42.5% and 41.6%, respectively as compared with control (Table 1). Adverse effect of NaCl on growth attributes of plants has also been reported by Khan et al,32 Siddiqui et al,33 Idrees et al,34 and Khan.35 Application of NO donor SNP alleviated the detrimental effects of salt stress and improved these growth parameters significantly. In order to validate the alleviating effect of NO on NaCl, NO scavenger cPTIO was used. Addition of cPTIO to SNP+NaCl suppressed the effect of NO and caused reduction in all the growth parameters studied (Table 1). The data showed that SNP improved the activity of CA (Table 1),4 the enzyme which maintains constant supply of CO2 to rubisco.36 Moreover, improvement in Chl content (Table 1) also facilitated the plants with improved photosynthetic efficiency. Therefore, enhanced CA activity and Chl content in salt-stressed plants treated with SNP maintained normal functioning of photosynthesis system and thus accumulated more dry matter which is witnessed by improved fresh and dry weights of plants (Table 1).
Effect of SNP and NaCl on Physiological and Biochemical Parameters
The perusal of the data shows that NaCl stress significantly reduced Chl content and CA activity by 39.1% and 16.0%, respectively as compared with the control (Table 1). Decrease in Chl content in salt-stressed plants was probably due to impaired protein complexes, required for chlorophyll synthesis and/or due to loss of chlorophyll by increased activity of chlorophyll degrading enzyme chlorophyllase37,38 that resulted in the destruction of chlorophyll. Application of SNP alone or to the salt-stressed plants showed enhanced level of Chl and exhibited 55.6% and 26.6% increase in Chl content when applied alone (SNP) and in combination with NaCl (SNP+NaCl), respectively (Table 1). These results are in agreement with the findings of Liu et al39 and Khan et al.4 who also reported improved CA activity and Chl content. On the other hand, NO scavenger cPTIO inverted the impact of NO and again decreased CA and Chl content. It is noteworthy that the values for CA and Chl content registered in salt-treated plants and cPTIO-treated plants did not differ statistically (Table 1).
Table 1: Effect of sodium nitroprusside and NaCl on shoot and root fresh and dry weight, leaf chlorophyll (Chl) content and carbonic anhydrase (CA) activity in barley (Hordeum vulgare L.)
| Treatments | Shoot FW (g) | Shoot DW (g) | Root FW (g) | Root DW (g) | Leaf Chl content (mg g-1 FW) | CA activity (µM CO2 kg-1 leaf FW s-1) |
| Control | 10.69±1.05b | 3.82±0.07b | 3.52±0.06b | 1.26±0.04bc | 2.35±0.49b | 318.57±4.39b |
| NaCl | 6.32±0.89d | 2.60±0.05c | 2.47±0.13cd | 0.89±0.008d | 1.69±0.05d | 274.60±5.61cd |
| SNP | 12.70±0.65a | 4.51±0.16a | 4.69±0.02a | 1.80±0.06a | 2.63±0.32a | 388.31±3.97a |
| SNP+NaCl | 8.62±1.12c | 2.22±0.08d | 2.68±0.06c | 1.35±0.02b | 2.14±0.29bc | 297.52±2.58bc |
| SNP+NaCl+cPTIO | 5.69±0.71de | 1.42±0.02e | 1.16±0.11e | 0.81±0.007de | 1.61±0.04de | 268.08±3.64de |
Average of three determinations is presented with ± indicating S.E. Data within the same column followed by the same letter do not differ statistically at P< 0.05 (Duncan Multiple Range Test). Double distilled water (Control), 200 mM NaCl (NaCl), 0.2 mM sodium nitroprusside (SNP), 0.2 mM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO).
Over production of ROS is one of the fatal effects of salinity that leads to the oxidative stress. In the present investigation H2O2 and O2−· were measured as oxidative stress markers. The results showed that NaCl stress caused a considerable increase of 46.6% and 68.8% in H2O2 and O2−· content, respectively compared with the control (Figure. 1 A and B). Excessive generation of ROS causes peroxidation of membrane lipids4,6 reflected by higher values of TBARS (Figure 1C). Salt-stressed plants also showed higher level of electrolyte leakage and lower LRWC as compared with the control (Figure 1D). Salt stress-induced peroxidation of membrane lipids causes disorganization of membranes and results in the leakage of electrolytes.3,4 Treatment of plants with SNP at the rate of 0.2 mM SNP suppressed oxidative stress in non-stressed as well as stressed plants. SNP reduced H2O2 and O2−· content by 20.8% and 24.1%, respectively compared with NaCl-stressed plants (Figure 1A and B). These results are in agreement with the findings of Egbichi et al,40 Ahmad et al41 and Fatma et al.42 Reduction in oxidative stress by SNP was also reflected in the form of reduced TBARS and electrolyte leakage (Figure 1C and D). Excessive accumulation of salts in the soil also creates osmotic stress that diminishes the water uptake capacity of plants.1,2 Salt-stressed plants treated with SNP (NaCl+SNP) exhibited 16.9% and 23.1% reduction in TBARS and electrolyte leakage, respectively compared with the NaCl-treated plants (Figure 1C and D). On the contrary, presence of cPTIO in growth medium overcame the effect of NO and created a condition similar to salt stress. The values for TBARS and electrolyte leakage in the plants treated with NaCl+SNP+cPTIO were statistically at par with that of the values of NaCl treated plants. It has been well established that NO detoxifies ROS either directly interacting with O2−·43 or by improving the activities of antioxidant defense system44 leading to decreased generation of H2O2 and O2−·45 (Figure 2C and D). Application of SNP to salt stressed plants also maintained normal water level of plants as shown by LRWC (Figure 1D). Although, the exact mechanism of NO-induced elevation in LRWC is not clear but Ke et al. (2013) suggested a decrease in solute potential, while increasing water potential in osmotic-stressed plants when treated with NO which facilitates enhanced water status of plants under salt stress. Khan et al.4,18 also reported improved water status in NaCl-stressed plants supplemented with NO. Furthermore, to counter osmotic stress plants accumulate higher levels of osmolytes33,4 for instance Pro and GB in the present investigation. But enhanced levels of these osmolytes were not sufficient enough to counter NaCl-induced osmotic stress as shown by decreased LRWC of NaCl treated plants (Figure 1D). Whereas, salt-stressed plants treated with NO donor SNP further enhanced the levels of Pro and GB and made the plants capable to face osmotic stress and maintained higher LRWC (Figure 2A and B; Figure 1D).
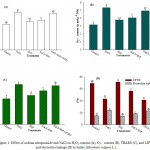 |
Figure 1: Effect of sodium nitroprusside and NaCl on H2O2 content (A), O2_ . content (B), TBARS (C), and LRWC and electrolyte leakage (D) in barley (Hordeum vulgare L.).
|
Average of three determinations is presented with bars indicating S.E. Bars followed by the same letter do not differ statistically at P< 0.05 (Duncan Multiple Range Test). [DDW (Control), 200 mM NaCl (NaCl), 0.2 mM sodium nitroprusside (SNP), 0.2 mM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO); hydrogen peroxide (H2O2), superoxide (O2_ .), thiobarbituric acid reactive substances (TBARS), leaf relative content (LRWC)].
Effect of NO and NaCl on antioxidant enzyme activities was assessed by measuring the levels of SOD, POX and CAT. The results revealed that 200 mM NaCl enhanced the activities of these antioxidant enzymes (Figure 2C and D). In spite of enhanced activities of these enzymes plants showed negative effect of salt stress in the form of reduced fresh and dry weight, CA activity, leaf Chl content, LRWC and enhanced levels of TBARS and electrolyte leakage. Whereas, application of 0.2 mM SNP to NaCl-suffered plants further enhanced the enzyme activities (Figure 2C and D) and protected the plants against NaCl-induced oxidative stress. It has been recognized that NO detoxifies ROS.4,18,43,44 It is well known that SOD dismutates O2−· radicals to H2O2 whereas POX and CAT convert H2O2 into water and oxygen. In the present investigation, NO donor SNP reduced the level of O2−· which indicates that the rate of conversion of O2−· to H2O2 by SOD was greater than the generation of O2−·. Furthermore, SNP treated salt-stressed plants also showed enhanced activities of POX and CAT which helped the plants to scavenge H2O2 by converting it to water and oxygen. All these together maintained normal functioning of cellular system and assisted plants to accumulate more dry matter (Table 1) even under stressed conditions. On the contrary, the combination of NaCl+SNP+cPTIO showed a reduction in the activities of antioxidant enzymes and again forced the plants to stressful environment.
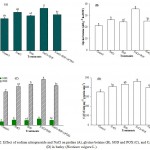 |
Figure 2: Effect of sodium nitroprusside and NaCl on proline (A), glycine betaine (B), SOD and POX (C), and CAT (D) in barley (Hordeum vulgare L.).
|
Average of three determinations is presented with bars indicating S.E. Bars followed by the same letter do not differ statistically at P< 0.05 (Duncan Multiple Range Test). [DDW (Control), 200 mM NaCl (NaCl), 0.2 mM sodium nitroprusside (SNP), 0.2 mM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO); superoxide dismutase (SOD), peroxidase (POX), catalase (CAT)].
Conclusion
The results of the experiment showed that application of NO donor SNP maintained normal functioning cellular machinery and improved the performance of plants under salt stress. Plants grown under salt stress tried to tolerate salt stress through enhancing their defense system as witnessed by elevated levels of osmolytes (Pro and GB) and higher activities of antioxidant enzymes. However, this activation of defense system was not sufficient to counter 200 mM NaCl stress and suffered plants showed reduced fresh and dry weights, CA activity, leaf Chl content and LRWC and enhanced levels of H2O2 and O2−· content, TBARS and electrolyte leakage. However, application of NO donor SNP to salt-stressed plants further elevated the defense system of plants to the level required to counter damaging effects of NaCl stress. In order to validate the alleviating effect of NO on salt stress, cPTIO was used as NO scavenger. Application of cPTIO overturned the effect of NO and again created a condition similar to salt stress.
Acknowledgment
The author is thankful to King Khalid University Hospital for providing the bacterial isolate and to Prof. Suliman Ali Alharbi, College of Science for providing laboratory facilities to perform the research and to his guidance and expertise.
References
- Glenn E.P, Brown J.J and Khan M.J. Mechanisms of Salt Tolerance in Higher Plants, in: A.S. Basra R.K. Basra (Eds.), Mechanisms of Environmental Stress Resistance in Plants, Harwood Academic Publishers, The Netherlands. 1997;83–110.
- Munns R, James R.A and Läuchli A. Approaches to Increasing the Salt Tolerance of Wheat and Other Cereals. Journal of Experimental Botany. 2006;57:1025–1043.
CrossRef - Zhu J.K. Plant Salt Tolerance. Trends in Plant Science. 2001;6:66–71.
CrossRef - Khan M.N, Siddiqui M.H, Mohammad F and Naeem M. Interactive Role of Nitric Oxide and Calcium Chloride in Enhancing Tolerance to Salt Stress. Nitric Oxide. 2012;27:210-218.
CrossRef - Hasegawa P.M, Bressan R.A, Zhu J.K and Bohnert H.J. Plant Cellular and Molecular Responses to High Salinity. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:463–499.
CrossRef - Foyer C.H and Noctor G. Oxygen Processing in Photosynthesis: Regulation and Signaling. New Phytologist. 2000;146:59–388.
CrossRef - Tester M and Davenport R. Na+ Tolerance and Na+ Transport in Higher Plants. Annals of Botany. 2002;91: 503–527.
CrossRef - Kopyra M and Gwóźdź E.A. The Role of Nitric Oxide in Plant Growth Regulation and Responses to Abiotic Stress. Acta Physiologiae Plantarum. 2004;26:459–472.
CrossRef - Beligni M and Lamattina L. Nitric Oxide Stimulates Seed Germination and De-Etiolation, and Inhibits Hypocotyl Elongation, Three Light inducible Responses in Plants. Planta. 2000;210:215-221.
CrossRef - Sirova J, Sedlarova M, Piterkova J, Luhova L and Petrivalsky M. The Role of Nitric Oxide in the Germination of Plant Seeds and Pollen. Plant Science. 2011;181:560-572.
CrossRef - He Y, Tang R, Hao V, Stevens R, Cook C, Ahn S, Jing L, Yang Z, Chen L, Guo F, Fiorani F, Jackson R, Crawford N and Pei Z. Nitric Oxide Represses the Arabidopsis Floral Transition. Science. 2004;305:1968-1971.
CrossRef - Jasid S, Galatro A, Villordo J, Puntarulo S and Simontacchi M. Role of Nitric Oxide in Soybean Cotyledon Senescence. Plant Science. 2009;176:662-668.
CrossRef - Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D and Wilson I. (2008) Nitric Oxide, Stomatal Closure, and Abiotic Stress. Journal of Experimental Botany. 2008;59:165–176.
CrossRef - Lanteri M, Pagnussat G.C and Lamattina L. Calcium and Calcium-Dependent Protein Kinases are Involved in Nitric Oxide and Auxin-Induced Adventitious Root Formation in Cucumber. Journal of Experimental Botany. 2006;57:1341–1351.
CrossRef - Sheokand S, Bhankar V and Sawhney V. Ameliorative Effect of Exogenous Nitric Oxide on Oxidative Metabolism in NaCl Treated Chickpea Plants. Brazilian Journal of Plant Physiology. 2010;22:81–90.
CrossRef - Kader M.A and Lindberg S. Uptake of Sodium in Protoplasts of Salt-Sensitive and Salt-Tolerant Cultivars of Rice, Oryza sativa L. Determined by the Fluorescent Dye SBFI. Journal of Experimental Botany. 2005;56: 3149–3158.
CrossRef - Siddiqui M.H, Al-Whaibi M.H and Basalah M.O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress. Protoplasma. 2011;248:447–455.
CrossRef - Khan M.N, Mobin M, Abbas Z.K and Siddiqui M.H. Nitric Oxide-Induced synthesis of Hydrogen Sulfide Alleviates Osmotic. 2017.
- Stress in Wheat Seedlings through Sustaining Antioxidant Enzymes, Osmolyte Accumulation and Cysteine Homeostasis. Nitric Oxide. In. Press. http://doi.org/10.1016/j.niox.2017.01.001.
CrossRef - Smith G.S, Johnston C.M and Cornforth I.S. Comparison of Nutrient Solutions for Growth of Plants in Sand Culture. New. Phytologist. 1983;94:537-548. http://dx.doi.org/10.1111/j.1469-8137.1983.tb04863.x
CrossRef - Lichtenthaler H.K and Buschmann C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In: Wrolstad R.E, Acree T.E, An H, Decker E.A, Penner M.H, Reid D.S, Schwartz S.J, Shoemaker C.F and Sporns P, Eds. Current Protocols in Food Analytical Chemistry, John Wiley and Sons, New York. 2001. F4.3.1-F4.3.8.
- Dwivedi R.S and Randhawa N.S. Evaluation of Rapid Test for Hidden Hunger of Zinc in Plants. Plant and Soil. 1974;40:445-451. http://dx.doi.org/10.1007/BF00011531
CrossRef - Velikova V, Yordanov I and Edreva A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Science. 2000;151:59-66.
CrossRef - Elstner E.F and Heupel A. Inhibition of Nitrite Formation from Hydroxyl Ammonium Chloride., A Simple Assay for Superoxide Dismutase. Annals of Biochemistry. 1976;70:616-620.
CrossRef - Cakmak I and Horst J.H. Effects of Aluminum on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiologia Plantarum. 1991;83:463-468.
CrossRef - Yamasaki S and Dillenburg L.C. Measurements of Leaf Relative Water Content in Araucaria angustifolia. Revista Brasileira de Fisiologia Vegetal. 1999;11:69–75.
- Lutts S, Kinet J.M and Bouharmont J. Changes in Plant Response to NaCl During Development of Rice (Oryza sativa L.) Varieties Differing in Salinity Resistance. Journal of Experimental Botany. 1995;46:1843–1852.
CrossRef - Bates L.S, Walden R.P and Teare I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant and Soil 39, 205–207.
CrossRef - Grieve C.M and Grattan S.R. Rapid Assay for Determination of Water Soluble Quaternary Ammonium Compounds. Plant and Soil. 1983;70;303–307.
CrossRef - Beauchamp C and Fridovich I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Annals of Biochemistry. 1971;44:276-287. http://dx.doi.org/10.1016/0003-2697(71)90370-8.
CrossRef - Upadhyaya A, Sankhla D, Davis T.D, Sankhla N and Smith B.N. Effect of Paclobutrazol on the Activities of Some Enzymes of Activated Oxygen Metabolism and Lipid Peroxidation in Senescing Soybean Leaves. Journal of Plant Physiology. 1985;121:453-461. http://dx.doi.org/10.1016/S0176-1617(85)80081-X
CrossRef - Cakmak I and Marschner H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiology. 1992;98:1222-1227. http://dx.doi.org/10.1104/pp.98.4.1222.
CrossRef - Khan M.N, Siddiqui M.H, Mohammad F and Naeem M. Salinity Induced Changes in Growth, Enzyme Activities, Photosynthesis, Proline Accumulation and Yield in Linseed Genotypes. World Journal of Agricultural Sciences. 2007;3:685-695.
- Siddiqui M.H, Khan M.N, Mohammad F and Khan M.M.A. Role of Nitrogen and Gibberellin (GA3) in The Regulation of Enzyme Activities and Osmoprotectant Accumulation in Brassica juncea L. under salt stress. Journal of Agronomy and Crop Science. 2008;194:214–224.
CrossRef - Idrees M, Naeem M, Khan M.N, Aftab T, Khan M.M.A. Moinuddin. Alleviation of Salt Stress in Lemongrass by Salicylic Acid. Protoplasm. 2011;249:709-20. 10.1007/s00709-011-0314-1.
CrossRef - Khan M.N. Growth and Physiological Attributes of Tomato (Lycopersicon esculentum Mill.) Genotypes as Affected by NaCl Stress. American Journal of Plant Sciences. 2016;7:453-460.
CrossRef - Badger M.R and Price G.D. The Role of Carbonic Anhydrase in Photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1994;45:369–392.
CrossRef - Reddy M.P and Vora A.B. Changes in Pigment Composition, Hill Reaction Activity and Saccharides Metabolism in Bajra (Pennisetum typhoides S&H) Leaves under NaCl Salinity. Photosynthetica. 1986;20:50-55.
- Dela-Rosa I.M and Maiti R.K. Biochemical mechanism in glossy sorghum lines for resistance to salinity stress. Journal of Plant Physiology. 1995;146:515–519.
CrossRef - Liu Y, Wu R, Wan Q, Xie G and Bi Y. Glucose-6-Phosphate Dehydrogenase Plays A Pivotal Role in Nitric Oxide-Involved Defense Against Oxidative Stress Under Salt Stress in Red Kidney Bean Roots. Plant and Cell Physiology. 2007;48:511–522.
CrossRef - Egbichi I, Keyster M and Ludidi N. Effect of exogenous application of nitric oxide on salt stress responses of soybean. South African Journal of Botany. 2014;90:131–136.
CrossRef - Ahmad P, Latef A A.A, Hashem A, Abd_Allah E.F, Gucel S and Tran L.S.P. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Frontiers in Plant Science. 2016;7: 347. doi: 10.3389/fpls.2016.00347.
CrossRef - Fatma M, Masood A, Per T.S and Khan N.A. Nitric Oxide Alleviates Salt Stress Inhibited Photosynthetic Performance by Interacting with Sulfur Assimilation in Mustard. Frontiers in Plant Science. 2016;7:521. doi: 10.3389/fpls.2016.00521.
CrossRef - Nakazawa H, Genka C and Fujishima M. Pathological Aspects of Active Oxygens/Free radicals. The Japanese Journal of Physiology. 1996;46:15–32. 10.2170/jjphysiol.46.15.
CrossRef - Tewari R.K, Hahn E.J and Paek K.Y. Function of Nitric Oxide and Superoxide Anion in the Adventitious Root Development and Antioxidant Defence in Panax ginseng. Plant Cell Reports. 2008;27:563–573. 10.1007/s00299-007-0448-y.
CrossRef - Bai X.Y, Dong Y.J, Wang Q.H, Xu L.L, Kong J and Liu S. Effects of Lead and Nitric Oxide on Photosynthesis, Antioxidative Ability, and Mineral Element Content of Perennial Ryegrass. Biologia Plantarum. 2015;59:163–170. 10.1007/s10535-014-0476-8.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





