How to Cite | Publication History | PlumX Article Matrix
Mohamed E. Osman , Om-kolthoum H. Khattab, A. A. Abo-Elnasr and S. Abdel Basset
, Om-kolthoum H. Khattab, A. A. Abo-Elnasr and S. Abdel Basset
Department of Botany and Microbiology, Faculty of Science, Helwan University, Cairo, Egypt.
Corresponding Author E-mail: sally_science@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2710
ABSTRACT: A microbial fuel cell (MFC) has great potential for azo dyes decolorization and electricity generation by using filamentous fungi as biocatalysts. In this study, Aspergillus niger and Trichoderma harzianum were inoculated in anode chamber of double-chamber MFC to decolorize azo dye acid black 172 with Potassium Ferricyanide in the cathode chamber. During MFC operations, Acid black 172 oxidized and produced a maximum open-circuit voltage of 890 mV, and maximum current density of 163 mA/m2 with an external resistance of 1000Ω. Also, variable parameters such as dye concentration, Co-substrate and dye as a sole carbon source were studied to improve microbial fuel cell performance.
KEYWORDS: Acid Black 172; Bioelectricity; Decolorization; Microbial Fuel Cell
Download this article as:| Copy the following to cite this article: Osman M. E, Khattab O. H, Abo-Elnasr A. A, Basset S. A. Acid Black 172 Dye Decolorization and Bioelectricity Generation by Microbial Fuel Cell With Filamentous Fungi on Anode. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Osman M. E, Khattab O. H, Abo-Elnasr A. A, Basset S. A. Acid Black 172 Dye Decolorization and Bioelectricity Generation by Microbial Fuel Cell With Filamentous Fungi on Anode. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32369 |
Introduction
Azo dyes are a group of poorly biodegradable synthetic colorants that are often found in the textile wastewater (Cai et al.,2014). These compounds are highly resistant to aerobic biodegradation by microorganisms but can be easily reduced by anaerobic microorganisms to form aromatic amines, which are a group of carcinogens that are stable under anaerobic conditions and must be returned to aerobic conditions before they can be further degraded and mineralized (Garcia et al., 2015). A range of physicochemical methods exists to remove color from dye containing effluents (Mu et al., 2004). The most extensively used are coagulation and flocculation processes. They require significant quantities of chemicals and produce notable amounts of sludge, requiring further handling and disposal. Enzymatic decolorization is now widely used for the decolorization of dye wastewater. However, this method is also facing several problems such as cost of enzymes, enzymes stability and product inhibition (Husain, 2010). On the other hand, biological processes provide a low-cost and efficient alternative for simultaneous color removal. In recent years, microbial fuel cell (MFC) technology has explored extensively for their innovative features and environmental benefits (Rozendal et al., 2009). This work presents an efficient and cost-effective approach to establish an MFC-assisted electrochemical oxidation process for the decolorization of azo dye.
Methodology
Dyes
Five model textile dyes (obtained from Moket Mac Company at 10th of Ramadan City, Egypt) were used for testing decolorization capacity of Aspergillus niger and Trichoderma harzianum including: Acid red 399, Acid yellow 235, Acid yellow 218, Acid blue 296, and Acid black 172.
Sample collection and cultivation conditions
A fungal species was collected from wastewater of an Egyptian Company for artificial Carpet at 10th of Ramadan City, in sterile clean glass bottles then, stored at 4°C, cultivated in petri-dishes containing Dox᾽s agar medium at 25°C and stocked in slant tubes containing Czapek᾽s yeast agar solid medium under refrigeration at 4°C. Initial selection was based on maximum voltage production and dye decolorization. For submerged cultures, a pre-inoculum of young mycelium disk of 0.5cm of diameter was obtained from 2 days-old colonies for Aspergillus niger, while in case of Trichoderma harzianum two young mycelium disks of 0.5cm of diameter were obtained from 4 days-old colonies in solid medium. Disks were inoculated into flasks with 50 ml of fresh medium containing 15g/L glucose, 2g/L NaNo3, 1g/L KH2PO4, 0.5g/L KCL and 5g/L yeast extract in 50 mM phosphate buffer (PH7) and incubated at 25°C for 96h for Trichoderma harzianum, while Aspergillus niger was incubated at 35°C for 48h. After this period of time, flasks were used for inoculation of 250 ml flasks (bottles) containing 50 ml Acid black 172 dye (which has been prepared using 10 mg/L) of anodic compartment of fungal microbial fuel cells. Each culture was incubated under maximum incubation temperature.
Construction of fungal microbial fuel cell
The dual chambered MFC was constructed using two air tight laboratory glass bottles (anode and cathode) of 250 ml capacity connected via a glass tube (Length꞊11 cm, width= 6.6 and diameter꞊0.5 cm) that is heated and bent into a u-shaped, filled with 2% agar gel in saturated KCL solution and inserted through the lid of each bottle. The anodic and cathodic electrodes were graphite rods (surface area꞊5.5cm). Copper (Cu) wires were soldered to the electrodes by using a conductive epoxy. A multimeter with a data logger system (MT-1820 ProsʹKit, Taiwan) was used as the automated data acquisition system.
Operation of a fungal microbial fuel cell
The fuel cell compartments were cleaned and the electrodes were autoclaved for 20 min. The anode solution (50mL) contained the following components (per liter) 15g C6H12O6, 2g NaNO3, 1g KH2PO4, 0.5g KCL , 5g yeast extract in 50 Mm phosphate buffer (pH 7) and fungal biomasses. The compartment was inoculated with 50 ml acid black 194 which prepared as mentioned previously, finally anode volume (100 ml).
The cathode solution on the other hand, consisted of 100 ml of Potassium Ferricyanide (K3(Fe(CN)6) solution (10 mmol/L) prepared in distilled water. The initial pH of Potassium Ferricyanide solution (pH 2) at cathode was adjusted using 0.5M HCL which acted as the electron acceptor.
The MFCs with inoculation were first incubated with electrolytes described above for 4 days, then evaluated the decolorization rate of Acid black 172 and electricity generation. The external resistance was fixed at 1000Ω and in some case the MFCs were operated under different external resistances to obtain the polarization curves. The temperature of MFCs was kept at 35°C for Aspergillus niger and at 25°C for Trichoderma harzianum using the laboratory incubators both the two inoculated MFCs and the two control sets were operated under identical conditions respectively, and average results were reported.
Electrochemical Analyses
Cell voltages were recorded every 20 minute after a stable open circuit potential was achieved using a data logger system. Current (I) was calculated at a resistance(R) from the voltage (V) by I=V/R, and current density (I/A2), where A is the projected anode surface area.
Acid Black 172 Decolorization
The absorption of A. black 172 in the visible light range peaks at a wavelength of 572 nm was measured using a T60 UV-VIS spectrophotometer and used to calculate A.black 172 from Eq. (1) where A0 and A are the initial and final absorptions, respectively.
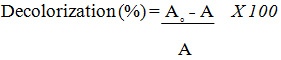
Results and Discussion
 |
Scheme 1
|
Decolorization of dyes using Aspergillus niger and Trichoderma harzianum
Aspergillus niger and Trichoderma harzianum were selected to investigate their effect on decolorization rate of the following dyes: (1) Acid red 399, Acid yellow 235, Acid yellow 218, Acid blue 296, and Acid black 172. The initial concentration was (10 mg/L) amended in flasks containing 100 ml of Dox’s broth medium. The flasks were inoculated as previously mentioned. The results in Fig (2) show variation in decolorization abilities. Aspergillus niger was able to remove more than 50% of these acid dyes. On other hand, Trichoderma harzianum was able to remove less than 50% of acid red 399, acid yellow 235, acid yellow 218 and acid blue 296 dyes. The results showed that, Aspergillus niger and Trichoderma harzianum were effectively able to remove more than 85% of acid black 172. Accordingly, it is selected for further investigations. These results may be due to chemical structures of dyes significantly affecting the performance of dye biodecolorization. For similar structures of azo dyes, monoazo dyes are easily biodecolrized than polyazo dyes (Hsueh et al., 2009). Also, our results agree with Mohamed et al., 20105 who state that the best decolorizing dye was acid black 194 by Aspergillus flavus, Aspergillus tamarii and Aspergillus parasiticus.
Acid black 172 concentration effect on dye decolorization and voltage output (OCV)
Comparison on performance of voltage output in a fungal microbial fuel cell at various concentrations of acid black 172 (i.e., 5, 10, 30 and 50mg/L; Table.1) showed that the decolorization percent and voltage output(OCV) for Aspergillus niger and Trichoderma harzianum reached its maximum value (95%) and (890 mV) respectively at dye concentration 10 mg/L. Also showed that increasing in the dye concentration resulted in a decrease in the decolorization percent and voltage output (OCV). The results are in agreement with the findings of Bor et al. (2010) that observed the decreased peak output voltage at high loading of Reactive Blue 160 might explain that decolorization and bioelectricity generation of Proteus hauseri are competitive to each other as both reactions all utilized electrons released from bacterial oxidation of organic matter. In addition, possibly due to accumulation of the decolorized intermediates of Reactive Blue 160 as mediators, the steady-state (SS) cell-voltages were stabilized at slightly higher levels than the SS-voltage in Reactive blue 160 free cases. In general, the performance of an MFC depends on the concentration and the type of dye used; Mu et al. (2009) showed that during closed-circuit operation, decolorization efficiency decreased from 78.7% to 35% with an increase in influent dye concentration. Sun et al. (2009) reported that the percent decolorization decreased with increase in Active Brilliant Red X-3B concentration.
Co-substrate effect on decolorization of Acid black 172 and voltage output (OCV)
Different co-substrates such as glucose, and sucrose were individually added as equimolecular to 30g sucrose per liter were tested to determine the optimum co-substrate which supported the maximum decolorization and voltage output of the tested fungi. The results in Table (2) showed that, the decolorization percent for Aspergillus niger and Trichoderma harzianum reached its maximum value (95% and 98%) respectively using glucose as co-substrate, also showed voltage output value reached its maximum value (899 mV and 895 mV) for Aspergillus niger and Trichoderma harzianum respectively. The results similar to those obtained by Sun et al. (2009) who stated that, the maximum decolorization rate of ABRX3 observed with glucose, also in agreement with our results Cao et al. (2010) found that, the power density was highest for glucose during decolorization of Congo red.
Simultaneous dye decolorization and current generation
Dye decolorization rate and current generation were measured after operation for three days. The results in Table (3) showed that decolorization rate and current density were decreased after sixty hours of operation. These results in contrast with Chen (2006) and Chen et al. (2009) who mentioned that impulse additions of C.I. reactive red 141 significantly enhanced increasing the rate of oxidative phosphorylation of P.hauseri to accelerate electron transport in the respiratory chain of immobilized cells on the anodic biofilm for bioelectricity production in MFC.
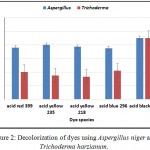 |
Figure 2: Decolorization of dyes using Aspergillus niger and Trichoderma harzianum.
|
Table 1: Acid black 172 concentration effect on dye decolorization and voltage output (OCV).
| Dye concentration
(mg/L) |
Decolorization (%) | Voltage(OCV) | ||
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| 5 | 93 | 94 | 850 mV | 870 mV |
| 10 | 95 | 95 | 890 mV | 890 mV |
| 30 | 80 | 75 | 810 mV | 800 mV |
| 50 | 65 | 61 | 750 mV | 720 mV |
Table 2: Co-substrate effect on decolorization of Acid black 172 and voltage output (OCV).
| Co-substrate | Decolorization(%D) | Voltage (OCV) | ||
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| Glucose | 95 | 98 | 899 mV | 895 mV |
| Sucrose | 90 | 92 | 820 mV | 800 mV |
| Control | 80 | 75 | 700 mV | 620 mV |
Table 3: Simultaneous dye decolorization and current generation.
| Time (h) | Decolorization (%) | Current density (mA/m2) | ||
| Aspergillus | Trichoderma | Aspergillus | Trichoderma | |
| 0 | 95 | 94 | 163 | 160 |
| 12 | 93 | 94 | 149 | 154 |
| 24 | 92 | 93 | 147 | 154 |
| 36 | 92 | 92 | 145 | 145 |
| 48 | 90 | 90 | 145 | 136 |
| 60 | 90 | 90 | 136 | 131 |
| 72 | 88 | 85 | 133 | 129 |
Conclusion
The microbial fuel cell (MFC) with a fungal anode achieved more than 84% decolorization of azo dye acid black 172 in three days of continuous operation with current density more than 128 MA/m2. Also, a maximum open circuit voltage(OCV) was obtained with 10 g/L of dye concentration was 890 mV and maximum open circuit voltage(OCV) was obtained with Co-substrate with glucose was more than 894mV for the two fungal species.
Acknowledgements
The authors would like to thank Helwan university of Egypt, for supporting this research as academic study. The authors also appreciate technical supports by scientific laboratory of Helwan university. Significant comments from the anonymous reviewers are also very much appreciated.
References
- Bor Y. C., Meng Z., Chang T. C., Yongtao D., Kae L. L., Chyow S. C., Chung C. H., Huizhong X. Assessment upon azo dye decolorization and bioelectricity generation by proteus hauseri. Bioresource Technology. 2010;101:4737-4741.
- Cai M. Q., Wei X. Q., dUch., Ma X. M., Jin M. C. Novel amphiphilic polymeric ionic liquid-solid phase micro-extraction membrane for the preconcentration of aniline as degradation product of azo dye Orange G under sonication by liquid chromatography- tandem mass spectrometry. J Chromatograghy A. 2014;1349:24-9.
- Cao Y.,Hu Y.,Sun J., Hou B. Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air- cathode single-chamber microbial fuel cell. Bioelectrochemistry. 201;79,71-76.
- Chen B. Y., Yen C. Y., Chen W. M., Chang C. T., Wang C. T., Hu Y. C. Exploring threshold operation criteria of biostimulation for azo dye decolorization using immobilized cell systems. Bioresour. Technol. 2009;100:5763-5770.
- Chen B. Y. Toxicity assessment of aromatic amines to Pseudomonas luteola: chemostat pluse technique and dose-response analysis. Process. Biochem. 2006;41:1529-1538.
- Garcia M. Y,, Bengoa C., Stuber F., Fortuny A., Font J., Fabregat A. Biodegradation of acid orange 7 in an anaerobic-aerobic sequential treatment system. Chem Eng Process. 2015;99-104.
- Hsueh C. C., Chen B. Y., Yen C. Y. Understanding effects of chemical structure on azo dye decolorization characteristics by. Aeromonas hydrophila. J. Hazard. Mater. 2009;167:995-1001.
- Husain Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: a review. Rev. Environ. Sci. Biotechnol. 2010;9:117-140.
- Mohamed E., Om-Kolthoum H., Amany A., Sally A. Optimization of different parameters for decolorization of acid black 194 dye using the selected fungal species. Int. Curr. Microbial. App . Sci. 2015;4(3):866-891.
- Mu Y., Rabaey K., Rozendal R., Yuan Z., Keller J. Decolorization of azo dyes in bioelectrochemical systems. Environ. Sci. Technol. 2009;34:5137-5143.
- Mu Y., Yu H. Q., Zheng J. C., Zhang S. J. TiO2-mediated photocatalytic degradation of orange II with the presence of Mn2+ in solution. J. Phtochem.Photobiol. 2004;163:311-316.
- Rozendal R. A., Leone E., Keller J., Rabaey K. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Commun. 2009;11:1752-1755.
- Sun J., Hu Y., Bi Z., Cao Y. Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour. Technol. 2009;100:3185-3192.

This work is licensed under a Creative Commons Attribution 4.0 International License.





