How to Cite | Publication History | PlumX Article Matrix
Abbas Mohamed Al-Azab1 and Essam Abdel-Salam Shaalan2
and Essam Abdel-Salam Shaalan2
1Biological Science Department, Faculty of Science, Sana'a University, Yemen.
2Zoology Department, Faculty of Science, Aswan University, Aswan 81528, Egypt.
Corresponding Author E-mail: abbasazab2000@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2693
ABSTRACT: A laboratory evaluation of three slow-release formulations (SRFs) of spinosad tablets, sumilarv granules and du-dim tablets was carried out in 20 liters plastic containers. The tested formulations were applied according to recommended doses to evaluate their efficacy and longevity against Ae. aegypti. The results revealed that the tested SRFs achieved complete inhibition of adult stages emergence of Ae. aegypti in the first four weeks and gave continuous effective with 50-100% for 98, 86 and 78 days post -treatment by using spinosad tablets, sumilarv granules and du-dim tablets respectively. The highest larval mortality was observed for spinosad followed by sumilarv and du-dim, it gave 1.3 folds than sumilarv and 1.1 folds with du-dium, whereas sumilarv and du-dium were more effective on pupal mortality than spinosad. On the other hand, morphological abnormalities were observed in larval and pupal stages of Ae. aegypti as delayed effects by tested formulations. This study highlighted that these SRFs could be used as potential larvicidal compounds in mosquito control programs as a single treatment and provide satisfactory results and continuous control against the dengue vector Ae. aegypti for several weeks.
KEYWORDS: Aedes Aegypti; Conditions; Evaluation; Persistence; Slow-Release Formulations
Download this article as:| Copy the following to cite this article: Al-Azab A. M, Shaalan E. A. S. Evaluation of Effectiveness and Persistence of Three Slow Release Formulations Against Aedes Aegypti (L) (Diptera: Culicidae) under Laboratory Conditions. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Al-Azab A. M, Shaalan E. A. S. Evaluation of Effectiveness and Persistence of Three Slow Release Formulations Against Aedes Aegypti (L) (Diptera: Culicidae) under Laboratory Conditions. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32185 |
Introduction
Mosquitoes are an annoying insects transmitting several diseases to human causing millions of deaths worldwide annually or lead to interfere with their work performance and spoil leisure time (WHO.2015). Dengue Fever is a viral disease which mainly transmitted by Ae. aegypti that became more widely dispersed than at any time in the past (Halstead, 2008). Outbreaks of dengue fever were reported in several tropical and sub-tropical countries and have increased 10 folds in the last 30 years (Simmons and Farrar, 2009 and WHO, 2015). Around 128 countries are at risk, with more than 3 billion infected people with dengue virus , and more than 20 thousand deaths annually(Bhatt, et al. 2013,WHO, 2015, Suresh , et al .,2015 and Rajaganesh et al .,2016).
In Saudi Arabia, the first dengue outbreak occurred in over 50 years. (Gubler, 2002) Since 1994, when the dengue fever infection was first officially documented in Jeddah, Saudi Arabia, it became a major public health problem in Jeddah city(Fakeeh and Zaki 2001). In 2006, dengue fever reported cases had risen drastically compared to the earlier recorded numbers9. Ghaznawi reported that the water storage containers, which found inside or outside construction sites, play an important role as breeding sites for Ae. aegypti and distribution of dengue virus infection in Jeddah (Aburas, 2007). Mosquito control strategy is the only effective method to control the disease, becuse there is no a specific drug to treat the dengue virus and no vaccine is available (Suresh et al., 2015 and Rajaganesh et al., 2016). The widespread and frequent usage of the chemical insecticides has led to resistant mosquito populations resulting in a human health concern (Benelli, 2016).
Basically, the effective control depends upon prevention and elimination of aquatic habitats that are necessary for the development of the immature stages of mosquitoes. Such habitats sometimes cannot be drained or removed to eliminate, therefore the application of IGRs formulation to these potential larval development sites consider the primary or the sole measure and sustainable way which will drastically reduce the population of Ae. aegypti and prevent disease outbreaks (WHO, 2004). Thus, the searching for alternatives, including bioregulators-synthetic analogs of insect hormones and biological-entomopathogenic bacteria products is very necessary and considerably important. Among these recommended products that could be used as alternatives to synthetic insecticides might be Spinosad, Sumilarv and Du-dium, due to their slow toxicity for humans and non-targets organisms and a highly effective against pests such as mosquitoes (Attaran et al., 2000, Suman et al., 2010 and WHO, 2010).
Spinosad contains 2 insecticidal factors, spinosyn A and spinosyn D, present in a 85:15 ratio within the final product (Kirst et al., 1992). It has very low acute mammalian toxicity. It had established a new standard for low environmental and other non-target fauna, human risks and degraded rapidly in the environment (Miles and Dutton, 2000, Cisneros et al., 2002 and Williams et al., 2003). It’s mode of action is unique against larvae involving the postsynaptic nicotinic acetylcholine and gamma-amino butyric acid receptors (GABA ), acted as a stomach , contact poison and neurotoxin and affecting on these receptors function(Watson, 2001 and Cisneros et al., 2002).Mosquito larvicidal activity of Spinosad was first reported in laboratory bioassays screening extracts from the fermentation broth of Saccharopolyspora Spinosa.
Bond et al. (2004) reported the Tracer®, a commercial suspension concentrate formulation (Tracer® Naturalyt® Insect Control) of spinosad, had high larvicidal toxicity against Ae. aegypti L. and Anopheles albimanus in the laboratory (Bond et al. 2004). The 24-h LC50 against 3rd and 4th instars of Ae. aegypti and An. Albimanus was estimated at 0.025 mg ( AI ) /liter and 0.024 mg (AI ) /liter, respectively. Several researchers evaluated the naturally – derived insecticide spinosad against larvae of mosquitoes species (Romi et al., 2006; Bahgat et al., 2007 ; Jiang and Mulla, 2009 ; Thavara et a1.,2009 and Hertlein et al., 2010). Semi-field and field studies have confirmed that different formulations of SRFs products could produce satisfactory effective control against Ae. aegypti and Culex spp (WHO, 2008, and Hertlein et al., 2010).
Diflubenzuron was the first bioinsecticides benzoylurea compound. Different studies on its mode of action against insects indicated that metabolism played a major role in the determination of the toxicological efficiencies of these compounds (Neumann and Guyer, 1987). Diflubenzuron has been recommended for controlling mosquitoes in drinking water. Its insecticidal effect came from inhibition of chitin synthesis in the cuticle of treated larvae (WHO, 2008).Numerous of studies showed that the diflubenzuron compounds have good efficacy for controlling the larvae of mosquitoes (Mulla et al. 2003; Thavara et al., 2007; Seccacini et al., 2008; Romeo et al., 2009; Silva et al., 2009 and Suman et al., 2010 ).
Pyriproxyfen (a growth regulator) is a juvenile hormone analogue which is mainly active against the developmental pupal stages of mosquitoes. Its mode of action to disrupt the regulation insects hormones leading to the inhibition of development, disturbed behaviour, decrease in adult fertility, inhibitor of embryogenesis, metamorphosis, and adult formation (Ishaaya and Horowitz ,1992). It has low toxicity for mammals with an LD50 above 5000 mg/kg for rats36. Pyriproxyfen had been evaluated for its action against mosquitoes and recommended to use for the control of some mosquito species (WHO, 2001). Concentrations of less than or equal to one part per billion of pyriproxyfen cause inhibition of adult Ae. aegypti emergence and remains effective up to five months, longer than Bacillus thuringiensis israeliensis, methoprene, or temephos. Adult mosquitoes emerged from larvae exposed to pyriproxyfen have decreased fecundity and such contaminated adults can disseminate lethal doses from treated to untreated sites ( Sihunincha et al., 2005).. Field and laboratory studies have revealed that pyriproxyfen have good residual activity against the pupal stage of Ae. aegypti (Nayar et al., 2002 ; Seccacini et al., 2008 and Aziz , 2017 ).
The present work was planned to evaluate the efficacy and longevity of three SRFs, spinosad, du-dium and Sumilarv, against the developmental stages of Ae. aegypti, the primary vector of dengue fever.
Materials and Methods
Mosquito Strain
This investigation involved evaluation and determining the efficacy and longevity of three selected slow release formulations under laboratory conditions. Larval stages of Ae. aegypti were collected from breeding habitats in Al-Balad-Jeddah city and had maintained in the laboratory under controlled conditions of 27±1°C and 70±5% R.H., with 14:10 (L:D) photoperiod.
Insecticides
Three slow release formulations (SRFs) were used: Spinosad (7.4% tablet) and Du-Dim (diflubenzuron 2 % tablet). They were supplied by Mosquito Research laboratory of the Municipality of Jeddah and Sumilarv (pyriproxyfen 0.5 % WDG) manufactured by Summit Chemical Company Japan, was obtained from Agricultural office-Jeddah, Saudi Arabia.
Test Experiments
Test procedures were conducted according to standard WHO guidelines (2005).Experiments were carried out in transparent plastic containers (40× 31× 21 cm) containing 20 liters of tap water. Lids for covering the plastic containers were cut from the middle and muslin were glued in all lids which were in place all the time to prevented either debris or adult mosquitoes from entering the containers. The lids just opened during addition of larvae and inspecting the efficacy of tested formulation. Each containers received 25 second instar larvae of A. aegypti from lab rearing colonies and the tested formulations. The amount of each formulation (0.14gm Spinosad; 1 gm Du-dim tablet; and 0.2gm Sumilarv) required for treating mosquito larvae was determined according to the recommended dosages for field trials as well as by calculating the total volume of water in the container. The larvae were given the larval food during these tests. Three untreated containers were kept as control. The treatments were made in four replicates. The plastic containers were inspected daily and larval mortalities were recorded until all larvae either pupated or died. Alive pupae were counted and transferred to the lab rearing untreated water in glass beakers to observe adult emergence or mortality. New sets of alive larvae were added to the test containers when complete larval mortality occurred. Every two weeks one third of water volume in all containers was removed and refilled, to simulate the field conditions. Temperature (25-27 C) and PH (7.6-8.2) of water in all treatments were measured every day during experiments. Fifty percentage inhibition of adult emergence was calculated and taken as criteria for low level of larvicidal effectiveness activity and persistence of tested formulations mortality percentage of larvae in all containers as well as the reduction percentage and inhibition of emergence (IE%) of adult.
Data Analysis
Percentages of mortality were corrected using Abbot’s formula. The data were statistically analyzed to obtain ANOVA and least significant different using SAS (V 9.0).
Results and Discussion
Different artificial containers such as water-storage that used for either holding or storing water consider suitable habitats and play an important role for the development of Ae. aegypti inside or outside buildings (Thavara et al., 2004).Evaluation of efficacy and longevity of three slow release formulations against the field strain of Ae. aegypti was conducted. The results demonstrated that the mortality occurred either in the immature stages (larvae and pupae) or at inhibition adult emergence.
Efficacy and longevity of slow release formulation of spinosad
As shown in table(1) and Figs (1and2) the efficacy of slow release formulations Spinosad, Nature DT against the immature and adult stages during the period of experiment (98 days) achieved 95-100% mortality of larval stages and 90-100 % inhibition of adult stages emergence in the first eight weeks. Spinosad showed higher larval mortality compared with sumilarv and du-dium. It produced the highest larval mortality and gave 1.3 fold than sumilarv and almost the same effectiveness with du-dium but its effectiveness started to decrease gradually after 77 days post treatment.
Table 1: Efficacy and longevity of Spinosad 7.4% on developmental stages of dengue fever vector Ae. aegypti from 1-98 days.
| No. of tests | larva mortality(%)a
Means ±SD |
Pupation% | Adult emergence% | Inhibition% | Duration effectiveness (days) |
| 1 | 100.0 ± 0.0* (7)b | 0.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | 98 |
| 2 | 100.0 ± 0.0* (6)b | 0.0 ± 0.0* | 0.0 ± 0.0* | 100.0± 0.0* | |
| 3 | 100.0 ± 0.0* (6)b | 0.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 4 | 100.0 ± 0.0* (7)b | 0.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 5 | 98.0 ± 0.8(7)b | 2.0 ± 0.8 | 1.0 ± 1.0 | 99.0 ± 1.0 | |
| 6 | 100.0 ± 0.0* (8)b | 0.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 7 | 100.0 ± 0.0* (7)b | 0.0 ± 0.0* | 2.0 ± 1.7 | 98.0 ± 1.7 | |
| 8 | 100.0 ± 0.0* (6)b | 0.0 ± 0.0* | 1.0 ± 0.8 | 99.0 ± 0.8 | |
| 9 | 98.0 ± 1.4(8)b | 2.0 ± 1.4 | 2.0 ± 0.8 | 98.0 ± 0.8 | |
| 10 | 95.0 ± 0.8 (7)b | 5.0 ± 0.8 | 10.0 ± 1.6 | 90.0 ± 1.6 | |
| 11 | 86.0 ± 2.1(8)b | 14.0 ± 2.1 | 20.0 ± 8.1 | 80.0 ± 8.1 | |
| 12 | 82.0 ± 8.1(7)b | 18.0 ± 8.1 | 22.0 ± 1.8 | 78.0 ± 1.8 | |
| 13 | 80.0 ± 3.1(7)b | 20.0 ± 3.1 | 25.7 ± 3.0 | 74.2 ± 3.0 | |
| 14 | 65.0 ± 2. 4(7)b | 35.0 ± 2.4 | 48.0 ±3 .6 | 52.0 ± 3.6 |
a: 25 larvae for each replicate and four replicates for each test.
b: Days post-treatment until complete mortality or pupation.
*: Not significantly different within the same column (P < 0.0001).
Efficacy and longevity of slow release formulation of Sumilarv
The results in table (2) and Figures (1and 2) revealed the efficacy of the juvenoid action of sumilarv against the immature and adult stages during the period of experiment (86 days). The mortality recorded of larval stages was 2-6%, whereas the inhibition of adult stages emergence was 80-100% in the first six weeks. Sumilarv showed higher pupal mortality compared with spinosad and its effectiveness started to decrease gradually after 56 days post treatment.
Table 2: Efficacy and longevity of Sumilarv 5% WDG on larvae of dengue fever vector Ae. aegypti from 1-86 days.
| No.
of tests |
Larval mortality (%)a Means ±SD | Pupation (%) | Adult emergence(%) | Inhibition (%). | Duration effectiveness (days) |
| 1 | 0.0 ± 0.0* (9)b | 100.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 2 | 0.0 ± 0.0* (10)b | 100.0.± 0.0* | 0.0 ± 0.0* | 100 .0 ± 0.0* | 86 |
| 3 | 0.0 ± 0.0* (9)b | 100.0 ± 0.0* | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 4 | 6.0 ± 0.8(9)b | 94.0 ± 0.8 | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 5 | 4.0 ± 0.8(9)b | 96.0 ± 0.8 | 16.0 ± 0.8 | 84.0 ± 0.8 | |
| 6 | 2.0 ± 1.6(10)b | 98..0 ± 1.6 | 20..0 ± 0.8 | 80.0 ± 0.8 | |
| 7 | 5.0 ± 0.8(11)b | 95.0 ± 0.8 | 26.0 ± 2.1 | 74.0 ± 2.1 | |
| 8 | 6.0 ± 0.8(9)b | 94.0 ± 08 | 40.0 ± 1.6 | 60.0 ± 1.6 | |
| 9 | 3.0 ± 0.8(10)b | 97.0 ± 0.8 | 50.0 ± 1.4 | 50.0 ± 1.4 |
a: 25 larvae for each replicate and four replicates for each test.
b: Days post-treatment until complete mortality or pupation.
*: Not significantly different within the same column (P < 0.0001).
Efficacy and longevity slow release formulation of du-dium
The results in Table (3) and Figures (1and 2) represented the efficacy of slow release formulation of du-dium against the immature and adult stages during the period of experiment (78 days). The larval mortality yielded 14-32% whereas the inhibition of adult stages emergence was 08-100% in the first eight weeks, the IE fluctuated between 54-100% during the period of study. Du-dium showed higher pupal mortality compared with spinosad and its effectiveness started to decrease gradually after 62 days post treatment.
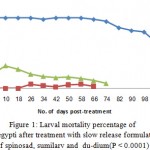 |
Figure 1: Larval mortality percentage of Ae. aegypti after treatment with slow release formulations of spinosad, sumilarv and du-dium(P < 0.0001). |
Table 3: Efficacy and longevity of Du-dium 2% on larvae of dengue fever vector Ae. aegypti from 1-78 days during.
| No. of tests | Larval mortality (%)a Means ±SD | Pupation (%) | Adult emergence (%) | Inhibition (%). | Duration effectiveness (days) |
| 1 | 20.0 ± 0.8(9)b | 80.0 ± 0.8 | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 2 | 32.0 ± 3.5(8)b | 68.0 ± 3.6 | 0.0 ± 0.0* | 100.0 ± 0.0* | 78 |
| 3 | 27.0 ± 2.5(8)b | 73.0 ± 2.6 | 0.0 ±0.0* | 100.0 ± 0.0* | |
| 4 | 20.0 ± 1.6(7)b | 80.0 ± 1.6 | 0.0 ± 0.0* | 100.0 ± 0.0* | |
| 5 | 17.0 ± 1.4(8)b | 83.0 ± 1.4 | 2.0 ± 1.4 | 98.0 ± 1.4 | |
| 6 | 15.0 ± 1.8(8)b | 85.0 ± 1.8 | 4..0 ± 0.8 | 96.0 ± 0.8 | |
| 7 | 16 .0 ± 1.3(7)b | 84.0 ± 1.2 | 5.0 ± 0.8 | 95.0 ± 0.8 | |
| 8 | 14.0 ± 1.5(7)b | 86.0 ± 1.5 | 19.0 ± 1.8 | 81.0 ± 1.8 | |
| 9 | 11.0 ± 0.8(8)b | 89.0 ± 0.8 | 25.0 ± 1.8 | 75.0 ± 1.8 | |
| 10 | 7.0 ±.1.5(8)b | 93.0 ± 1.5 | 46.0 ± 2.9 | 54.0 ± 2.9 |
a: 25 larvae for each replicate and four replicates for each test.
b: Days post-treatment until complete mortality or pupation.
*: Not significantly different within the same column (P < 0.0001).
Results of the present study revealed that the three SRFs achieved complete inhibition of adult stages emergence of Ae. aegypti in the first four weeks and gave continuous effective with 50-100% for 98, 86 and 78 days post treatment by using spinosad tablets ,sumilarv granules and du-dim tablets, respectively. The results demonstrated that the spinosad, sumilarv and du-dium achieved 100% inhibition of adult emergence up to 28 days post treatment (Fig.1). The complete adult emergence observed in the present study could be due to morphological aberration that lead to failure in adult emergence (Mulla, 1995. They began to gradually decreasing their effectiveness after 77, 62 and 56 days post treatment for spinosad, sumilarv and du-dium respectively. The reason for this fluctuating of decreasing might be to different mode of actions and the type of formulations (Thavara, 2009). Even with replacement of third of water in the containers in all treatments, the three tested formulations in this study provided high efficacy and long-term adult inhibition. This agrees with findings of Chang et al. (2006) and Seng et al. (2008) who reported that the removing and replacing two-thirds of the volume of the water didn’t reduce the duration efficacy of a controlled-release formulation of pyriproxyfen against Ae. aegypti mosquito in simulated domestic water storage containers.
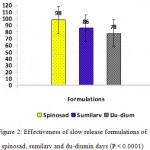 |
Figure 2: Effectiveness of slow release formulations of spinosad, sumilarv and du-diumin days (P < 0.0001). |
As shown in tables 2 and 3, both sumilarv and du-dium have poor larvicidal effects against Ae. aegypti compared with spinosad , in contrast the spinosad (Tab. 3) revealed the highest larval mortality. It gave 1.3 folds than sumilarv and 1.1 folds effectiveness than du-dium. Both sumilarv and du-dium were more effective on pupal mortality than spinosad because they have complementary mode of action (Darriet and Cobel, 2006 and Lee et al., 2005) . Sumilarv inhibits adults and prevent their emergence, conversely spinosad showed highly larval mortality, despite that there were some survivorship of larvae. On the other hand, morphological abnormalities were observed in larval and pupal stages of Ae. aegypti as delayed effects by tested formulations (Fig.3) which agrees with Vythilingam et al. (2005) who reported that the IGRs induce abnormalities or delayed effects on mosquitoes larvae lead to decline in reproduction or fecundity.
 |
Figure 3: Morphological abnormalities in the larval and pupal stages of Ae. aegypti post-treatment with spinosad. (A) Sumilarv, (B) (intermediate stage) and spinosad, (C) Sumilarv, (D) Black area, (E) Incompletely emerged pupa, (F) Normal untreated larvae (Control). |
Overall, in this study the three SRFs showed high efficacy and longevity against Ae. aegypti for several weeks in water containers and have low risk to human, environment and non-target organisms(Williams et al., 2003 and WHO, 2010).Nemours similar studies were carried out by several researchers using different SRFs of insecticide such as pyriproxyfen, diflubenzuron and spinosad against different species of mosquito vectors (El-Shazly and Refaie, 2002 Thavara et al., 2007, Vythilingam et al., 2005 ; Seng et al., 2008; Martins, et al., 2008; Kamal and Khater, 2010 and Aziz, 2017 , Al-Azab and Shaalan, 2018).
Conclusion
It could be concluded that, three SRFs showed high efficacy and longevity against Ae. aegypti mosquito for several weeks in water storage under lab conditions. Thus we suggest that the slow-release formulations used in this study, could be used as a suitable bioinsecticides alternative for chemical insecticides in vector control program by a single application. Furthermore, mixture of spinosad with either sumilarv or du-dium formulations could be applied in mosquito breeding sites especially artificial water containers or tanks to achieve larval and pupal mortality and prevent their survivorship and consequently.
Acknowledgements
The authors are grateful to all work colleagues and friends for their co-operation and encouragement during this work. The authors also thankful to editor and reviewers of this Journal for their valuable comments and suggestions to the improvement of this paper.
Conflict Of Interest
There is no conflict of interest.
References
- Abbott W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:256-269.
CrossRef - Aburas H. M. ABURAS Index : A Statistically Developed Index for Dengue- Transmitting Vector Population Prediction . PWASET. 2007;23:151–154.
- Al-Azab A., Shaalan E. Efficacy of Spinosad and Flubex against Dengue Fever Vector Aedes aegypti in Jeddah governorate, Saudi Arabia. Vet. Res. 2018;3(2):15-21.
- Attaran A., Roberts D. R., Curtis C. F., Kilama W. L. Balancing risks on the backs of the poor. Nature Medicine. 2000;6:729–731.
CrossRef - Aziz A. T. Insecticidal activity of three insect growth regulators towards the dengue and Zika virus vector Aedes aegypti in Saudi Arabia. J Entomol and Zool Stud . 2017;5(1):961-966.
- Bahgat I., El Kady G., Tamerak S., Lysandrou M. The natural bio-insecticide spinosad and its toxicity to combat some mosquito species in Ismailia Governorate , Egypt. World J Agric Sci. 2007;3(4):396-400.
- Benelli G. Plant-mediated synthesis of nanoparticles: A newer and safer tool against mosquito-borne diseases? Asia Pacif J Trop. Biomed. b. 2016;6:353-354.
- Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., et al. The global distribution and burden of dengue.Nature. 2013;496:504-507. http://dx.doi.org/10.1038/nature 12060.
- Bond J. G., Marina C. F., Williams T. The naturally derived insecticide spinosad is highly toxic to Aedes and Anopheles mosquito larvae. Med Vet Entomol. 2004;18:50-56.
CrossRef - Chang M., Setha T., Chantha N., Socheat D., Guillet P., Nathan M. B. Inhibition of adult emergence of Aedes aegypti in simulated domestic water storage containers by using a controlled-release formulation of pyriproxyfen. J Am Mosq Control Assoc. 2006;22:152-4.
CrossRef - Cisneros J., Goulson D., Derwent L. C., Penagos D. I., Hernandez O., Williams T. Toxic effects of spinosad on predatory insects. Bioi Control. 2002;23:156-163.
CrossRef - Darriet F., Corbel V. Laboratory Evaluation of Pyriproxyfen and Spinosad, Alone and in Combination, Against Aedes aegypti Larvaedes J. Med. Entomol. 2006;43(6):1190-1194.
CrossRef - El-Shazly M. M., Refaie B. M. Parricidal effect of the juvenile hormone mimic pyriproxyfen on Culex pipiens. J. Am. Mosq. Cont. Assoc. 2002;18(4):321-8.
- Fakeeh M., Zaki A. M. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia 1994-1999. Am. J. Trop. Med. Hyg. 2001;65(6):76-767.
CrossRef - Ghaznawi H. I., Al-Khateeb T. O., Akbar N., Afifi H., Nasser A. Surveillance for dengue fever in Jeddah East Mediterr. Health J . 1997;3:567-570.
- Gubler D. J. Epidemic dengue /dengue hemorrhagic fever as public health , social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103.
CrossRef - Halstead S. B. Dengue-Mosquito Interactions. Ann. Rev. Ento. 2008;53:273-291.
CrossRef - Hertlein M. B., Mavrotas C., Jousseaume C., Lysandrou M., Thompson G. D., Jany W., et al. A review of Spinosad as a natural product for mosquito control. J Am Mosq Control Assoc. 2010;26:67–77.
CrossRef - Ishaaya I., Horowitz A. Novel phenoxy juvenile hormone analog (pyriproxyfen) suppresses embryogenesis and adult emergence of sweet potato whitefly (Homoptera: Aleyrodidae). J Eco Entomolo. 1992;85(6):2113-217.
CrossRef - Jiang Y., Mulla M. S. Laboratory and field evaluations of spinosad a biorational natural product, against larvae of Culex . J. Am Mosq Control Assoc. 2009;25:456 – 466.
CrossRef - Kamal H., Khater E. The biological effects of the insect growth regulators; pyriproxyfen and diflubenzuron on the mosquito Aedes aegypti. J. Egypt. Soc. Parasitol. 2010;40(3):565-574.
- Kirst H. A., Michel K. H., Mynderse J. S., Chio E. H., Yao R. C., Nakatsukasa W. M., et al. Discovery, isolation and structure elucidation of a family of structurally unique fermentation-derived tetracyclic macrolides. In: Baker D.R., Fenyes J. G., Steffens J. J., eds. Synthesis and chemistry of agrochemicals. Washington, DC. Am Chem Soci. 1992;3:214-225.
CrossRef - Lee Y., Zairi J., Yap H., Adanan C. Integration of Bacillus thuringiensis israeliensis H – 14 formulations and pyriproxyfen for the control of larvae of Aedes aegypti and Aedes albopictus. J Am Mosq Control Assoc. 2005;2(1):84-89.
CrossRef - Martins A. J., Belinato T. A., Lima J. B., Valle D. Chitin synthesis inhibitor effect on Aedes aegypti populations susceptible and resistant to organophosphate temephos. Pest Man. Sci. 2008;64(6):676-80.
CrossRef - Miles M., Dutton R. Spinosad-a naturally derived insect control agent with potential for use in glasshouse integrated pest management systems. Mededel Facult Landbouwkund Toegep Biolog Wetensehap Univ Gent. 2000;65(2):393 – 400.
- Mulla M. S., Thavara U., Tawatsi A., Chompoosri J., Zaim M.,Su T. Laboratory and field evaluation of Novaluron a new acylurea insect growth regulator against Aedes aegypti (Diptera: Culicidae). J Vect Ecol. 2003;28(2):241-254.
- Mulla M. S. The future of insect growth regulators in vector control. J Am Mosq Control Assoc. 1995;11:269-273
- Nayar J. K., Ali A., Zaim M. Effectiveness and residual activity comparison of granular formulation of insect growth regulators Pyriproxyfen and S-Methoprene against Florida mosquitoes in Laboratory and outdoor conditions. J Am Mosqu Control Assoc. 2002;18(3):196-201.
- Neumann R., Guyer W. Biochemical and toxicological differences in the modes of action of the benzoylureas. Pestic. Sci. 1987;20:147-156.
CrossRef - Rajaganesh R., Murugan K., Panneerselvam C., Jayashanthini S., Roni M., et al. (Fern-synthesized silver nanocrystals: Towards a new class of mosquito oviposition deterrents? Res Vet Sci. 2016;109:40-51.
CrossRef - Romeo B., Alessandro A., Marco C., Roberta C., Luciano D., Maurizio M. Efficacy and lasting activity of four IGRs formulations against mosquitoes in catch basins of northern Italy. J Euro Mosq Control Assoc. 2009;27:33-46.
- Romi R., Proietti S., Diluca M., Cristofaro M. Laboratory Evaluation of the Bioinsecticide Spinosad for Mosquito Control. J Am Mosquito Control Assoc. 2006;22(1 ):93-96.
CrossRef - Seccacini E., Lucia A., Harburger L., Zerba E., Licastro S., Masuh H. Effectiveness of pyriproxyfen and diflubenzuron formulations as larvicides against Aedes aegypti. J Am Mosq Control Assoc. 2008;24(3):398-403.
CrossRef - Seng C., Setha T., Nealon J., Socheat D., Nathan M. Six month of Aedes aegypti control with a novel controlled- release formulations of pyriproxyfen in domestic water storage containers in Combodia. South. Asian J. Trop. Med. Pub. Heath. 2008;39(5):822-826.
- Sihunincha M., Zamora-Perea E., Orellana-Rios W., Stancil J. D., Lopez-Sifuentes V., Vidal-Oré C. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J Med Entomol. 2005;42(4):620-630.
CrossRef - Silva J., Mendes J., Lomônaco C. Effects of sublethal concentrations of diflubenzuron and methoprene on Aedes ægypti ( Diptera : Culicidae ) fitness . Int J Trop Insect Sci. 2009;29(1 ):17-23.
CrossRef - Simmons C., Farrar J. Changing Patterns of Dengue Epidemiology and Implications for Clinical Management and Vaccines. PLoS Med Journal. 2009;6(9).
- Suman D., Parashar B., Shri P. Effect of Sublethal Dose of Diflubenzuron and Azadirachtin on Various Life Table Attributes of Culex quinquefasciatus (Diptera : Culicidae ). J Med Entomol. 2010;47(6):996–1002 (7).
- Suresh U., Murugan K., Benelli G., Nicoletti M., Barnard D. R., Panneerselvam C. et al. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2015;114:1551-1562.
CrossRef - Thavara U., Tawatsin A., Asavadachanukorn P., Mulla M. Field evaluation in Thailand of spinosad, a larvicide derived from Saccharopolyspora spinosa ( Actinomycetales ) against Aedes ægypti ( L.) larvae. Southeast Asian J. Trop Med Publie Health. 2009;40:235-242.
- Thavara U., Tawatsin A., Chansang C. Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti in water – stovage containers. Asian J. Trop. Med. And Pub. Heath. 2007;28(2):269-276.
- Thavara U., Tawatsin A., Kong-ngamsuk W., Mulla M. S. Efficacy and longevity of a new formulation of temephos larvicide tested in village-scale trials against Ae. aegypti larvae in water-storage containers. J Am Mosq Control Assoc. 2004;20(2):176-82.
- Tomlin C. D(ed). The pesticide manual. 12th ed. London, United Kingdom : British Crop Protection Council. 2000.
- Vythilingam I., Maria L., Luz B., Hani R., Siew R., Beng T., Huat T. Laboratory and field evaluation of the insect growth regulator pyriproxyfen (Sumilarv 0.5G) against dengue vectors. J. Am. Mosq. Cont. Assoc. 2005;21(3):296-300.
CrossRef - Watson G. Actions of insecticidal spinosyns on γ-aminobutyric acid receptors from small-diameter cockroach neurones. Pestic Biochem Physiol. 2001;71:20–28.
CrossRef - Williams T., Valle J., Vin˜ uela E. Is the naturally-derived insecticide Spinosad1 compatible with insect natural enemies? Bio Sci and Technol. 2003;13:459–475.
- World Health Organization specification 636/DT .Spinosad Tablets for Direct Application. 2008. http://www.who.int/whopes/quality/en/.
- World Health Organization. Report of the fourth WHOPES working group meeting. Document WHO/CDS/WHOPES/World Health Organization, Geneva. 2001;2.
- World Health Organization. Global Strategic Framework for Integrated Vector Management WHO / CDS / CPE / PVC / 2004;10.
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/CDPP/ 2005;13.
- World Health Organization. Diflubenzuron in drinking-water: Use for vector control in drinking-water sources and containers. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva. 2008. (WHO/HSE/AMR/08.03/6).
- World Health Organization. Spinosad DT in drinking water: use for vector control in drinking-water sources and containers. Geneva: World Health Organization. [Online] Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/spinosadbg. 2010.
- World Health Organization. Dengue and severe dengue. Factsheet N◦ 117. World Health Organization, Geneva. 2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.






