How to Cite | Publication History | PlumX Article Matrix
Nivedita Ghayal1, Megha Biware2 and Pallavi Gharpure3
1Department of Botany, Abasaheb Garware College, Pune-04, MS., India.
2Department of Chemistry, Abasaheb Garware College, Pune-04, MS., India.
3Department of Biodiversity, Abasaheb Garware College, Pune-04, MS., India.
Corresponding Author E-mail: gnivedita_ghayal@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2691
ABSTRACT: Many invasive weeds are known to create their deleterious effects on biological ecosystems and also rhizosphere soils. Weeds such as Cosmos and Xanthium have their existence near agricultural crops fields. Such weeds grow in abundance, releasing specific allelochemicals which have adverse effect on germination rate, physiological patterns and reproduction of crop plants. In present work, allelopathic effects of leaf leachates of Cosmos and Xanthium were observed on seed germination and seedling growth of Triticum aestivum, Vigna radiata and Trigonella foenum-graceum like crops. Seed germination was inhibited at higher concentration at 6% while lower concentrations showed stimulatory effect on Mungbean and Fenugreek from 1%-4% concentrations. But seed germination percentage of Vigna and Trigonella showed 70% and 60% growth in response to leaf leachates of Cosmos at 6% concentration. Triticum showed total inhibition of 40% to both leaf leachates. The qualitative phytochemical analyses showed presence of alkaloids, phytosterols, phenols, tannins and flavonoids. GCMS and IR studies revealed presence of major constituents such as esters, ethers, anhydride and polyalcohols. Cosmos and Xanthium showed the characteristic FTIR fingerprinting regions of various functional groups such as –OH, carbonyl, anhydride, ester and amides. The variations in phytochemicals of these invasive might be attributed to response of the plants to different environmental stresses.
KEYWORDS: Allelopathy; Asteraceae; GC-MS; IR Spectroscopy; Phytochemicals
Download this article as:| Copy the following to cite this article: Ghayal N, Biware M, Gharpure P. Phytotoxic Effects of Leaf Leachates of Invasive Weeds Cosmos Sulphureus and Xanthium Strumarium on Agricultural Crops. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Ghayal N, Biware M, Gharpure P. Phytotoxic Effects of Leaf Leachates of Invasive Weeds Cosmos Sulphureus and Xanthium Strumarium on Agricultural Crops. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32140 |
Introduction
Allelopathy majorly is described as any direct or indirect effect of one plant on another plants, animals or microorganisms through the production of chemical compounds that escape into the environment and influence the growth and development (Bezuidenhout; 2012, Sangeetha and Bhaskar; 2015, Rice and Pancholy; 1974). This phenomenon encompasses many aspects of physiology, biochemistry, observations and interactions occurring in nature. The effect of any alien or exotic species can cause loss of ecosystem balance, affecting its functioning incurring the loss of biodiversity through release of allelochemicals (Inderjit, 2005). Such plants can have stimulatory or inhibitory effects on crops. The secondary metabolites from such plants that are considered as allelochemicals do not have much physiological function in metabolism of plants. They serve only as agents of plant-plant competition during establishment in new ecosystem along with plant-microbe symbioses.
Exotic invasive weeds are non-natives due to their occurrence in different geographical regions. These invasive plants species have several motives for their successful establishment such as faster rate of reproduction, dispersal mechanisms, potency to establish large populations in short period of time with adaptive nature resulting in resource depletion. Such exotic plants show faster rate of establishment without any human assistance. Many recent investigations have shown that invasive plants have changed several community attributes like species diversity, richness, species composition and abundance (Keblawy et al. 2006). Dominance, higher growth rate throughout the invaded area, high tolerance towards abiotic environmental factors and also the allelopathic potential are probable favourable factors for their sustainability.The researches have indicated that some plant invaders become much more dominant in their invaded range than their native range. Frankel (1999) showed that alien plants displaced local flora, changed ecosystems and disturbed the biodiversity. The dominant plants by forming pure stands/ monothickets through interactions exhibit allelopathy and such plants generally are free of predators, parasites and diseases. They have influence on the growth and development of agricultural and biological ecosystem (Sangeetha and Bhaskar, 2015) through release of various allelochemicals or ecochemicals.
Allelochemicals are produced in all plant parts in different concentrations and released in the environment through leaching, volatilization, root exudation, decomposition etc. Their concentration depends on maturity stage of plant as younger plants are more toxic. Soil and soil factors as biotic and abiotic components are important in terms of determining the quality, quantity and availability of allelochemicals in the vicinity of neighbouring species (Inderjit, 2005). Concentration of allelochemicals is density-dependent factor (Inderjit 2005) present in soil, which shows changes in the soil characteristics, but to detect the exact concentrations of allelochemicals in field is difficult. It has been reported that even low concentrations of chemicals can cause significant effect on growth and germination of other plants. Therefore isolation and identification of chemicals is most significant part in demonstrating allelopathy (Inderjit and Dakshini,1994, Inderjit et al. 2005). According to (Rice, 1984) and Putnam and Tang (1986) the most widely used standard bioassay test is the influence of allelochemicals on germination parameters such as seed germination, seedling growth, root length and shoot length etc. under controlled laboratory conditions, which is undertaken in the present investigation as well. So also to establish phenomenon of allelobiogenesis, the assessment of allelochemicals by qualitative phytochemical analysis, GC-MS and IR methods was taken on.
Khadakwasla, Mulshi and Paud-Pirangut areas (18o30’42.30” N, 73o40’49.28” E) are agricultural fields on Lavasa road. According to field observations Cosmos has enormously invaded from Bhugaon upto Mulshi (about 30kms) covering the whole area along road side edges and rice crops. Xanthium also shows widespread expansion and has been observed near agricultural crop fields (Patil 2009). These areas are with moderate rainfall and have sandy and loamy soil which is favourable for growth of these two invasive species and compete with the kharif crops (Patil 2009). Cosmos and Xanthium both are considered toxic and non-palatable alien weeds unsuitable as fodder with no certain methods of eradications. Katraj ghat ranges (18027’27.12”N, 730 52’3.89”E) was another area selected for the study and found to be invaded greatly by the same two weeds Cosmos and Xanthium.
 |
Graph 1: Satellite image of selected study sites.
|
Cosmos sulphureus Cav. (Asteraceae) a native of Mexico, was accidentally introduced to Indian sub-continent. This annual, herbaceous, invasive weed, with non-fragrant flowers, is found gregariously growing on fields and hedges of crops, wastelands and marshy places. It can grow up to height of 8-10 feet, that can create shadow for short heighted crops inhibiting their light harvesting mechanism. It is a moderate reseeder, growing on sandy and loamy soils favor its profuse seed germination.
Xanthium strumarium L. (Asteraceae), a perennial shrub, native of North America and Eurasia. It germinates by reseeding itself. The plant seeds can sustain very harsh environmental conditions, by remaining dormant for about 2-10 years. Young seedlings of Xanthium release toxic chemicals that can inhibit germination and prove fatal to neighbouring plants. Specific environmental conditions such as optimum oxygen, high moisture and direct sunlight with moist, loamy or sandy soil are necessary requirements for its successful growth. Seeds and seedlings are highly toxic to mammalian herbivores.
Mungbean (Vigna radiata L) and Fenugreek/ Methi (Trigonella foenum-graceum L.) — (Both Members of Fabaceae), cultivated crops throughout India and also Wheat (Triticum aestivum L)–Wheat (Poaceae), a major cultivated staple food crop were used for standard seed germination bioassay studies.
Materials and Methods
Collection of Plant Materials
Fresh leaves of Cosmos and Xanthium at maturity stage were collected during monsoon period (July- October 2016, 2017) along agricultural fields from selected rural areas like Khadakwasla, Mulshi and Paud-Pirangut areas, Lavasa area and Katraj ghat area.
Preparation of Leaf Leachates
Fresh leaves were air shade dried, ground in a mixer to form a homogeneous powder and stored in air tight bottles. 10 g. of dried powders of Cosmos and Xanthium were mixed with 100 ml water in 250 ml beakers separately and kept in dark for 24 hours at room temperature. They were filtered through muslin cloth (size 2mm) to obtain aqueous leachates (10%). From this stock solution different concentrations were prepared (1% – 6% v/v) by making dilutions with distilled water and were used for seed germination bioassays.
The seeds of three crop plants Triticum, Trigonella, Vignawere procured from authentic source. Healthy seeds of wheat, fenugreek (methi) and mungbean were surface sterilized with 0.02% aqueous Hgcl2 (Mercuric chloride) for two minutes. Then the seeds were thoroughly washed with distilled water. Petri-plates (9 cm dia.) were sterilized with 70% alcohol and lined with germination paper moistened with different concentrations of leaf leachates of Cosmos and Xanthium. The seeds were placed in these petri-plates for germination. Experiments were carried out twicein laboratory conditions in triplicates with control of distilled water. Petri-plates were monitored daily and germination papers were moistened after 2-3 days with solutions of respective concentrations. After 8 days germination parameters such as number of seeds germinated (germination %), root length, shoot length, root : shoot ratio, vigour index were studied (Gupta et al; 1996).
 |
Graph 2: Seed germination bioassay by petriplate method.
|
Solvent Extraction Method for Phytochemicals Analyses
Powdered dried leaf materials of Cosmos and Xanthium weighing about 5mg was mixed with Ethanol solvent and allowed to homogenize for 3-4 hrs approximately. The mixtures were filtered through muslin cloth and filtrate was further tested for phytochemical analysis.
Detection of Allelochemicals by GC-MS Methods
Working procedure for test sample was as follows – 10 ppm solution was prepared in ethanol for Cosmos and Xanthium dry leaf powders separately. The solutions were filtered through 0.2µ nylon filter and filtrate sample was injected. GC-MS method- Make model: Agilent Technologies 7000 GC/MS Triple Quad The 7000 Series Triple Quad GC/MS is a standalone capillary GC detector for use with the Agilent 7890A Series gas chromatograph. Inbuilt Software installed for qualitative analysis of MS is Mass Hunter.
Detection of Allelochemicals by IR Method
Jasco FTIR (V-5300) model: Dry leaf powders of Cosmos and Xanthium were homogenised in mortar – pestle in a palette along with Potassium bromide powder (KBr) and about 1% of mixture was filled in cuvette. Control or Blank was run with Pottassium bromide (KBr) only followed with samples respectively. Wavelength from 400-4000 nm is generally an installed range for the detection of compound. Graph is overlay with API (Active Pharmacopeia Ingredients) standards to identify the peaks.
Statistical Analysis
Calculations of germination parameters were carried out as average of three determinants along with standard deviation. Analysis of data results were subjected to correlation to find out overall effect of increasing concentrations on germination % and graphs were performed in SPPS software.
Results and Discussion
Cosmos and Xanthium are invasive weeds, mainly found in the areas which are altered due to great human disturbances such as changes in land-use activities like agriculture, construction of road etc. Previous studies have revealed that these invasive weeds have deleterious effects on natural habitats like changes in soil composition due to release of allelochemicals which makes it unsuitable for growth of indigenous plants / crops. Different crop plants show different levels of tolerance towards allelochemicals which may be either beneficial or deleterious. Therefore to evaluate the effects of allelochemicals on germination and growth of crop seeds, standard laboratory germination bioassay method was considered as most important part of this study (Friedman 1995; Gogoi et al. 2002)
Seed germination and seedling growth are two focal parameters used in allelopathic bioassays (Rice, 1984), since the plant is considered to be most susceptible in its growth stage. The latter shows effective response to presence of allelochemicals as elongation of root. It is natural deciding factor that shows positive or negative effect on rate of germination.
Seed Germination Studies
Germination Percentage
Leaf leachates of Cosmos and Xanthium inhibited the seed germination with increased concentrations in all selected crops with varying degree of response. For Xanthium 5% and 6% concentrations showed significant changes in seed germination for all 3 crops, Wheat, Mungbean and Fenugreek whereas at lower concentrations (1%) seed germination was at par with control for Xanthium. Cosmos in comparison with Xanthium showed inhibition at 5% to Wheat whereas for Mungbean and Fenugreek LC50 was observed at 6% concentration. The graphs (Figures 1 and 2) showed a gradual decrease in germination for Cosmos as compared to Xanthium. Changing response of different plants may be due to concentration of inhibitory chemicals and mechanisms of inhibitory effects. The wheat seeds were found to be more receptive with respect to inhibition of germination than mungbean and Fenugreek crop seeds.
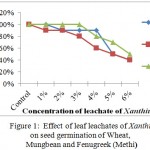 |
Figure 1: Effect of leaf leachates of Xanthium on seed germination of Wheat, Mungbean and Fenugreek (Methi). |
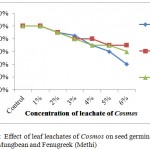 |
Figure 2: Effect of leaf leachates of Cosmos on seed germination of Wheat, Mungbean and Fenugreek(Methi). |
Effects of Cosmos and Xanthium leaf leachates on seed germination of Triticum aestivum (Wheat)
Triticum showed minimum i.e. (40%) germination at 6% concentration for both Xanthium and Cosmos. At 1% concentration effect was at par with control. The inhibitory effects were observed in all germination parameters with increasing concentrations of leachates. The effect of leachates on root length was similar to shoot length for both Cosmos and Xanthium with minimal differences. Shoot length was inhibited (9.2 ± 0.60) over control (12.2 ± 0.92) to Cosmos leachate at 1%concentration whereas effect of Xanthium leachates at 1% on germination was similar with that of control and it reduced to 90% of germination at 2% to 4% of leachates concentrations. Root: shoot ratio remained consistent for concentration upto 6% for Cosmos but it showed increasing trend with Xanthium. Vigor index showed drastic reduction with increasing concentrations as shown in both the Tables1 and 2.
Table 1: Effect of leaf leachates of Cosmos on seed germination of Triticum aestivum.
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
14.2
±1.06 |
12.2
±0.92 |
1.16
±0.09 |
1220
±91.50 |
| 1% | 100%
±0.06 |
7.2
±0.47 |
9.2
±0.60 |
0.78
±0.05 |
920
±59.80 |
| 2% | 90%
±0.05 |
7.06
±0.39 |
7.7
±0.42 |
0.91
±0.05 |
693
±29.64 |
| 3% | 85%
±0.08 |
6.6
±0.63 |
8.4
±0.80 |
0.78
±0.07 |
714
±55.86 |
| 4% | 70%
±0.07 |
6.73
±0.67 |
7.8
±0.78 |
0.86
±0.09 |
546
±54.60 |
| 5% | 60%
±0.05 |
3.1
±0.25 |
3.3
±0.26 |
0.93
±0.07 |
198
±15.84 |
| 6% | 40%
±0.03 |
2.8
±0.18 |
2.7
±0.18 |
1.03
±0.07 |
108
±91.50 |
Table 2: Effect of leaf leachates of Xanthium on seed germination of Triticum aestivum.
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
12.17 ±0.91 | 12.17 ±0.91 | 1 ±
0.07 |
1217
±91.27 |
| 1% | 100%
±0.06 |
10.83 ±0.70 | 11.03 ±0.72 | 0.98 ±0.06 | 1103
±71.69 |
| 2% | 90%
±0.05 |
9.77
±0.54 |
10.23 ±0.56 | 0.95 ±0.05 | 920.7
±50.64 |
| 3% | 90%
±0.09 |
9.4 ±
0.89 |
8.87
±0.84 |
1.05 ±0.10 | 798.3
±75.84 |
| 4% | 90%
±0.09 |
8.83
±0.88 |
8.67
±0.87 |
1.01 ±0.10 | 780.3
±78.03 |
| 5% | 50%
±0.04 |
5.07
± 0.41 |
3.73
±0.30 |
1.35 ±0.11 | 186.5
±14.92 |
| 6% | 40%
±0.03 |
3.67
±0.24 |
1.63
±0.11 |
2.25 ±0.15 | 65.2
±5.30 |
Effects of Cosmos and Xanthium leaf leachates on seed germination of Vigna radiata (Mungbean)
Germination was observed to be inhibitory at 6% for Xanthium (40%) but it remained upto (70%) for 6% concentration for Cosmos. Leaf leachates of Cosmos probably had stimulatory effect on germination percentage, root and shoot length at all concentrations as concentrations increased (Table 3). Shoot length was increased at 1% concentration (11.03cm) than that of control (9.6 cm) in response to leachate of Cosmos. Though Xanthium showed little increase in shoot length at 1% (8.07cm) as compared with control (8.17cm), but as concentration increases there was reduction in shoot length. Root: shoot ratio showed a constant trend of increase from control to 6% concentration for Xanthium but a reduced trend in case of Cosmos. Root: Shoot ratio is a highly dependent factor on length of root and shoot of plant and also dependent on concentrations of leachates. Vigour index showed gradual decrease over control as affected by leachates of Xanthium and Cosmos.
Table 3: Effect of leaf leachates of Cosmos on seed germination of Vigna radiata
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
7.1
±0.53 |
9.6
±0.72 |
0.73
±0.05 |
960
±72.00 |
| 1% | 100%
±0.06 |
6.8
±0.44 |
11.03
±0.72 |
0.61
±0.04 |
1103
±71.69 |
| 2% | 90%
±0.05 |
5.33
±0.29 |
7.4
±0.41 |
0.72
±0.04 |
666
±36.63 |
| 3% | 80%
±0.08 |
5.2
±0.49 |
7.06
±0.67 |
0.73
±0.07 |
564.8
±53.66 |
| 4% | 80%
±0.08 |
4.7
±0.47 |
6.9
±0.69 |
0.68
±0.07 |
552
±55.20 |
| 5% | 70%
±0.06 |
3.5
±0.28 |
5.3
±0.42 |
0.66
±0.05 |
371
±29.12 |
| 6% | 70%
±0.05 |
3.46
±0.22 |
5.2
±0.34 |
0.66
±0.04 |
364
±23.66 |
Table 4: Effect of leaf leachates of Xanthium on seed germination of Vigna radiata
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
4.57
±0.34 |
8.17
±0.61 |
0.57 ±0.04 | 817 ±60.53 |
| 1% | 90%
±0.06 |
7.93
±0.52 |
8.07
±0.52 |
0.98 ±0.06 | 726.3 ±47.21 |
| 2% | 90%
±0.05 |
7.57
±0.42 |
5.63
±0.31 |
1.34 ±0.07 | 506.7 ±27.87 |
| 3% | 80%
±0.08 |
7.3
±0.69 |
5.2
±0.49 |
1.4
±0.13 |
416 ±39.52 |
| 4% | 60%
±0.06 |
2.93
±0.29 |
4.07
±0.41 |
0.71 ±0.07 | 244.2 ±24.42 |
| 5% | 50%
±0.04 |
0.97
±0.08 |
3.53
±0.28 |
0.27 ±0.02 | 176.5 ±14.12 |
| 6% | 40%
±0.03 |
0.93
±0.06 |
1.73
±0.11 |
0.53 ±0.03 | 69.2
±60.53 |
Effects of Cosmos and Xanthium leaf leachates on seed germination of Trigonella foenum-graceum (Methi)
Both the weeds Cosmos and Xanthium showed inhibitory effects on germination percentage for Fenugreek 60% and 50% respectively over control. Minimum effect was observed for leachate concentrations of Cosmos on germination. LC50 for Xanthium was attained at 6% concentration only. Emergence of shoot length was drastically inhibited at 5% (2.23cm) and 6% (1.07cm) concentrations of leaf leachates of Xanthium as compared to Cosmos. Cosmos showed minimal inhibitory effect on all measurable parameters. Root: shoot ratio showed reduction for Cosmos over control to 6% concentration. But Xanthium showed increase in root length though shoot growth was hampered. Reduction in vigor index upto 70% from control to 5% and 6% for Cosmos was observed but in Xanthium reduction of vigor index was concentration dependent from 5% to 6%. Values are mentioned in tables 5 and 6 respectively.
Table 5: Effect of leaf leachates of Cosmos on seed germination of Trigonella foenum-graceum.
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
5.6
±0.42 |
6.3
±0.47 |
0.88
±0.07 |
630
±47.25 |
| 1% | 100%
±0.06 |
3.7
±0.24 |
5.1
±0.33 |
0.72
±0.05 |
510
±26.52 |
| 2% | 90%
±0.05 |
3.26
±0.18 |
4.8
±0.26 |
0.67
±0.04 |
432
±21.12 |
| 3% | 80%
±0.08 |
2.9
±0.28 |
4.66
±0.44 |
0.63
±0.06 |
372.8
±35.43 |
| 4% | 70%
±0.07 |
2.4
±0.24 |
4.1
±0.41 |
0.58
±0.06 |
287
±28.90 |
| 5% | 70%
±0.06 |
2.6
±0.21 |
4.2
±0.34 |
0.61
±0.05 |
294
±23.84 |
| 6% | 60%
±0.04 |
2.03
±0.13 |
3.86
±0.25 |
0.41
±0.03 |
231.6
±15.05 |
Table 6: Effect of leaf leachates of Xanthium on seed germination of Trigonella foenum-graceum.
| Conc. of leachates | Germination % | Root length | Shoot length | R:S ratio | Vigor index |
| Control | 100%
±0.07 |
3.33
±0.25 |
5.4
±0.41 |
0.61
±0.05 |
540
±40.50 |
| 1% | 100%
±0.06 |
4.8
±0.31 |
5.17
±0.34 |
0.92
±0.06 |
517
±33.60 |
| 2% | 100%
±0.06 |
4.03
±0.22 |
4.6
±0.25 |
0.87
±0.05 |
460
±25.30 |
| 3% | 100%
±0.09 |
3.97
±0.38 |
4.07
±0.39 |
0.97
±0.09 |
407
±38.66 |
| 4% | 80%
±0.08 |
3.6
±0.36 |
3.9
±0.39 |
0.92
±0.09 |
1216.8
±31.20 |
| 5% | 70%
±0.06 |
3.27
±0.26 |
2.23
±0.18 |
1.46
±0.12 |
156.1
±12.49 |
| 6% | 50%
±0.03 |
2.47
±0.16 |
1.07
±0.07 |
2.3
±0.15 |
53.5
±3.48 |
Discussion
Allelopathic plants are described as alien, invasive weeds from different geographical areas. They establish successfully through release of various allelochemicals, according to the contiguous environmental stress conditions. Dominance, higher growth rate and adaptability to existing environments are some of the attributes for their successful establishment. Most of the allelochemicals are secondary metabolites of plants playing a significant role as stress tolerant compounds. Allelo/ ecochemicals present in several parts of such plants have their own mechanisms of release into environment. They may have inhibitory or stimulatory effect on growth and germination of neighboring plants or crops. This effect can be monitored Leather and Einhellig (2005) through seed germination bioassay studies by considering parameters such as germination %, root length and shoot length. According to Inderjit and Nilsen (2003) laboratory bioassays and field studies are fundamental part of allelopathy research, because they are fast and repeatable tools for exploring the potential for different types of interactions.
The study was to evaluate the phytotoxic effect of such invasive weeds on physiological growth of cereals and pulses. The allelopathic impact of leachates or extracts is more harmful to radicle (Friedman; 1995). The phytotoxicity was directly proportional to the increasing concentrations of leachates. Many researchers have shown such inhibition of seed germination as affected by leaf leachates of various invasive weeds. 10% of aqueous leaf extract of Parthenium hysterophorus has shown total inhibition of Triticum aestivum (Seerjana Maharajan et al., 2007). Aqueous extract and leachate may have different response towards the germination of Wheat. Preliminary studies had revealed significant inhibitory effect on seed germination and seedling growth of selected crops from different geographical regions. The previous studies showed that aqueous extracts of hearleaf cocklebur (Xanthium) was effective on crops such as Wheat and Barley even with low density of presence of weeds (IzzetKadioglu, 2004). Cutler and Cole reported that potassium carboxyactractyloside, a glycoside isolated from the residues of Xanthium strumarium L. strongly inhibiting coleoptiles growth of Wheat (Benyas et al, 2010).
Similar inhibitory effects of aqueous extracts of Ranunculus arvensis, Sinapis arvensis, Smilax aspera were observed with wheat germination percentage and rate as compared to inhibitory effects of Cosmos and Xanthium leaf leachates. The effect of aqueous extracts on root growth was more than shoot growth (Qasem, 2017). Different plants showed variable effects on target species and their responses.
Clerodendrum infortunatum showed highest inhibitory effect on Vigna radiata seeds at 100% concentration. And for Trigonella foenum-graceum maximum inhibitory effect on germination was at 100% concentration and in case of Triticum aestivum and Brassica campestris highest inhibitory effect started from 75% and 50% concentration respectively (Gopal Debnath et al., 2016). The results of present study showed partial stimulatory effects in comparison with studies conducted on Clerodendrum infortunatum in Tripura region. Similar supporting results were observed with ethanolic leaf extract of Solanum nigrum (Girija, 2015) on seed germination, radical length and protein content of Trigonella foenum-graceum. Higher concentrations showed inhibitory effect whereas lower concentrations had no effect.
Vigna radiata indicated both stimulatory and inhibitory response effect to some extent to leachate concentration of Cosmos and Xanthium respectively. Reduction in net yield of fallow-mungbean was reported due to allelochemicals released by Sunflower (Vishwajit, 2017). Sunflower showed significant allelopathic effect on receptor plant Mungbean in sunflower-mungbean crop rotation. Cosmos and Xanthium though are not cultivated plants but show inhibitory effects due to release of various allelochemicals. The root length of Mungbean was inhibited at 91.91% and seedling growth as inhibited at 52.05% at 50mg/ml concentration of leaf extracts of Diospyros kaki (Cui, 2017) similar to results of effects of Cosmos and Xanthium leaf leachates.
Correlation graph and standard deviation calculations and observations resulted in significant inhibition of hypocotyls and coleoptiles growth of all three selected crops with difference in variation to response to the allelochemials. Stimulatory effect could be observed at 1% and 2% concentration at par with control. Inhibition concentration was observed at 6% concentration of leaf leachates of Xanthium and Cosmos.
Increased concentrations of allelochemicals suppress the mitotic activity of young cells or embryo resulting in inhibition of seed germination. Bioassay conditions play a major direct role in seed germination such as light conditions and humidity (Chen Feng et al. 2017, NEERI REPORT, 2000). There was direct effect of temperature on emergence of hypocotyl and coleoptile throughout the experiment.
Phytochemical Analysis
Phytochemicals revealed that alkaloids, saponins, phenols, tannin, flavonoids and proteins are present in the extract.
Results
Phytochemical analysis conducted on the plant extracts revealed the presence of constituents which may probably show medicinal bioactivities. Analysis of the plant extracts of Cosmos revealed the presence of phytochemicals such as phenols, phytosterols, tannins, flavonoids and alkaloids with absence of saponins, carbohydrates and proteins. Whereas phytochemical analysis results for Xanthium plant extract revealed the presence of alkaloids, tannins and flavonoids but absence of saponins and phenols.
Discussion
Biologically active molecules present in plants are phytochemicals. Phytochemical analysis is considered as major analytical part for detection of various compounds.These compounds are useful to understand the ecology and stress conditions of plants.Phytochemical analysis conducted on Xanthium strumarium (Farooq Umer, 2014) revealed the presence of similar secondary metabolites such as alkaloids, phenols, flavonoids tanninsand terpenoids but with variations in saponins, glycosides, steroids.
Phytochemical screening tests on Cosmos sulphureus plant extract (Jadav, 2017) revealed the presence of proteins, flavonoids, phenols, alkaloids, tannins, saponins which are similar to the results obtained from tests conducted on Cosmos from Pune region. Similarities and differences in results suggest different responses to environmental stress conditions from two different geographical areas.
GC – MS results
The allelopathic potential exhibited by both the weeds might be due to different types of allelochemicals existing in them. The dominance of these two weeds and their inhibitory activity on the other plants and also the crops can be attributed to the presence of different types of ecochemicals existing in them which are detected with GC-MS. GC-MS analysis of ethyl acetate extract of both samples revealed the presence of some similar compounds. The major constituents were esters, ethers, anhydride and polyalcohols.
Table 7: Functional group peaks from GC-MS study of Cosmos sulphureus.
| Sr.No | Retention Time | IUPAC | Molecular formula | Molecular Weight |
| 1. | 26.334-27.049 | Carnegine | C13H19NO2 | 221 |
| 2. | 17.559-18.553 | Malonic acid,2-formamido-2{4-(4-hydroxy-3-methyl-2-butenyl)indol-3}methyl-dimethyl ester | C20H24N2O6 | 388 |
| N-{5-(3-hydroxy-2-methylpropenyl)-1,3,4,5-tetrahydrobenzo(cd) indol-3-yl}-N-methylacetamide | C18H22N2O2 | 298 | ||
| 3. | 26.250-27.001 | Carnegine | C13H19NO2 | 221 |
| Hexanal,(2,4-dinitrophenyl) hydrazone | C12H16N4O4 | 280 | ||
| (2-Methyl-3-nitrophenyl) methanol,dimethylpentaflu-orophenylsiyl ether | C16H14F5NO3Si | 391 |
Table 8: Functional group peaks from GC-MS study of Xanthium strumarium.
| Sr. No | Retention Time | IUPAC | Molecular formula | Molecular weight |
| 1. | 8.921 | Salmeterol | C25H37NO4 | 415 |
| 2. | 26.581 | Tricylotetradecan-6-one,4-ethenyl-3-hydroxy-2,4,7,14-tetramethyl | C20H32O2 | 304 |
| 3. | 8.860 | 4-Acetyloxyimino-6-6-dimethyl-3-methylsulfanyl-4,5,6,7-tetrahydro-benzo(c)thiophene-1-carboxylic acid methyl ester | C15H19NO4S2 | 341 |
| 8.980 | Brnzene,2,3,4,5-tetramethyl-1-(2,3,4,5-tetarmethyl benzyl) | C21H28 | 280 | |
| Corynan-17-ol,18,19-didehydro-10-methoxy | C20H26NO2 | 326 | ||
| 4. | 13.996-14.241 | Carda-5-20(22)-dienolide,3-(6-deoxy-alpha-L-mannopyranosy)oxy)-14-hydroxy-(3beta) | C29H42O8 | 518 |
| 11-beta,19-Cyclopregn-5-ene-3,20-dione,11-hydroxy-cyclic bis (ethylene acetal ) | C25H36O5 | 416 | ||
| Carnegine | C13H19NO2 | 227 | ||
| 5. | 15.801-16.681 | Malonic acid,2-formamido-2{4-(4-hydroxy-3-methyl-2-butenyl)indol-3-y}methyl-dimethyl ester | C20H24N2O6 | 388 |
| 4a-Methyl-1-methylene-1,2,3,4,4a,9,10,10a-octahydrophenanthrene | C16H20 | 212 | ||
| Benzeneethanamine,2-fluoro-beta,3,4-trihydroxy-N-isopropyl | C11H16FNO3 | 229 | ||
| 6. | 17.524-18.835 | Malonic acid,2-formamido-2{4-(4-hydroxy-3-methyl-2-butenyl)indol-3-yl) methyl-dimethy lester | C20H24N2O6 | 388 |
| Benzenamine,4-(1-methylethyl)-N-phenyl | C15H17N | 211 | ||
| 1,7-di-iso-propylnapthalene | C16H2O | 212 | ||
| 7. | 20.200-20.425 | Carnegine | C13H19NO | 221 |
| 9,12,15-Octadecatrienoic acid,2,3-bis{(trimethylsily)oxy} propyl ester | C27H52O4Si2 | 496 | ||
| 2-Oxo-4-6-diphenyl-3-(4-tolyl)-1,2,3,4-tetrahydropyrimidine | C23H20N2O | 340 |
IR spectroscopy Results
FTIR analysis of Cosmos and Xanthium in the range 4000-400 cm-1 showed the characteristic fingerprinting regions of various functional groups such as –OH, carbonyl, anhydride, ester, amide. For example 3517 cm-1for free –OH group, 2323 cm-1 and 2917 cm-1 for –C-H stretching. FTIR data suggested the probability of presence of compounds containing these functional groups. GC-MS analysis of both samples ethyl acetate plant extract, revealed the presence of similar compounds. The major constituents were esters, ethers, anhydride and polyalcohols. (Tables 9 and 10)
Table 9: FTIR spectral peak values and functional groups for the leaf extract of Xanthium strumarium
| Peak Values Wave number (Cm–1) | Functional groups |
| 3517.52 | Free -OH |
| 2923.56 | -CHO ( C-H Stretch) |
| 1895.68 | C=O of anhydride, ester |
| 1746.23 | =CH2, C=O of acid anhydride |
| 1541.81 | Aromatic region, N-H for prim and sec amide |
| 1407.78 | Aromatic region |
| 1041.37 | C-O-C |
| 437.439 | C-N,C-C,C-O |
| 440.665 | C-N,C-C,C-O |
| 632.537 | Aromatic |
| 525.507 | Aromatic |
| 466.689 | C-N,C-C,C-O, aliphatic C-I, |
| 422.334 | C-N,C-C,C-O |
| 412.692 | C-N,C-C,C-O, aliphatic C-I, |
| 327.194 | Bending vibration depends on substitution Pattern |
| 308.555 | Bending vibration depends on substitution Pattern |
| 212.131 | Bending vibration depends on substitution Pattern |
| 159.099 | Bending vibration depends on substitution Pattern |
| 95.4591 | Bending vibration depends on substitution Pattern |
Table 10: FTIR spectral peak values and functional groups for the leaf extract of Cosmos sulphureus.
| Peak Values Wave number (Cm–1) | Functional groups |
| 3639.02 | Free -OH |
| 2917.77 | -CHO ( C-H Streach ) |
| 1656.55 | C=C in alkens or Aromatic region, |
| 1425.14 | Aromatic region |
| 1014.37 | C-O-C(Ethers) |
| 782.958 | =C-H(m),CH=CH, |
| 476.331 | Aliphatic C-I region. |
| 445.476 | Bending vibration depends on substitution Pattern |
| 431.012 | Bending vibration depends on substitution Pattern |
| 415.585 | Bending vibration depends on substitution Pattern |
| 820.563 | =C-H |
| 538.042 | -C-X, |
| 501.401 | -C-X, |
| 466.689 | -C-X, |
| 422.334 | Bending vibration depends on substitution Pattern |
| 411.728 | Bending vibration depends on substitution Pattern |
| 372.194 | Bending vibration depends on substitution Pattern |
| 295.055 | Bending vibration depends on substitution Pattern |
| 282.52 | Bending vibration depends on substitution Pattern |
| 223.702 | Bending vibration depends on substitution Pattern |
| 194.775 | Bending vibration depends on substitution Pattern |
| 159.099 | Bending vibration depends on substitution Pattern |
| 123.422 | Bending vibration depends on substitution Pattern |
| 95.4591 | Bending vibration depends on substitution Pattern |
Discussion
Isolation, detection and identification of functional groups is important part of allelopathic studies that helps to recognize nature of allelochemicals released by plant in response to fluctuating environmental conditions. These chemicals are from different groups as phenolic acids such as ferulic, p-coumaric acids, p-hydroxy benzoic acids, cyanogenic glycosides, tannins etc. Studies reveal that allelopathy is result of compounds having different actions as antagonistic, synergistic on plants in the vicinity.
Conclusions
With increasing emphasis on organic farming and to ensure sustainable and healthy method of agriculture, it was necessary to understand the mechanism of allelopathy in nature. Main objective was to derive conclusions on effective applications of allelopathy in agriculture production though it showed negative effect on growth of agriculture crops through release of allelochemicals. In the experiments conducted it was mainly concluded that Xanthium had significant effect on crops than Cosmos. The effects are directly related to expanse or density of weed and age of plant. Both being members of Asteraceae impact was different, which may probably due to the different combinations of allelochemicals. Hence, further evaluation of detection of allelochemicals and such other qualitative phytochemical methods of analysis must be done to understand the ecological role of this weeds in future. Qualitative analysis of phytochemicals forms the basic idea of presence of bioactive molecules. But quantitative analysis of allelochemicals study should be conducted in further studies to understand the nature of environmental response of the plant to conclude.
Acknowledgements
The authors are thankful to Dr. Ankur Patwardhan, Vice Principal, M.E.S Abasaheb Garware College and Head, Annasaheb Kulkarni Department of Biodiversity, for his constant support in carrying out this research.
Conflict of Interest
There is no conflict of interest.
Funding Source
The authors have not received support from any funding agency. This research work was self-financed.
References
- Bezuidenhout S. R. Allelopathy as a Possible Cause for Crop Yield Reductions. 2012; KZN Agri and Rural devt.
- Sangeetha C. and Bhaskar P. Allelopathy in weed management: A critical review. African J. of Agri. Res. 2015; 10(9):1004-1015.
CrossRef - Inderjit., Weston L. A and Duke S. O. Challenges, Achievements and Opportunities in allelopathy research. J. of Plt. Int. 2005;1(2):69-81.
- El-Keblawy A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plt. Ecol. 2006;190(1):23-35.
CrossRef - Friedman J. Allelopathy. Autotoxicity and Germination. In Seed Development and Germination (Eds., J. Kigel, and G. Galili) Marcel Dekker. New York. 1995;629-644.
- Maharjan S., Shrestha B. B and Kumar P. J. Allelopathic Effects of Aqueous Extract of Leaves of Parthenium hysterophorus L. on Seed Germination and Seedling Growth of Some Cultivated and Wild Herbaceous Species. Sci. World. 2007;(5).
- Kadioglu I. Effects of Hearleaf Cocklebur (Xanthium strumarium L.) extract on some crops and weeds. Asian J. of Plt. Sci. 2004;3(6):696-700.
CrossRef - Benyas E., Hassanpouraghdam M. B., Zehtabsalmasi S.,Oskooei O. S. K. Allelopathic Effects of Xanthium strumarium L. Shoot Aqueous Extract on Germination, Seedling Growth and Chlorophyll Content of Lentil (Lens culinaris Medic). Romanian .Biotec. Letters. 2010;15(3).
- Qasem J. R. A survey on the phytotoxicity of common weeds, wild grown species and medicinal plants on Wheat. Allelopathy. J. 2017;42(2):179-194.
CrossRef - Debnath G., Das P and Krishna S. A. Allelopathic effect of Clerodendrum infortunatum L. Leaf extract on seed germination and seedling growth of some agricultural crops of Tripura, India. Int. Res. J. Pharma. 2016;8(1).
- Girija G and Gowri S. Allelopathic effect of Solanum nigrum on Pisum sativum, Eleusine corocana and Trigonella foenum-graecum. Biomed Pharma J. 2015;1(1).
- Halgalimath V and S. P. Ganajaxi Math. Allelopathic effects of Sunflower on succeeding Mungbean (Vigna radiata Wilezek ) crop. Allelopathic. J. 2017;42(1):37-48.
CrossRef - Cui C., Liu B.,Hou L and Zhang S. X. Allelopathy effects of persimmon (Diospyros kaki) leaves extracts on germination, seedling growth and enzymatic activities of receptor plants. Allelopathy. J. 2017;42(1):49-64.
CrossRef - Feng C.,Yong J. M., Hai W. S., Xiao F. L.,Wen G. Z., Jian W. L.,Wen Y. Y., Kai S. Effect of plant allelochemicals on seed germination and its ecological significance. Chinese J. of Eco-Agri. 2017;25(1):36-46.
- Kruse M. Ecological Effects of Allelopathic Plants – a Review. NEERI. Tech. Report No. 315. 2000.
- Umer F. A Comparative Study of Phytochemical Investigation of Xanthium strumarium on Medicinal Plant. Int. J. Res. in Pharma and Chem. 2014;4(1):96-100.
- Krishna J and Gowda K. N. N. Preliminary Phytochemical analysis and in vitro antioxidant activity of Araucaria columnaris bark peel and Cosmos sulphureus. Int. J. of Current Pharma. Res. 2017;0975-7066(9):4.

This work is licensed under a Creative Commons Attribution 4.0 International License.





