How to Cite | Publication History | PlumX Article Matrix
Iqra Qayyum1, M. Fazal-ur-Rehman1 and M. S. Ibrahim2
and M. S. Ibrahim2
1Department of Chemistry, University of Education, Lahore-Vehari Campus, Vehari-61100, Punjab, Pakistan.
2Department of Chemistry, School of Science Education, Sa’adatu Rimi College of Education, Kumbotso P.M.B 3218, Kano State, Nigeria (NYSC).
Corresponding Author E-mail: fazalurrehman517@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2688
ABSTRACT: Nicotine is obtained from the tobacco plant, Nicotiana tabacum L. This plant comes from the nightshade family which has other members including red peppers, eggplant, tomatoes and potatoes. In this study, Nicotine was extracted from tobacco leaves separated from Gold Live Classic BrandTM cigarettes using liquid-liquid solvent extraction method with ether by dissolving the leaves in NaOH solution. The percentage yield determined after the whole extraction method was 0.6%. Calculated percent recovery was 0.6 %, this percentage yield clarified that in this brand, very small nicotine is investigated, this deduces a significant loss of product throughout the procedure which are due to formation of emulsions and not due to washing thoroughly with ether to extract maximum yield, so repeated the process three times. In order to verify the nicotine, other physical properties were determined, MW;162.23g/mol, MP; -79oC, and BP; 246.8oC. While the [α]D of nicotine; -168.5o at the temperature of 293.15K was determined. Distinct peaks on the IR spectra indicated the bond frequencies of certain functional groups, which also confirm the nicotine.
KEYWORDS: Addiction; Nicotiana Tabacum; Reflux Extractions; Solvent Extraction
Download this article as:| Copy the following to cite this article: Qayyum I, Fazal-ur-Rehman M, Ibrahim M. S. Extraction of Nicotine (3-(1-methyl-2-pyrrolidinyl) pyridine) from Tobacco Leaves Separated from Gold Live Classic BrandTM Cigarettes by Solvent Extraction Approach and Characterization Via IR Analysis. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Qayyum I, Fazal-ur-Rehman M, Ibrahim M. S. Extraction of Nicotine (3-(1-methyl-2-pyrrolidinyl) pyridine) from Tobacco Leaves Separated from Gold Live Classic BrandTM Cigarettes by Solvent Extraction Approach and Characterization Via IR Analysis. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32190 |
Introduction
Tobacco plant; Nicotiana tabacum. L (NTL) is a source of nicotine. This plant comes from the nightshade family which has other members including red peppers, eggplant, tomatoes and potatoes (Lawson, 2015) as well as in coca leaves (Hossain & Salehuddin, 2013). “Nicotine” the word comes from tobacco plants, now called nicotiana tabacum, as changed after the name of French ambassador in Portugal, jean nicotode villemain (1560) who transferred tobacco leaves and seeds to Paris and publicized their usage in medicines. From brazil by luis de gois the tobacco and seeds were brought to ambassador nicot (Kocha, 2013). NTL is well-known extensively planted commercial crop (Hu et al., 2015) (Zhang, Gao, Zhang, Liu, & Ye, 2012). China produced and consumed 400 to 500-million-ton tobacco yearly. Additionally, over than 200 million ton of tobacco waste stuffs are produced per annum in tobacco farming and cigarette manufacturing industries. The wastes of tobacco, including its low quality leaves, stem, leaf vein and tobacco roots, have severely irritating odor and contributes to severe ecological pollution (Hu et al., 2015).
Worldwide, highest cultivation and production of tobacco are seeing in China among other countries. It has been reported that China was produced 2.5 million tons of tobacco leaf in 2008, in comparison of 2007 its increase in production of 22.9% (Zhang et al., 2012).
Along with China, in world, second is Indonesia which is also one of the biggest manufacturers of tobacco, which harvested 166,262 tons per year and from total production of tobacco 99% of this is used for cigarette manufacturing. It has been previously reported that as its active compound tobacco leaf consists of three alkaloids named as D-limonene, indole, and nicotine. In order to extract nicotine from tobacco leaf, various methods have been exploiting such as column chromatography extraction and binary-sustained liquid membranes, reflux abstractions and ingestion (Fathi, Fauzantoro, Rahman, & Gozan, 2018).
All over the Bangladesh throughout the season diverse types of tobacco shrubs are maximum often used as they are cheap and available. In United States (US), instantaneous consumption of tobacco with alcohol is one of popular drug mixture. Usually, nicotine seems to minimum reinforce in females than in males for retaining cigarette addiction (Hossain & Salehuddin, 2013). In last of 19th span, legislators had commenced to recognize adverse impacts of nicotine. Surgeon General of the U.S. in 1964, introduced a research findings associating smoking with lung cancer and cardiac disease (Felman, 2018).
Nicotine is a nitrogen-containing chemical, extremely poisonous compound, being appropriate to the tobacco alkaloid (DeVito, Herman, Waters, Valentine, & Sofuoglu, 2014). It is prepared by numerous varieties of plants containing plant of tobacco. It can also be prepared synthetically (Lawson, 2015). Nicotine, 3-(1-methyl-2-pyrrolidinyl) pyridine is a colorless, approaching to pale yellow, hygroscopic oily liquid existing in NTL leaves (Hossain & Salehuddin, 2013). For a while, small quantity of nicotine stimulates the nervous system. For smoking purposes low nicotine content tobacco is used (Hopkins, Ruiz-Tiben, Eberhard, Roy, & Weiss, 2017). Molecular formula C10H14N2 for nicotine has been derived from elemental analysis and molecular weight determination. It absorbs two molecules of CH3I, suggesting the tertiary nature of both the nitrogen atoms. On oxidation with chromic acid, nicotine yields nicotinic acid (Fig.3).
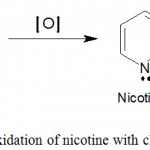 |
Figure 3: Oxidation of nicotine with chromic acid.
|
This demonstrate that the alkaloid contains a pyridine nucleus with a side chain at 3-position (Hopkins et al., 2017). Therefore, the formula of nicotine (Fig.4) may be written as:
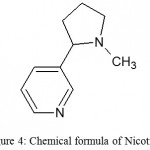 |
Figure 4: Chemical formula of Nicotine
|
Medically, Nicotine is described to recover the health conditions of abnormals of schizophrenia (Goniewicz et al., 2017) and dementia patients, dopaminergic neurons and axons, levodopa induced dyskinesia, skin mild cognitive dysfunction, (Benowitz et al., 2018), and to decrease the consumption of injurious ingredients of smokers in nicotine assisted smoking interruption. Nicotine has antimicrobial and insecticidal actions (Heydari, Mobidi, Mohammadi, Forouzandeh, & Rashidzadeh, 2017) and applied as a natural pesticide with features of certainly degradable, harmless to humans and cause no environmental pollution (Hu et al., 2015).
 |
Figure 5: Cigarette
|
During cigarette (Fig.5) manufacturing, over than 20% of leaves of tobacco used, are wasted and removed. This discarded material of tobacco contaminates the surrounding and are not used for any other purposes. Consequently, it is significant to investigate and exploit the discarded leaves of tobacco removed during manufacturing (Zhang et al., 2012). Tobacco necessary oil (TNO) is usually unlike as of other necessary oils, generally comprises of numerous extraordinary aromatic mixtures and a proper solvent. It is chiefly utilized to lessen aggressive flavor and irritancy of products of tobacco. Furthermore, TNO can be utilized in fragrances as well as smoking termination goods (Zhang et al., 2012). Addiction of tobacco causes many diseases in developing countries which leads to death (DeVito et al., 2014) (Khan, 2014). (Jarvik, 1991). According to WHO, 1.3 billion smokers are present now a day. Death of 5 million peoples each year has been reported sue to smoking. Tobacco will kill 10 million people each year by 2020 according to present situation of smoking. (Khan, 2014). If worldwide tobacco intake persisted at present rate, it accounts 5.4 million deaths per year (Mishra et al., 2015). In insecticides and against parasites toxic effect of nicotine has been used (Jarvik, 1991). Increase in heart rate, memory, alertness and reaction time due to chemical reactions produce by nicotine in the nerve endings. Neurotransmitters called dopamine and later endorphins are released in the brain producing feelings of pleasure and satisfaction. As an addictive drug, nicotine has been used as stimulant as well as depressant (Khan, 2014). Nicotine can be intake in different ways; Orally (not readily absorbed from digestive system) or Sniffed/snuffed (absorbed through mucous membranes of nasal cavity) or via smoking (90 percent of inhaled nicotine absorbed through mucous membranes of lungs), Patches, Nicotine inhaler, Lozenges, and Gum (Khan, 2014). For the extraction of nicotine, various techniques of chromatography were being performed to abstract the nicotine from numerous plants extracts. Great advantageous methods engaged for extraction of nicotine in tobacco leaves are several solvent extraction methods associated with gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-ultraviolet absorption spectroscopy (LC-UV) (DeVito et al., 2014).
Nicotine produces both positive and negative effects. The capability of nicotine to get rid of anxiety and nervousness is still a focus of conflict. Several smokers think cigarettes aid them to focus. Moreover, only a few of laboratories from their experimental data support this fact (Jarvik, 1991). Modern research shows that nicotine has ability to boosted the prospective memory (Blog, 2017). It has been reported that nicotine has capability to shrink body mass in human being and wildlife is one of its utmost consistent and strong effect. Weight loss in wealthy Western civilization is frequently preferred particularly by females. Nicotine works to decrease mass by minimum two ways: It decreases hunger and enhances metabolism (Jarvik, 1991). Nicotine shows remarkable effects in curing of Alzheimer’s disease as no remedy has not yet found (Blog, 2017). Schizophrenia is a severe psychological condition causing hallucinations, misunderstandings and changed communication ways in affected ones. Furthermore, nicotine has been exposed to recover the action on affected ones with Schizophrenia (Blog, 2017). A study on 11,000 older Australian men revealed that persons who addicted of nicotine, over than 51% less expected to requisite operation to substitute hips and knees spoiled by arthritis, when mass and obesity was taken into consideration (Blog, 2017). Nicotine creates numerous kinds of negative impacts in major tissues and structures of human body (Felman, 2018). The most important severe health effects of nicotine addiction include Cardiovascular disorder, cancer disease and respirational syndromes. If females continue to intake in pregnancy, harmful effect of nicotine can commence formerly the delivery. Now, maternal addiction is only great main threat issue for Sudden Infant Death Syndrome (SIDS). A recent preclinical shocking study has realized not only maternal addiction but, moreover, grand maternal addiction is associated with higher pediatric asthma hazards in kids (Leslie, 2013). Cardio active, and heart hazards of mouth quench and grazing tobacco have researched. Alterations in heart beat rate and blood pressure were analogous to those of nicotine addictive’s (Benowitz, Porchet, Sheiner, & Jacob, 1988). Predominant immediate harmful impacts as perceived in animal research works and in individuals increase in blood pressures and heart beat rates (Mishra et al., 2015). Further hazards contains lung contractions, pneumonia, muscle pains, rise in sugar levels, increasing threat of diabetes and bone aching (Felman, 2018).
Experimental
Chemicals/Apparatus Used
Gold Live Classic Brand™ cigarettes, Diethyl Ether, Sodium Hydroxide (NaOH), K2CO3 (1-2g), Distilled Water, Separatory Funnel (SF), Beakers, Filter papers, IR Spectrophotometer, Polarimeter
Method
The Liquid-Liquid Solvent Extraction method (Kataoka, Inoue, Yagi, & Saito, 2009) was applied to extract the nicotine from tobacco leaves. Weighed about 1 g of tobacco leaves from Gold Live Classic Brand™ cigarettes (Fig.5) in a beaker and added about 100 mL of 5% NaOH solution and shake it well for about 15 minutes. Filtered the mixture by filter paper and pressed the filter paper until all the liquid residue was filtered. Transferred the tobacco leaves again in first beaker added about 30 mL of water, stirred well and filtered again. Collected the filtrate together in one beaker. Transferred the filtrate into SF and added 25 mL of ether (Diethyl ether) into SF. Extracted the organic layer three times, collected them in one beaker. Dried the organic layer by using one tea spoon of anhydrous K2CO3. Filtered and evaporated the ether on water bath. Measured the volume of liquid nicotine and calculated the percentage yield of nicotine. To verify the nicotine, physical properties like BP and density of the product were determined respectively. IR Analysis for functional group identification was done by IR Spectrophotometer at Institute of Chemical Sciences-BZU, Multan, Punjab, Pakistan.
Results and Discussion
The percentage yield determined after the whole extraction method was 0.6%. In order to verify the nicotine, different properties; Molecular Weight (MW), Melting Point (MP), Boiling Point (BP), Optical Rotation ([α]D), Density, Refractive Index (RI) were also determined (table.1). The boiling point and density of product were determined which were 247oC, and 1.01 gcm-3 respectively. Other properties determined as the MW was 162.23g/mol, MP was -79 oC, and BP was 246.8 oC. While the [α]D of nicotine was -168.5o at the temperature of 293.15K. These determined results verified the extracted product as nicotine.
Calculated percent recovery was 0.6 %, this percentage yield clarified that in this brand, very small nicotine is investigated, this deduces a significant loss of product throughout the procedure which are due to formation of emulsions (Fauzantoro, Dalimunthe, & Gozan, 2017) and not due to washing thoroughly with ether to extract maximum yield (Ustick, Chuang, Muesse, & Russ, 2018). It is also significant to be considered that reactions of precursor with solvent pair may not be completed, so 100% yield is not conceivable. Due to much transfers in all processes, this loss might be occurred (Xie et al., 2018). It is also revealed that as much water was added which decreased the concentration of nicotine. Other properties (Table.1) determined as the MW was 162.23g/mol, MP was -79 oC, and BP was 246.8 oC. While the [α]D of nicotine was -168.5o at the temperature of 293.15K. These determined results verified the extracted product as nicotine.
Table 1: Chemical properties of nicotine.
| Sr.No | Property | Value |
| 1 | MW | 162.23 g/mol |
| 2 | MP | -79 oC |
| 3 | BP | 246.8 oC (~247 oC) |
| 4 | [α]D | -168.5 o at 293.15 K |
| 5 | Density | 1.01 gcm-3 |
| 6 | RI | 1.53 |
Liquid nicotine product was allowed to be analysed through an IR Spectrophotometer for identification. IR spectra of photon energy having the peaks at different frequencies (Hz) was obtained (Table.2).
Table 2: IR Spectra values of Analysis.
| Sr.No | Frequency (cm-1) | Prediction |
| 1 | 2970-2781 | Presenc of C-H single bond |
| 2 | 1677 | Presenc of Aryl (Aromatic) C=N double bond |
| 3 | 1691 | Presenc of Aryl (Aromatic) C=C double bond |
| 4 | 717-904 | Presenc of C-H bond of mono substituted pryidine ring |
Distinct peaks on the IR spectra indicated the bond frequencies of certain functional groups. Peak at f=2970-2781 cm-1 specifies the existence of C-H single bond stretch. Peak at f=1677 cm-1 shows the availability of Aryl C=N double bond stretch. Peak at f=1691 cm-1 indicates the presence of Aryl C=C double bond stretch. Peak at f=717-904 cm-1 pointed out presence of C-H single bond stretch of mono substituted pyridine ring. Same results were predicted by some other research studies (Hua et al., 2017; Prückner, 2017; Stanfill et al., 2018).By these peaks obtained by IR spectroscopy, the composition of final product was predicted.
Conclusion
Nicotine was extracted from tobacco leaves separated from Gold Live Classic Brand™ cigarettes. The percentage yield determined after the whole extraction method was 0.6%. Calculated percent recovery was 0.6 %, this percentage yield clarified that in this brand, very small nicotine is investigated, this deduces a significant loss of product throughout the procedure which are due to formation of emulsions (Fauzantoro et al., 2017) and not due to washing thoroughly with ether to extract maximum yield (Ustick et al., 2018). So, repeated the process three times. It is also significant to be considered that reactions of precursor with solvent pair may not be completed, so 100% yield is not conceivable. It is also revealed that as much water was added which decreased the concentration of nicotine. The boiling point and density of product were determined which were 247oC, and 1.01 gcm-3 respectively. Other properties determined as the MW was 162.23g/mol, MP was -79 oC, and BP was 246.8 oC. While the [α]D of nicotine was -168.5o at the temperature of 293.15K. These determined results verified the extracted product as nicotine. Distinct peaks on the IR spectra indicated the bond frequencies of certain functional groups. Peak at f=2970-2781 cm-1 pointed out available C-H single bond stretch, at f=1677 cm-1 shows the presence of Aryl C=N double bond stretch, and at f=1691 cm-1 specifies the existence of Aryl C=C double bond stretch, f=717-904 cm-1 indicates the presence of C-H single bond stretch of mono substituted pyridine ring. Same results were predicted by some other research studies (Hua et al., 2017; Prückner, 2017; Stanfill et al., 2018).
Acknowledgments
All used chemicals and instruments were taken from chemistry Lab of University of Education, Vehari Campus, Punjab, Pakistan. This study was performed under the supervision of Dr. Rashad Mehmood (Assistant Professor of Chemistry, Department of Chemistry, University of Education, Vehari Campus). Authors say thanks to him for his affectionate guidance during this study.
References
- Benowitz N. L., Pipe A., West R., Hays J. T., Tonstad S., McRae T., Anthenelli R. M. Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch in Smokers: A Randomized Clinical Trial. JAMA internal medicine. 2018;178(5):622-631.
CrossRef - Benowitz N. L., Porchet H., Sheiner L & Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clinical Pharmacology & Therapeutics. 1988;44(1):23-28.
CrossRef - Blog A. <Benefits of Nicotine.pdf>. Retrieved from https://www.ecigarettedirect.co.uk/ashtray-blog/2014/11/10-benefits-of-nicotine.html. 2017.
- DeVito E. E., Herman A. I., Waters A. J., Valentine G. W & Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431.
CrossRef - Fathi R. M., Fauzantoro A., Rahman S. F & Gozan M. Column chromatography isolation of nicotine from tobacco leaf extract (Nicotiana tabaccum L.). Paper presented at the AIP Conference Proceedings. 2018.
CrossRef - Fauzantoro A., Dalimunthe A. A & Gozan M. Production of Biopesticide From Tobacco Leaves (Nicotiana Tabacum) With Digestion and Reflux Extractions. Paper Presented At The The 6 Th Indonesian Biotechnology Conference. 2017.
- Felman A. Everything you need to know about nicotine. from https://www.medicalnewstoday.com/articles/240820.php. 2018.
- Goniewicz M. L., Gawron M., Smith D. M., Peng M., Jacob P & Benowitz N. L. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes a longitudinal within-subjects observational study. Nicotine & Tobacco Research. 2017;19(2):160-167.
CrossRef - Heydari R., Mobidi A. M., Mohammadi R., Forouzandeh Z & Rashidzadeh S. Effect of emotional distress and academic stress on level of nicotine addiction among medical students. Nova Journal of Medical and Biological Sciences. 2017;6(1).
- Hopkins D. R., Ruiz-Tiben E., Eberhard M. L., Roy S. L & Weiss A. J. Archive for the ‘Guinea worm disease/Dracunculiasis’ Category. Management. 2017;500.
- Hossain A. M & Salehuddin S. M. Analytical determination of nicotine in tobacco leaves by gas chromatography–mass spectrometry. Arabian Journal of Chemistry. 2013;6(3):275-278.
CrossRef - Hu R. S., Wang J., Li H., Ni H., Chen Y. F., Zhang, Y. W., Li H. H. Simultaneous extraction of nicotine and solanesol from waste tobacco materials by the column chromatographic extraction method and their separation and purification. Separation and Purification Technology. 2015;146:1-7.
CrossRef - Hua Q., Lu W., Zheng S., Zhang Y., Zhang W., Wu D & Shen Y. Thermal release of nicotine and its salts adsorbed on silica gel. Thermochimica Acta. 2017;656:53-58.
CrossRef - Jarvik M. E. Beneficial effects of nicotine. British journal of addiction. 1991;86(5):571-575.
CrossRef - Kataoka H., Inoue R., Yagi K & Saito K. Determination of nicotine, cotinine and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. Journal of pharmaceutical and biomedical analysis. 2009;49(1):108-114.
CrossRef - Khan A. <nicotinepresentation-141110074149-conversion-gate02.pdf>. from https://www.slideshare.net/Afsana Khan1/nicotine-presentation-41351291. 2014.
- Kocha J. <pptonnicotine-131011144541-phpapp01.pdf>. from https://www.slideshare.net/jashankochar/nicotine-a-harmful-drug?from_action=save. 2013.
- Lawson N. S. <nicotinesumedited2-151115082556-lva1-app6891.pdf>. from https://www.slideshare.net/imtfuzz/introduction-about-nicotine. 2015.
- Leslie F. M. Multigenerational epigenetic effects of nicotine on lung function. BMC medicine. 2013;11(1):27.
CrossRef - Mishra A., Chaturvedi P., Datta S., Sinukumar S., Joshi P & Garg A. Harmful effects of nicotine. Indian journal of medical and paediatric oncology: official journal of Indian Society of Medical & Paediatric Oncology. 2015;36(1):24.
CrossRef - Prückner K. “Aus dem Gebiete der gesammten Heilkunst.” Die Heidelberger Klinischen Annalen und die Medicinischen Annalen. Eine medizinische Fachzeitschrift zwischen Naturphilosophie und Naturwissenschaft. 2017;15. Springer-Verlag.
- Stanfill S. B., Croucher R. E., Gupta P. C., Lisko J. G., Lawler T. S., Kuklenyik P., Peuchen E. H. Chemical characterization of smokeless tobacco products from South Asia: Nicotine, unprotonated nicotine, tobacco-specific N′-Nitrosamines and flavor compounds. Food and Chemical Toxicology. 2018;118:626-634.
CrossRef - Ustick J., Chuang C., Muesse D & Russ J. Analysis of nicotine in clay samples by solvent extraction and gas chromatography mass spectrometry. Paper presented at the Abstracts of Papers of the American Chemical Society. 2018.
- Xie J., Zhou B., Zhang T., Zeng X., Yang M., Wang W & Yang J. Preparation of nicotine surface molecularly imprinted polymers for selective solid-phase extraction of nicotine from zero-level refill liquids of electronic cigarettes. Analytical Methods. 2018.
CrossRef - Zhang X., Gao H., Zhang L., Liu D & Ye X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Industrial crops and products. 2012;39:162-169.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





