How to Cite | Publication History | PlumX Article Matrix
Investigation on Growth of Oil Degrading Thermophilic Bacteria Isolated from Hot Spring
Iqbal Ansari1 , Ritesh Kumar2, Muniyan Sundararajan2, Deblina Maiti1, Mohd Ashraf Rather3 and Meemshad Ali4
, Ritesh Kumar2, Muniyan Sundararajan2, Deblina Maiti1, Mohd Ashraf Rather3 and Meemshad Ali4
1Academy of Scientific and Innovative Research (AcSIR), CSIR-CIMFR, Dhanbad, Jharkhand, India.
2Scientist, CSIR-CIMFR, Dhanbad-826015, India.
3Department of Fisheries Biology, College of Fisheries, Ratnagiri, India.
4Department of Biotechnology, VBU, Hazaribagh, India.
Corresponding Author E-mail id:iqbal.cimfr@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2689
ABSTRACT: Oil spills are one of the key concerns of oil industry. The main distress of environmental specialists is the hazard to the marine ecosystem, caused due to offshore oil spills. In the present study the oil degrading potential of isolated bacteria on different media composition was carried out. From the study, it has been found that oil degrading thermophilic bacteria are capable of degrading soyabean oil, olive oil, tween 20, glycerol and crude petroleum oil. The culture of thermophilic was performed in thermus agar media and it was observed from the experimental study that the growth of thermophilic bacteria was moderately good at the range of 50oC to 60oC and 60oC to 70oC but declines after 70oC; no growth was observed in the range of 25oC to 50oC. It has also detected that the decaying capability of the thermophilic bacteria in Olive oil is initially better than soyabean oil during first six hours culture afterward its performance is better in soyabean oil than Olive oil. The present study is of special environmental significance as it can be efficiently used for bioremediation of oil polluted water.
KEYWORDS: Broth Media; Crude Petroleum Oil; Hot Water Springs; Thermophilic Bacteria
Download this article as:| Copy the following to cite this article: Ansari I, Kumar R, Sundararajan M, Maiti D, Rather M. A, Ali M. Investigation on Growth of Oil Degrading Thermophilic Bacteria Isolated from Hot Spring. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Ansari I, Kumar R, Sundararajan M, Maiti D, Rather M. A, Ali M. Investigation on Growth of Oil Degrading Thermophilic Bacteria Isolated from Hot Spring. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32330 |
Introduction
Thermophilic bacteria is defined as such microorganisms which are capable of living at soaring temperature and not only surviving in such stressed environment although they still flourish in hot water.1 Doolittle2 described the thermophilic bacteria are heat lover microorganisms and capable of growing at high temperature (more than 55°C) .They are found within boiling springs and water heaters. Such extreme conditions of their existence give rise to some exceptional characteristics about their evolution. One theory suggests that they were the first living organisms that have been evolved on the earth during the prehistoric birthing day of earth when the temperature on the earth was quite hot and they are called as “Universal inherited”. Bergey3 has opined as thermophiles which show no growth or only fragile growth below 40ºC to 45ºC. Their maturity requires above 50°C and some are capable of growing at a temperature of 80ºC though the most abundant growth was shown in ranges between 60ºC to 70ºC. Thermophiles are capable of growing at high temperatures and in the process give rise to more stable extracellular enzyme. Such characteristics make them acceptable for increasing enzyme utilization through genetic manipulation and consequently they were first suitable candidates for enzyme production for industrial applications.4
The first investigation on the characterization of thermophilic bacteria was carried out by Miquel.5 The existence of thermophilic bacteria has been previously reported in such temperate environmental conditions.6,7,8 Several authors9,10,11 reported that protein produced from thermophilic bacteria are thermostable and resist along with denaturation and proteolysis process. In addition it was found that thermophilic bacteria, archaea and actinomycetes are surviving at elevated temperatures due to their increased hydrophobic interactions, electrostatic and disulphide interactions in protein structure.
Various study12,13,14 on lipases and their characteristics stated that thermophilic isolates viz. Bacillus were used to purify and characterize several lipases. Thermophilic bacteria contain a thermostable enzyme, Taq-polymerase, which has been used in polymerase chain reactions (PCR) for the amplification of DNA in molecular biology research studies.15,16,17,18,19According to Sharma et al.20 lipases play a major role in agricultural based industry, cosmetics and the pharmaceutical industry and their applications also has noticed in the synthesis of new molecules. Gomes21 reported an enzyme which degrades naturally occurring starch and cellulose are the area of interest due to their industrial potential and a very useful enzyme, pullulanase which is valuable in the production of maltooligosaccharides is also capable of degrading starch. A numbers of authors22,23,24 reported that thermophilic bacteria which produces extracellular lipase enzymes acts as lipid-degrading and generally works in the presence of inducers likes Tween 20, olive oil, oleic acid and palm oil. Hard and long chain of polymeric substrates like starch, cellulose, xylan, pectin and chitin can be degraded enzymatically in the presence of thermophilic bacteria.25,26,6
Perfumo et al., 200627 reported the high degradation rates of hydrocarbons were observed when a thermophilic biosurfactant-producing oil degrading P. aeruginosa AP02- 1 was used and which efficiently utilised crude oil and diesel within a short period (<7 days ) at 450C. Similarly numbers of co-workers have also reported that species of thermophilic bacteria can degrade crude oil and aromatic hydrocarbons at different temperatures such as Bacillus sp. (40-45°C) Al-Maghrabi et al, (1999),28 B.stearothermophilus (60°C) Sorkhoh et al. (1993)29 and Consortium of Pseudomonas sp. (40-42°C) Lugowsky et al (1997).30 The finding of an experimental studied showed that theromophilic bacteria could be used in either the treatment of oil spill or in-situ stimulation of heavy oil wells. The bacteria have proved its ability to degrade crude oil containing asphaltene (Abdulrazag & Chaalal 2005).31
To our knowledge, very few studies has been focused on isolation of Thermophilic bacteria from India hot water, still no continuous investigation had focused on further application of these thermophiles bacteria. In India, from different hot water spring the thermophilic bacteria have been investigated and isolated. Four thermophilic bacterial strains were isolated from Manikaran hot water spring of northern Himalayan region of Himachal Pradesh (Verma et al., 2014)32. In another study; the thermophilic bacteria (Bacillus sp.) have been isolated from hot spring of Tarabalo, India which could tolerate high temperatures (Mrunmaya et al., 2013).33 Similar study was conducted to isolate thermophilic bacteria from hot spring at Tarabalu Orissa, India. The bacterium was Gram-negative, motile rods, non-spore forming and generally occurred singly or in pairs. The growth temperature ranges from 570C to 1000C, optimum at 750C (Hemant et al., 2010).34 Therefore an experimental study was carried out to assess the decaying capability of thermophilic bacteria in different media. In this study, we reported the potential of thermophilic bacteria which can be harnessed to exploit their metabolism process for biotechnological exercises in degradation of different crude oils. ch thermophilic bacteria may be useful in degradation of crude oils at varying temperature by utilizing as a source materials for their growth.
Materials and Methods
Study Area
Surajkund hot-water spring is a natural spring of hot-water which is geologically situated at the latitude and longitude of 24°08′58″N and 85°38′44″E respectively at 364 m elevation from MSL in Belkapi village of Hazaribagh district in Jharkhand state of India. It is also called Surya Kund which means in local language “Pond of Sun”. The beauty of the Surajkund is that a kind of bacteria called thermophilic grows in this pond at higher temperature as it also provides a suitable environment for its growth. The average temperature of the subsurface just below the Surajkund is 165°C. The geographical location has been shown in figure 1.
 |
Figure 1: Location of Surajkund Hot Spring in Jharkhand State, India.
|
Experimental Design
The experiment was conducted on laboratory scale to study the growth of thermophilic bacteria in different media and their efficiency of release of lipase enzyme. The water sample was collected from one of the hot springs of India commonly known as Surajkund hot spring situated in Hazaribag district of Jharkhand state. The photograph of sampling point has been shown in figure 2.
 |
Figure 2: Sampling site of Surajkund Hot Spring where water samples were taken.
|
Collection and Culture
Water samples were collected from Surajkund hot springs in sterile bottles. Thermus agar media was prepared for proper growth of bacteria. Thermus agar media is a semi-synthetic media that contains pepton which is a protein hydrolydate and beef extract that provide amino- acid, organic acids, vitamins, minerals, NaCl and pH for stability. Since liquid and solid media contained in petriplates provide an artificial environment suitable for rapid growth of bacteria, thermus agar media is preferably chosen for isolation and maintenance of thermophilic bacteria as shown in figure. 3. The streak plate method was adopted to view the colony growth of the thermophilic bacteria colony as revealed in figure 4 and Gram’s staining technique of color observation was followed to identify whether the stain is Gram’s positive or negative as shown in figure 5.
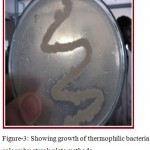 |
Figure 3: Showing growth of thermophilic bacteria colony by streak plate methods.
|
 |
Figure 4: Preparation of different media for culture of thermophilic bacteria.
|
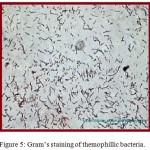 |
Figure 5: Gram’s staining of themophillic bacteria.
|
Serial Dilution
In a serial dilution, the original sample is diluted several times to reduce the microbial population sufficiently in order to obtain separate colonies when plated. This is an effective process to obtain individual colony and study its characteristics.
Pure Culture
Pure culture of thermophillic bacteria was attained by streak plate method. In this method, sterilized loop dipped into a suitable diluted suspension of sample is streaked on surface of already solidified agar plate. Petriplate containing thermus agar media were placed in an incubator to check contamination of serially diluted water sample of surajkund. Thermus agar plate and serially diluted soil sample were taken to laminar air flow to perform streaking in a zig- zag manner. Plates were then marked with 10-6 dilution incubated for 24-48 hours at 65ºC. Individual species were obtained from colonies of desired characteristic size and appearance.
Culture Condition for Lipase Production
Batch culture of the isolate thermophilic bacteria which is able to degrade lipids was carried out in a conical flask as a bioreactor with working capacity of volume 500 ml. The TYEM media was used for lipase production. The inoculums size was 1.0%, the growth temperature was 65ºC and the pH was maintained at 9.0 throughout the cultivation. Besides, DO was maintained by shaking at 120 rpm for proper aeration.
Lipase Purification
The cell free supernatant was prepared by centrifugation (10000 X g for 15 min) of culture broth. It was then passed through the filtered supernatant and slowly ammonium sulfate was added under stirring to 30% saturation. The suspension was centrifuged (10000 X g, 20 min) and then ammonium sulphate was added to supernatant to each 80% saturation at 4ºC. The final precipitate was collected by centrifugation (10,000X g, 20 min) 4oC and re -dissolved in a minimal volume of 20 mM Tris HCL buffer (pH 7.5) at 4oC. The dissolved enzyme solution was dialysed against the same buffer for 16 hours at 4°C to remove the residual ammonium sulphate. The dialyzed solution was applied to a DEAE sephacel column (Hi media) previously equilibrated with 20mm Tris –HCL buffer (pH 7.5) and eluted with a linear gradient of sodium chloride (0-250 mm) at a flow rate of 0.2ml min-1. Protein fraction containing lipase activity were pooled and passed through a column of sephacryl 5200 (Hi media) per equilibrated with 20mM Tris – HCl buffer (pH 7.5) at a flow rate of 0.1 ml min-1. Fraction containing lipase activity was then separated and viewed by sulphate polyacrylamide gel electrophoresis (SDS-PAGE) as shown in Figure 6.
 |
Figure 6: Thermophilic lipase enzyme isolation using SDS-PAGE.
|
Results and Discussion
The results of this research carried out on thermophilic bacteria, collected from SurajKund Hot Spring has been explained below. To understand its growth pattern and its morphology under various conditions like temperature, different media environment gram staining was done and tabulated in Tables 1 to 3.
Table 1: Growth of thermophilic bateria at different temperature.
| Sl. No. | Temperature | Nunber of colonies | Remarks |
| 1. | 25oC to 37oC | – | No growth |
| 2. | 37oC to 60oC | + | Moderate growth |
| 3. | 60oC to 70oC | ++ | Good growth |
| 4. | 70oC to 75oC | + | Moderate growth |
| 5. | >75oC | – | No growth |
Effect of Temperature
Temperature is one of the several decisive factors for growth of any thermophilic bacteria. The thermophilic property of the bacteria was proved from the result that at higher temperature good growth of the bacterial culture was observed which also revealed the particular range of temperature most suitable for optimum growth. It can be observed from Table 1 that the range of temperature from 60ºC to 70ºC, showed highest growth. At lower temperature i.e. from 25ºC to 37ºC, no growth was observed. No growth was observed above or below the temperature range of 50ºC to 75ºC. According to Mustafa et al.,35 the optimum growth temperatures for thermophilic bacteria are higher than 50°C. Similar study was reported by Abdulrazag and Chaalal28 in which the effect of temperature on the growth of thermophilic bacteria at 35-75ºC was noted. Arzu et al.36 had also isolated a gram-positive staining, rod-shaped thermophilic bacteria and observed its growth at 37–69ºC; according to their results they observed optimum growth at 60°C. Satoshi et al.37 reported a thermophilic bacterium growing optimally at 58°C and were of strain PBT, gram-positive as well as spore-forming property. Similarly optimal growth was observed at 55–58°C for Anoxybacillus rupiensis sp. Nov., a novel thermophilic bacterium isolated from Rupi basin, Bulgaria.38 The thermophilic bacterial adaptations in their genomes occurs natural selection of more designable folds, indicating to designability as a vital constituent of protein fitness.39 Similarly Donal & Singer40 has suggested that the natural selection acting on genomes, transcriptomes and proteomes and may be the reason for the survival of thermophilic bacteria in such extreme temperature. According to Yusuke et al.41 a thermophilic bacteria produces two types of unusual polyamine; long linear polyamines such as caldopentamine and caldohexamine, and branched polyamines such as quaternary ammonium compounds (tetrakis (3 aminopropyl) ammonium) in which the long linear polyamines are competent to stabilize DNA, and tetrakis(3-aminopropyl) ammonium plays a vital role in stabilizing RNAs in their cell structures. Another Grosjean & Oshima42 reported in vivo, the half-lives of both RNA and DNA of thermophilic bacteria possess longer than that estimated in vivo, attesting to cellular strategies which protects their nucleic acids against damaging effect of heat.
Gram Staining
The isolated bacteria were found to be blue in color and out of them only one group of thermophilic bacteria was found which were of bacillus shape. The selected strain was further observed morphologically by Gram’s staining technique43and their growth characteristics were studied to reveal their Gram-positive nature, rod shape and single arrangement (Table 2 and Figure 5).
Table 2: Gram’s staining of thermophilic bacteria.
| Observation No. | Shape | Color | Arrangement | Identification |
| 1. | Bacillus | Blue | Single | Less Color |
| 2. | Bacillus | Blue | Single | Blue colored |
Growth of Thermophilic Bacteria in TYEM + Different Media
The isolated thermophilic bacteria were taken from the batch culture for its growth in TYEM (Thermophilic Yeast Extracted Media) + soybean oil at 65°C. Reading of optical density was taken at 4 hours interval. Table 3 shows the comparative results of growth of thermophilic bacteria in TYEM + different media. Fig. 7 and Fig. 8 shows the plot of growth of thermophilic bacteria in different media with time in terms of OD and the wavelength of the refracted light passing through the different media. It was observed during the growth of thermophilic bacteria that different media such as the soybean oil, olive oil broth, Tween 20 broth, glycerol and crude petroleum oil were gradually degraded. This reveals that the isolated bacteria belonging to the thermophilic category is also capable of degrading lipid compounds and significant production of lipase enzyme with increased decaying capacity was also observed. Peng et al.44 reported that the Bacillus sp. strains of thermophilic bacteria are capable to degrade aromatic acids such as cinnamic, 4-coumaric, 3-phenylpropionic, 3-(p-hydroxyphenyl) propionic, ferulic, benzoic, and 4-hydroxybenzoic acids at 60°C. A similar thermophilic bacteria was also isolated and grown at 55°C by Nayak.45 Ibrahim et al.46 isolated a thermophilic bacteria growing optimally at 60-80°C while also degrading long chain of organic molecules. The growth of thermophilic bacteria was drastically augmented at high temperature of 80oC and such bacteria would be also useful for degrading of crude oil.28 Such thermophilic bacteria can degrade the crude oils at varying temperatures. A strain PBT, gram-positive thermophilic bacterium grew acetogenically on several alcohols, methoxylated aromatics, pyruvate, glycine, cysteine, formate and hydrogen or CO2.30 Thermophilic bacterium (Anoxybacillus rupiensis sp. Nov) is capable of degrading sugars, polyols, and polysaccharides such as xylan, glycogen and starch at 55–58°C.31 Another gram positive rod shaped strain of thermophilic bacteria ‘Bacillus justea I’ was isolated and was capable to degrade various sugars such as glucose, fructose, manose, maltose, lactose, sucrose, trehalose, mannitol, melibiose, raffinose, xylose and cellobiose as a carbon source.47 The strain Pseudomonas aeruginosa (AP02-1) was isolated from hot springs, growing optimally at 45°C and degraded 99% of crude oil 1% (v/v) and diesel oil 2% (v/v) when supplied to the basal mineral medium within 7 days of incubation (Amedea et al.).48 Similarly Christos et al.49 isolated a thermophilic bacteria, phylogenetically affiliated with Bacillus sp., were capable of degrading long chain crude oil alkanes by 47% and 88% when provided as a carbon source. Further another study reported that an aerobic, thermophilic, halotolerant, gram-positive bacterium, was capable to degrade benzoic, p-hydroxybenzoic, protocatechuic, vanillic, p-hydroxyphenylacetic, 3,4 -dihydroxyphenylacetic, cinnamic, ferulic acids, phenol and m-cresol.50 Hence thermophilic bacteria can be efficiently used for bioremediation of oily contaminants.
Table 3: Profile of thermophilic bacteria in terms of OD during culture in different media.
| Sl. No. | Time | OD profile of different media at 580 nm wave length of light | ||||
| Soyabean Oil | Olive Oil | Tween 20 | Glycerol | Petroleum | ||
| 1. | 0 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 |
| 2. | 4 | 0.009 | 0.009 | 0.007 | 0.006 | 0.008 |
| 3. | 8 | 0.076 | 0.096 | 0.082 | 0.009 | 0.072 |
| 4. | 12 | 0.222 | 0.310 | 0.130 | 0.082 | 0.120 |
| 5. | 24 | 0.372 | 0.452 | 0.351 | 0.122 | 0.311 |
| 6. | 28 | 0.434 | 0.521 | 0.411 | 0.233 | 0.421 |
| 7. | 32 | 0.546 | 0.522 | 0.412 | 0.235 | 0.486 |
Table 4: Correlation of biodegradation of different medium in terms of OD.
| Media | Equation | R2 |
| Soyabean Oil | d(t) = 0.017t – 0.029 | 0.979 |
| Olive Oil | d(t) = 0.018t – 0.008 | 0.944 |
| Tween20 | d(t) = 0.014t – 0.027 | 0.979 |
| Glycerol Oil | d(t) = 0.007t – 0.023 | 0.923 |
| Petroleum Oil | d(t) = 0.015t – 0.042 | 0.981 |
Note: ‘t’ stands for time and ‘d(t)’ stands for OD at time t (hr).
 |
Figure 7a: Concentration of thermophilic bacteria in terms of wave length ratio of the refracted light to the wave length of light at 580nm during culture in different media with time; (b) to (f) Linear trends in growth of the bacteria in individual medium with time. |
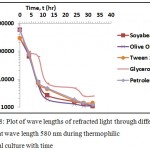 |
Figure 8: Plot of wave lengths of refracted light through different media at wave length 580 nm during thermophilic bacterial culture with time.
|
Comparative Study of Different Media for its significance using ANOVA
The summary of mean and variance of different media is shown in (Table 5). The comparative study of the five different media was carried out to establish a hypothesis that all the media are equally performing. ANOVA study carried out suggests that the hypothesis cannot be rejected. F (test results) <F (critical) as can be seen from Table 6. Also, P-value > 0.05 (α: Level of Significance) therefore, hypothesis cannot be rejected.The positive correlation was observed by strains of thermophilic bacteria the stains such as H5 and H8 abilities to utilize arabinose,ribose, xylose, sorbose, mannitol, mannoside, starch,glycogen, and fucose (P < 0.05) having linking protease production.51
Table 5: Mean and average of different media.
| ANOVA observed during statistical analysis for different media | ||||
| Groups | Count | Sum | Average | Variance |
| Soyabean Oil | 9 | 2.223 | 0.2470 | 0.0366 |
| Olive Oil | 9 | 2.553 | 0.2837 | 0.0423 |
| Tween20 | 9 | 1.849 | 0.2054 | 0.0265 |
| Glycerol | 9 | 0.894 | 0.0993 | 0.0080 |
| Petroleum | 9 | 1.857 | 0.2063 | 0.0305 |
Table 6: ANOVA for different media data set.
| Source of Variation | ANOVA observed during statistical analysis | |||||
| SS | df | MS | F | P-value | F (critical) | |
| Between Groups | 0.172 | 4 | 0.0430 | 1.4944 | 0.22212797 | 2.61 |
| Within Groups | 1.151 | 40 | 0.0288 | |||
| Total | 1.323 | 44 | ||||
Conclusion
The experimental study reveals that the growth of thermophilic bacteria was moderate in the range of 50oC to 60oC and good at the range of 60oC to 70oC but declines after 70oC; no growth was found at 25oC to 50oC. It has also been observed that the decaying capacity of the thermophilic bacteria for Olive oil was initially better than soyabean oil during first six hours in the culture afterwards its performance was comparatively better in soyabean oil. The purpose of this research was to isolate microbial lipases from thermophilic bacteria. The results showed that the isolated bacteria are capable of degrading oil containing different medial composition. This approach will be fruitful for bioremediation of oil containing hydrocarbons. This lipase also has a significant role in various industrial processing. In future, this study can be applied in preparation of anti-ageing protein as it is able to survive and is not denatured at highest temperature which is found in the hot spring environment. This study would also be helpful in the treatment of waste water containing hydrocarbons, especially oil spills as from experimental study it has been shown that it may be prevent the deterioration of water quality of aquatic body such as rivers and marine. It will be a sustainable tool for clean environment, particularly treating waste containing hydrocarbons and oil, which requires further research to establish the techno-economic feasibility. The consortia will be helpful in degradation of crude oil.
References
- Brock T. Introduction: an overview of the thermophiles. In, Brock, T. Thermophiles general, molecular and applied microbiology – John Wiley and Sons, New York. 1986;2-15.
- Doolittle W. F. Phylogenetic classification and universal tree. Sciences. 1999;284:2124-2128.
CrossRef - Bergey D. H. Thermophilic bacteria. Laboratory of hygiene, University of Pennsylvania. Journal of bacteriology. 1919;4:4.
- Hisotsuyanagi K. Stepwise introduction of regulatory genes stimulating production of α-amylase into Bacillus subtilis, Construction of α- amylase extra hyper producing strain. Agric boil Chem. 1979;43:2343-2349.
- Miquel P. Monographic d’un Bacille Vivant Au-Dela de70oAnn. Micrographic. 1888;1:3-10.
- Mutzel A., Reinscheid U. M., Antranikian G., Müller R. Isolation and characterization of a thermophilic strain that degrades phenol and cresols as sole carbon and energy source at 70o Appl. Microbiol.Biotechnol. 1996;46:593-596.
CrossRef - Wiegel J., Ljungdahl L. G., Rawson J. R. Isolation from soil and properties of the extreme thermophile Clostridium thermo hydro sulfuricum. J Bacteriol. 1979;139:800-810.
- Rahman T. J., Marchant R., Banat I. M. Distribution and molecular investigation of highly thermophilic bacteria associated with cool soil environment. Biochem Soc Transactions. 2004;32:209-213.
CrossRef - Kumar S., Nussinov R. How do theromophilic proteins deal with heat? – Mol .Life. Sci. 2001;58:1216-1233.
CrossRef - Pebone E., Limauro D., Bartolucci S. The machinery for oxidative protein folding in thermophiles. Antioxid Redox Signal. 2008;10(1):157-169.
CrossRef - Ladenstein R., Ren B. Protein disulfides and protein disulfide oxidoreductase in hyperthermophiles. Federation of European Biochemical Society Journal. 2006;273(18):4170-4185.
CrossRef - Schmidt-Dannert C., Sztajer H., Stocklein W., Menge U., Schmid R. D. Screening, purification and properties of a thermophilic lipase from Bacillus thermocatenulatus. Biochim Biophys Acta. 1994;1214:43-53.
CrossRef - Luisa R. M., Dannert C. S.,Wahl S.,Sprauer A., Schmid R. D. Thermo-alkalopilic Lipase of Bacillus thermocatenulatus Large scale production, purification and properties, aggregation behavior and its effects on activity. Biotechnol. 1997;56:89-102.
- Kim H. K., Sung M. H., Kim H. M., OH T. K. Occurrence of thermostable Lipase in thermophilic. Bacillus sp. strain 398-Biosci. Biochem. 1994;58:961-962.
- Rather M. A., Bhat I. A., Gireesh-Babu P., Chaudhari A., Sundaray J. K & Sharma R. Molecular characterization of kisspeptin gene and effect of nano–encapsulted kisspeptin-10 on reproductive maturation in Catla catla. Domestic animal endocrinology. 2016;56:36-47.
CrossRef - Rathor P. K., Bhat I. A., Rather M. A., Gireesh-Babu P., Kumar K., Purayil S. B. P & Sharma R. Steroidogenic acute regulatory protein (StAR) gene expression construct: Development, nanodelivery and effect on reproduction in air-breathing catfish, Clarias batrachus. International journal of biological macromolecules. 2017;104:1082-1090.
CrossRef - Rather M. A., Bhat I. A., Rathor P. K., Gireesh-Babu P., Chaudhari A., Kumar S. J & Sharma R. In silico analysis and expression studies of kisspeptin gene in C. catla. Journal of Biomolecular Structure and Dynamics. 2017;35(11): 2485-2496.
CrossRef - Sharma N., Rather M. A., Ajima M. N., Gireesh-Babu P., Kumar K & Sharma R. Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nano particles. Food and chemical toxicology. 2016;96:122-132.
CrossRef - Nandanpawar P., Badhe M., Rather M. A & Sharma R. Chitosan nanoparticles for gene delivery in Macrobrachium rosenbergii (DE MAN 1879). Journal of Cell and Tissue Research. 2013;13(2):3619.
- Sharma R., Soni S., Vohra R. Production of extracellular alkaline lipase from a Bacillus sp. RSJ 1 and its application in ester hydrolysis. Indian Journal of Microbiology. 2002;42(1):49-54.
- Gomes I., Gomes J., Steiner W. Highly thermostable amylase and pullulanse of the extreme thermophilic Eubacterium Rhodothermus Marinus, Production and Partial Characterization. Bioresour Tech. 2003;90:2007-2014.
CrossRef - Shabtai Y. Isolation and characterization of a lipolytic bacterium capable of growing in a low water content emulsion. Applied and Environmental Microbiology. 1991;57(6):1740-1745.
- Shabtai Y., Daya-Mishne N. Production, purification and properties of a lipase from a bacterium (Pseudomonas aeruginosa YS-7) capable of growing in water restricted environments. Applied and Environmental Microbiology. 1992;58(1):174-180.
- Sigurgísladóttir S., Konráòsdóttir M., Jónsson Á. Lipase activity of thermophilic bacteria from icelandic hot springs. Biotechnology Letters. 1993;15(4):361-366.
CrossRef - Stetter K. O. Hyperthermophilic prokaryotes. FEMS Microbial Rev. 1996;18:149-158.
CrossRef - Kristjansson J. K., Hreggvidsson G. O. Ecology and Habitats of Extremophiles. World J Microbiol. Biotechnol. 1995;11:17-25.
CrossRef - Perfumo A., Banat I. M & Marchant R. The use of thermophilic bacteria in accelerated hydrocarbon bioremediation. Environmental Problems in Coastal Regions VI.WIT. Transactions on Ecology and the Environment. 88;67-77.
CrossRef - Al-Maghrabi I. M. A., Aqil A.O. B., Islam M. R., Chaalal O. Use of thermophilic bacteria for bioremediation of petroleum contaminants. Energy Sources. 1999;21:17-29.
CrossRef - Sorkhoh N. A., Ibrahim A. S., Ghannoum M. A., Radwan S. S. High-temperature hydrocarbon degradation by Bacillus stearothermophilus from oil-polluted Kuwait desert. Appl Microbiol Biotechnol. 1993;39:123-126.
CrossRef - Lugowsky A. J., Palamteer G. A., Boose T. R., Merriman J. E. Biodegradation process for detoxifying liquid streams. Patent US. 1997;5656169.
- Y. Z.,Chaalal O. Effect of temperature on biodegradation of crude oil. Energy Sources. 2005;27:233–244.
CrossRef - Ambika V., Monika G and Poonam S. Isolation And Characterization Of Thermophilic Bacteria In Natural Hot Water Springs Of Himachal Pradesh (India). The Bioscan. 2014;9(3):947-952.
- Mrunmaya K. P., Mahesh K. S., Kumananda T. Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. 2013;5(2):159-165.
- Hemant K. K., Suryakanta S., Rajesh K. S., Santanu K. K., Bibhuti B. P., Kanchan M. B. Isolation and characterization of thermophilic bacteria from hot spring in Orissa, India .Biosciences, Biotechnology Research Asia. 2010;7(1):449-451.
- Mustafa O. B., Genc S. A. B., Gulsah A., Ahmet A. Isolation and Characterization of Thermophilic Bacteria from Geothermal Areas in Turkey and Preliminary Research on Biotechnologically Important Enzyme Production. Geomicrobiology Journal. 2017;34(1):53-62.
CrossRef - Abdulrazag Y., Zekri O. C. Effect of Temperature on Biodegradation of Crude Oil. Energy Sources. 2005;27:233–244.
CrossRef - Arzu C. C., Birgul O., Cumhur C. Anoxybacillus salavatliensis nov., an α-glucosidase producing, thermophilic bacterium isolated from Salavatli, Turkey. Journal of Basic Microbiology. 2011;51:136–146.
CrossRef - Satoshi H., Yoichi K., Satoshi H., Shoun H. Thermacetogenium phaeum nov. sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. International Journal of Systematic and Evolutionary Microbiology. 2000;50:1601–1609.
CrossRef - Derekova A., Sjøholm C., Mandeva R., Kambourova M. Anoxybacillus rupiensis Nov., a novel thermophilic bacterium isolated from Rupi basin (Bulgaria). Extremophiles. 2007;11:577–583.
CrossRef - Jeremy L. E., Boris E., Eugene I. S. S. Natural selection of more designable folds: A mechanism for thermophilic adaptation. PNAS. 2003;100(15):8727–8731.
CrossRef - Donal A., Gregory H., Singer A. C. Genomic and proteomic adaptations to growth at high temperature .Genome Biology. 2004;5(10). Article 117.
- Terui Y., Ohnuma M., Etsuko K. H., Kawashima., Tairo O. Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophiles. J. 2005;388:427–433.
- Grosjean H., Oshima T. How Nucleic Acids Cope with High Temperature,In Gerday C., Glansdorff N (ed), Physiology and Biochemistry of Extremophiles. ASM Press, Washington, DC. 2007;39-56.
CrossRef - Smibert R. M., Krieg N. R. Phenotypic characterization. In Methods for General and Molecular Bacteriology, Edited by Gerhardt P. Murray R. G. E.,Wood W. A & Krieg N. R. Washington, DC: American Society for Microbiology. 607–654.
- Peng X., Misawa N.,Harayama S. Isolation and characterization of thermophilic bacilli degrading cinnamic, 4-coumaric, and ferulic acids. Applied and Environmental Microbiology. 2003;69(3):1417–1427.
CrossRef - Nayak S. P. Identification and characterization of thermophillic bacteria from hot springs of odisha and their potential applications. A PhD thesis. Department of microbiology Orissa. University of agriculture and technology, Bhubaneswar, Odisha, India. 2013.
- Ibrahim M. A., Al-Maghrabi A. O.,Aqil M. R. B., Islam O. C. Use of Thermophilic Bacteria for Bioremediation of Petroleum Contaminants. Energy Sources. 1999;21(1-2):17-29.
CrossRef - Elnasser Z. M.,Khraisat A. O. W. A. Isolation and Characterization of New Thermophilic Bacteria in Jordan. The Internet Journal of Microbiology. 2006;3(2):1-7.
- Amedea P., Ibrahim M. B., Francesco C., Roger M. Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. Appl Microbiol Biotechnol. 2006;72:132–138.
CrossRef - Christos M., Kalliopi I., Chalkou K. A., Kormas A. D. Karagouni. Biodegradation of crude oil by thermophilic bacteria isolated from a volcano island. Biodegradation. 2006;1-7.
- Chamkha M., Mnif S., Sami S. Isolation of a thermophilic and halophilic tyrosol-degrading Geobacillus from a Tunisian high-temperature oil field. FEMS Microbiol Lett. 2008;283:23–29.
CrossRef - Balsam T. M., Hala I. A., Daghistani A. J., Saleh A. L., Christian K. Isolation and Characterization of Thermophilic Bacteria from Jordanian Hot Springs: Bacillus licheniformis and Thermomonas hydrothermalis Isolates as Potential Producers of Thermostable Enzymes. International Journal of Microbiology. 2017;1:1-12.

This work is licensed under a Creative Commons Attribution 4.0 International License.





