How to Cite | Publication History | PlumX Article Matrix
miRNA-1, miRNA-145 As A Myocardial Infarction Diagnostic Biomarker
Ibrahim Abdul-Majeed Altamemi1 , Aqeel Raheem Hassan2 and Alawi Jawad3
, Aqeel Raheem Hassan2 and Alawi Jawad3
1Department of Medical Microbiology, College of Medicine, University of Al-Qadisiyah.
2Department of Medicine, College of Medicine, University of Al-Qadisiyah.
3M. B. CH. B Resident Physician at Al-Dewaniyah Teaching Hospital.
Corresponding Author E-mail: ibrahim.altamemi@qu.edu.iq
DOI : http://dx.doi.org/10.13005/bbra/2712
ABSTRACT: Many myocardial infarction biomarkers currently available but they are a lack of specificity, therefore present study suggests to evaluate the significant importance of miRNA-1, miRNA-145 as biomarkers for early diagnosis of myocardial infarction. A blood sample was collected from three groups. The first group was patients with acute myocardial infarction (MI), the Second group was patients who have a risk factor for MI, and the Third group included healthy volunteers. Serum blood of this sample used to RNA purification and cDNA application with stem-loop specific primer then miRNA-1, and miRNA-145 was quantitated by using RT-PCR. The level of miR-1 fold change was significantly highest in the MI group followed by risk group and then by control group (P<0.05). while of miRNA-145 fold change was significantly lowest in the MI group followed by risk group and then by control group (P<0.05). A receiver operator characteristic (ROC) analysis; the cut off value was identified at miRNA-1 of >5.28 fold change with a sensitivity of 91.67 % and a specificity of 90.7%, while the cut off value of miRNA-145 has cut off ≤ 0.7 fold change with a sensitivity of 95.83 % and a specificity of 89.47%. miRNA-1, miR145 has high sensitivity and Specificity in this study which was bushed to using them as an alone biomarker or supported for Another biomarker in AMI diagnosis.
KEYWORDS: miRNA-1; miRNA-145; MI
Download this article as:| Copy the following to cite this article: Altamemi I. A, Hassan A. R, Jawad A. miRNA-1, miRNA-145 As A Myocardial Infarction Diagnostic Biomarker. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Altamemi I. A, Hassan A. R, Jawad A. miRNA-1, miRNA-145 As A Myocardial Infarction Diagnostic Biomarker. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32612 |
Introduction
AMI diagnosis is based on ECG findings and measurements of blood biomarkers of myocardial damage, among which cardiac troponins (cTns), but they suffer from a lack of specificity since the elevation of cTn levels can be due to non-cardiac causes.1 Therefore, there is a need for novel, early and specific biomarkers of AMI as miRNAs.
MicroRNAs (miRNAs) are endogenous, noncoding, single-stranded RNAs of ∼22 nucleotides and constitute a novel class of gene regulators.2 Recent studies have revealed that miRNAs exist in circulating blood. In contrast to our original thought, the cell-free miRNAs are relatively stable due to binding with other materials such as exosomes in circulating blood.3 In summary, the diversity and stability of miRNAs in combination with technical availabilities establish miRNAs as novel and promising biomarkers for the diagnosis of human diseases.4 In the rat, the circulating miR-1 level is rapidly increased 1 hr after coronary artery ligation and peaked at 200-fold higher than the baseline 6 hrs after AMI. The elevated miR-1 level returned to basal levels 3 days after AMI, a time course earlier than traditional AMI biomarkers, such as troponin.5-7 Another study suggests that miR-145 could be a valuable biomarker for cardiovascular diseases,8-10 therefore miRNA-1, miRNA-145 were included in the present study.
Material and Method
A case control study has been conducted based on 24 patients with ST-elevation myocardial infarction (MI), 24 persons who have risk factors for ischemic heart disease and 24 healthy control subjects. A blood sample was collected from three groups. The first group was 50 patients with acute myocardial infarction which include (28 male and 22 female), who were observed in CCU of Al-Diwaniyah teaching hospital / Iraq, Second group was 50 patients who have risk factor for MI (Hypertension, Hyperlipidemia, and Diabetes mellitus) the Third group included 50 healthy volunteers (non coronary artery diseases). A blood sample was collected by vein puncture from these groups. Each blood sample of three groups was collected to 3 ml of blood collected directly in a sterile tube (without EDTA) for total RNA were extracted from serum samples by using (TRIzol® reagent kit. Bioneer. Korea) and done according to company instructions.
The extracted RNA was treated with DNase I enzyme to remove the trace amounts of genomic DNA from the eluted total RNA by using samples (DNase I enzyme kit) and done according to the method described by Promega company, USA instructions.
Table 1: miRNA-1 F and R primer.
| Primer | Sequence | |
| miR-1RT primer | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACATACAT | |
| miR-1 primers | F | GTGCAGGGTCCGAGGT |
| R | GTTGGGTGGAATGTAAAGAAGT | |
| miR-1probe | FAM-CAGAGCCAACATACAT-MGB | |
>hsa-miR-1-3p MIMAT0000416
Table 2: miRNA-145, F and R primer.
| Primer | Sequence |
| miR-145 RT primer | GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAGGGAT |
| miR-145 primers | GTGTCCAGTTTTCCCAGGA |
| GTGCAGGGTCCGAGGT | |
| miR-145 probe | FAM-CAGAGCCAACAGGGAT-MGB |
>hsa-miR-145-5p MIMAT0000437.
The Taq Man MicroRNA assays were used looped-primer RT-PCR, a new real-time quantification method, for detection of mature miRNAs. Total RNA containing miRNA was the starting material in RT-PCR reaction which was performed in two steps.
For reproducible and accurate results in miRNA quantification, a normalization control was used in real-time PCR, where it was crucial to normalize the target miRNA amount by the use of an appropriate endogenous reference RNA. This method is called a relative quantification. Factors that may result in inaccurate quantification are corrected through this normalization. These factors include differences in the quantity of RNA input, the probability of degradation of RNA, the availability of inhibitors in the samples of RNAs, as well as the variation in sample handling. Furthermore, normalization makes it possible to compare different samples directly. In this experiment, RNU6-2 was used as a reference gene according to the manufacturer recommendations. (TaqMan small RNA assays protocol, 2011). The F and R primer for miRNA-1 and miRNA-145 are shown in table 1, and 2.
Results
The mean age of patients with MI was 58.83±11.04 years, the mean age of risk group was 53.04± 9.65 years and that of the control group was 60.00±9.87 years. There was no significant difference in mean age among study and control groups enrolled in the present study (P=0.058), which ensures age matching that is mandatory for such a study.
Regardingto gender distribution about 16 patients (66.7%) were male, and 8 patients(33.3%) were female, while the risk group included 10 (41.7%) male and 14 (58.3%) female and the control group included 16 (66.7%) male and 8 (33.3%) women. There was no significant difference in mean age among the three groups regarding the distribution of patients according to gender (P=0.121), which ensures gender match that is mandatory for such a study.
The median fold change and inter-quartile range of miRNA-1 in patients groups were 11.4 (6.95), and 3.6 (2.7) of the risk group respectively. and the value of fold change of control group was fixed at 1. Thus, the level of miRNA-1 fold change was significantly highest in the MI group followed by risk group and then by control group (P<0.05), as shown in figure (1).While, the median fold change and inter-quartile range of miRNA-145 were 0.055 (0.12), 0.375 (0.78) in patients and risk group respectively. and the value of fold change of control group was fixed at 1. The level of miRNA-145 fold change was significantly lowest in the MI group followed by risk group and then by control group (P<0.05), as shown in figure (2).
Receiver operating characteristic curves (ROC) carried out. The area under the curve (AUC), were 0.94, and 0.92 for miRNA-1, and miRNA-145 respectively as shown in figure (3) and figure (4), The cut off value was identified at the miRNA-1 level of >5.28 fold change with a sensitivity of 91.67 % and a specificity of 90.7%, as shown in table 2. Similarly, the cut off value was identified for the miRNA-145 level of ≤0.7 fold change with a sensitivity of 95.83 % and a specificity of 89.47%, as shown in table 4.
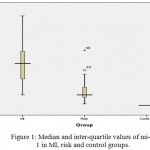 |
Figure 1: Median and inter-quartile values of mi-R 1 in MI, risk and control groups.
|
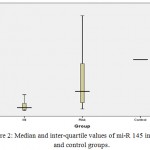 |
Figure 2: Median and inter-quartile values of mi-R 145 in MI, risk and control groups.
|
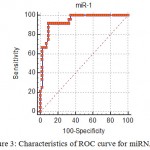 |
Figure 3: Characteristics of ROC curve for miRNA-1.
|
Table 3: ROC cutoff value of miRNA-1 that predict diagnosis of MI.
| Cut off value | >5.28 fold change |
| AUC (accuracy) | 0.940 (94%) |
| P | <0.001 |
| Sensitivity | 91.67 |
| Specificity | 90.70 |
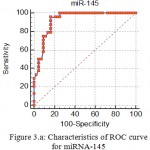 |
Figure 3.a: Characteristics of ROC curve for miRNA-145.
|
Table 4: ROC cutoff value of miRNA-1 that predict diagnosis of MI.
| Cut off value | ≤0.7 |
| AUC (accuracy) | 0.927 (92.7%) |
| P | <0.001 |
| Sensitivity | 95.83 |
| Specificity | 83.72 |
Discussion
Circulating levels of miRNA-1 were significantly increased in patients with AMI, this result came in agreement with Pan Z, Sun X et al. since they mention that miRNA-1 positively correlates with serum CK-MB level in AMI patient. miRNA-1, the level was increased with risk group because the most of this group are hypertensive patients as a result of Atherosclerosis which consider as an inflammatory process of endothelial blood vessels wall that stimulating apoptosis, the present study shows an increasing level of miRNA-1 in this group when compared with control group.11 Also, in addition to that, results are in consensus with published studies describing increased circulating levels of miRNA-1 and miRNA-133 in patients with MI.12-14 The source of elevated levels of miRNA-1 and miRNA-133 is likely in the damaged cardiac tissue.13 Moreover, present study mention that the level of miRNA-145 fold change was significantly lowest in the MI group, which came in agreement with the previous study that demonstrated a significantly lower miRNA-145 level in coronary artery diseases patients.14 Moreover, miRNA-145 fold change was significantly lower in the MI risk factor group (Hypertensive, hyperlipidemia, DM and others risk factor) compared with the control which confirms previously conducted study done by Cordes and colleagues since they demonstrated that miRNA-145 promotes differentiation and represses proliferation of smooth muscle cells (SMCs).15 Moreover, These results reiterate findings from the previous study that demonstrated significantly lower miRNA-145 levels in CAD patients.14
Rapid and correct diagnosis is crucial to treatment and prognosis of AMI. Up to date, cardiac troponins and creatine kinase-MB are the most commonly used biomarkers for AMI, but their clinical value is limited in many cases. Secreted by cardiac cells and accumulated in blood, miRNAs are expected to reflect cardiac injury in response to cardiovascular risk factors and various pathological conditions. Thus, the circulating cardiac-specific or -enriched miRNAs (such as miR- 1 and miR-145) may provide unique biomarkers for diagnostic of AMI.
In order to find a cutoff value of miRNA-1, and miRNA-145 that can predict a diagnosis of MI, More precisely, receiver operating characteristic curves (ROC) carried out. For the separation between non-MI and AMI patients An area (AUC) of 1 would represent a perfect test contrariwise to an area of 0.5 that would consist in a worthless test. The cut off value was identified for miRNA-1 with a sensitivity of 91.67 % and a specificity of 90.7%,. Similarly, the cut off value was identified for the miRNA-145 with a sensitivity of 95.83 % and a specificity of 89.47%.
Receiver operating characteristic analysis further indicated that these four miRNAs might be good biomarkers for AMI diagnosis. These results are partially supported by others report mention that that miR-1 level was significantly higher in plasma from AMI patients compared with non-AMI subjects.12 Therefore MiR-1 can act as AMI biomarker according to the above result and based on miRNA characterizes which are remain stable in serum and other body fluids.16 Circulating miRNAs are protected themselves from degradation by several mechanisms, including packing in membrane vesicles (such as microvesicles,17 exosomes, and apoptotic bodies,18 bound to transporter proteins, and inclusion in macromolecular complexes (such as high-density lipoproteins).19
Increasing evidence has shown that circulating miRNAs may function as diagnostic markers for coronary artery disease (CAD), diabetic heart diseases, myocardial infarction, hypertension, and heart failure.20-24
Current study confirm previously conducted study done by Long et al., since, they demonstrated that the ability of the miRNA-1-score to differentiate the AMI group from the control group according to ROC curve with an AUC of 0.92, 0.90, 0.94, 0.92, 0.96 and 0.90. they achieved a sensitivity of 93%, 93%, 94%, 93%, 93% and 90% and a specificity of 90%, 90%, 93%, 90%, 90% and 90%, respectively, measured at six time interval for the identification of AMI patients.25
Several studies have investigated the circulating miRNA-145 levels in patients with coronary artery disease.14,20 Fichtlscherer et al. reported that circulating miRNA-145 was down-regulated in patients with stable coronary artery disease compared with healthy controls.14
The miRNA-145 level in the total peripheral blood has been found to be elevated in patients with acute myocardial infarction and correlate with the infarction size estimated by troponin-T release.26 Since miRNA-145 is enriched in VSMCs, elevated miRNA-145 levels in AMI may reflect the vessel injury that occurs during atherosclerotic plaque rupture. Consistent with this idea, upregulation of miRNA-145 expression is found in atherosclerotic plaques in hypertensive patients.27
Thus, the present study demonstrates a unique signature of circulating miRNA for sensitive and specific diagnosis of MI that could be translated into non-invasive blood-based biomarker panels for patients. Considering that miRNA are active molecules, our study also suggests that miRNA-145, miRNA-1 may be instrumental in MI pathology and could represent new targets for therapeutic interventions.
Conclusion
The characteristic of ( miRNA-1,miR145) and their high sensitivity and Specificity in this study bushed forward to use them as alone biomarker or supported for Another biomarker in AMI diagnosis.
References
- Vella K., Vella R., Mifsud C. Sensitivity and specificity of Troponin I in the detection of acute myocardial infarction at the Emergency Department, Mater Dei Hospital. 2015.
- Chua J. H., Armugam A., Jeyaseelan K. Micro RNAs: biogenesis, function and applications. Current opinion in molecular therapeutics. 2009;11(2):189-99.
- Cortez M. A., Calin G. A. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert opinion on biological therapy. 2009;9(6):703-11.
- Kim Y. K. Extracellular microRNAs as biomarkers in human disease. Chonnam medical journal. 2015;51(2):51-7.
- Wang G. K., Zhu J. Q., Zhang J. T., Li Q., Li Y., He J., et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European heart journal. 2010;31(6):659-66.
- Cheng Y., Tan N., Yang J., Liu X., Cao X., He P., et al. A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clinical science. 2010;119(2):87-95.
- Fang Y. C., Yeh C. H. Role of microRNAs in vascular remodeling. Current molecular medicine. 2015;15(8):684-96.
- Weber M., Baker M. B., Patel R. S., Quyyumi A. A., Bao G., Searles C. D. MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiology research and practice. 2011;2011.
- Samples P. A Role for miR-145 in Pulmonary Arterial Hypertension. 2012.
- Wei Y., Nazari-Jahantigh M., Neth P., Weber C., Schober A. MicroRNA-126,-145 and-155. Arteriosclerosis, thrombosis and vascular biology. 2013;33(3):449-54.
- Creemers E. E., Tijsen A. J., Pinto Y. M. Circulating micro RNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circulation research. 2012;110(3):483-95.
- Ai J., Zhang R., Li Y., Pu J., Lu Y., Jiao J., et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochemical and biophysical research communications. 2010;391(1):73-7.
- Kuwabara Y., Ono K., Horie T., Nishi H., Nagao K., Kinoshita M., et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate the existence of myocardial damage. Circulation: Genomic and Precision Medicine. CIRCGENETICS. 2011;110:958975.
- Fichtlscherer S., De Rosa S., Fox H., Schwietz T., Fischer A., Liebetrau C., et al. Circulating microRNAs in patients with coronary artery diseasenovelty and significance. Circulation research. 2010;107(5):677-84.
- Cordes K. R., Sheehy N. T., White M. P., Berry E. C., Morton S. U., Muth A. N., et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705.
- Reid G., Kirschner M. B., van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Critical reviews in oncology/hematology. 2011;80(2):193-208.
- Hunter M. P., Ismail N., Zhang X., Aguda B. D., Lee E. J., Yu L., et al. Correction: Detection of microRNA Expression in Human Peripheral Blood Microvesicles. PloS one. 2010;5(3).
- Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81-ra.
- Vickers K. C., Palmisano B. T., Shoucri B. M., Shamburek R. D., Remaley A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature cell biology. 2011;13(4):423.
- D’Alessandra Y., Carena M. C., Spazzafumo L., Martinelli F., Bassetti B., Devanna P., et al. Diagnostic potential of plasmatic MicroRNA signatures in stable and unstable angina. PLoS One. 2013;8(11):e80345.
- Tijsen A. J., Pinto Y. M., Creemers E. E. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. American journal of physiology-heart and circulatory physiology. 2012;303(9):H1085-H95.
- Xu J., Zhao J., Evan G., Xiao C., Cheng Y., Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. Journal of molecular medicine. 2012;90(8):865-75.
- Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. BioMed research international. 2014;2014.
- Rawal S, Manning P, Katare R. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovascular diabetology. 2014;13(1):44.
- Long G., Wang F., Duan Q., Chen F., Yang S., Gong W., et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. International journal of biological sciences. 2012;8(6):811.
- Meder B., Keller A., Vogel B., Haas J., Sedaghat-Hamedani F., Kayvanpour E., et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic research in cardiology. 2011;106(1):13-23.
- Santovito D., Mandolini C., Marcantonio P., De Nardis V., Bucci M., Paganelli C., et al. Overexpression of microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert opinion on therapeutic targets. 2013;17(3):217-23.

This work is licensed under a Creative Commons Attribution 4.0 International License.





