How to Cite | Publication History | PlumX Article Matrix
Heavy Metal Analysis of Locally Available Anticancer Medicinal Plants
Khushnood ur Rehman1, Muhammad Hamayun*2 , Sumera Afzal Khan3, Amjad Iqbal4 and Anwar Hussain2
, Sumera Afzal Khan3, Amjad Iqbal4 and Anwar Hussain2
1Department of Botany, Islamia College Peshawar, Pakistan.
2Department of Botany, Abdul Wali Khan University Mardan, Pakistan.
3Center of Biotechnology and Microbiology, University of Peshawar, Pakistan.
4Department of Agriculture, Abdul Wali Khan University Mardan, Pakistan.
Corresponding Author E-mail: hamayun@awkum.edu,pk
DOI : http://dx.doi.org/10.13005/bbra/2727
ABSTRACT: Plant species are used in different forms either dry or fresh to extract the active ingredients that can be used for medicinal purposes. These active ingredients may or may not contain non-essential elements. One of the main non-essential elements includes heavy metals. The consumption of medicinal plants having larger amounts of heavy metals can affect the health of human beings. Currently, we have also assessed eight locally available medicinal plant species for endogenous heavy metals (i.e. cadmium, arsenic, mercury, lead and zinc). The results revealed that Saxifraga flagellaris, Moringa oleifera, and Fegonia cretica had no lead, whereas Melia azedarach had the highest concentration of lead. Similarly, Saxifraga flagellaris had lower concentration of arsenic, while Albizia lebbeck had zero and Melia azedarach had the highest accumulation of arsenic. Cadmium was absent in Saxifraga flagellaris, Withania coagulans, and Valeriana jatamansi. Moringa oleifera had lower and Melia azedarach had the greatest amounts of cadmium. Mercury concentration has been high in Melia azedarach (2.39±0.18 µg/g), followed by Hedera helix (0.26±0.02 µg/g), Saxifraga flagellaris (0.051±0.031 µg/g) and Albizia lebbeck (0.041±0.01 µg/g). The species, Fegonia cretica, Valeriana jatamansi, Withania coagulans, Moringa oleifera had no mercury. The highest zinc concentration was observed in Melia azedarach and the lowest concentration was found in Saxifraga flagellaris.
KEYWORDS: Atomic Absorption Spectroscopy; Heavy Metals Analysis; Medicinal Plant Species
Download this article as:| Copy the following to cite this article: Ur-Rehman K, Hamayun M, Khan S. A, Iqbal A, Hussain A. Heavy Metal Analysis of Locally Available Anticancer Medicinal Plants. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Ur-Rehman K, Hamayun M, Khan S. A, Iqbal A, Hussain A. Heavy Metal Analysis of Locally Available Anticancer Medicinal Plants. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2U0uB5x |
Introduction
Those elements with relative densities of which are higher than water are known as heavy metals (Fergusson, 1990). The elements having atomic weight equal to five or more water molecules are heavy metals (Tchounwou et al., 2012). The elements, which are present in trace amount in nature, are known as heavy metals, they occur in 10 ppm (Kabata-Pendias, 2010). There are biological factors which effects the bioavailability of heavy metals, include physiological adaptations and species characteristics and trophic interactions (Verkleji, 1993). On one hand heavy metals negatively affect cell organelles and on another effect processes of cell repair and detoxification (Wang and Shi, 2001). The effects of heavy metals as carcinogen need more elucidation (Tchounwou et al., 2012). Some heavy metals, including zinc (Zn) and copper (Cu) are needed by cell enzymes, but many of them are carcinogens and cause cancer and other ailments (Fergusson, 1990; Hambidge and Krebs, 2007). Plant generates reactive oxygen species (ROS) when they are exposed to heavy metals (Shahid et al., 2014). Plants have evolved detoxification mechanism which reduce the effects of heavy metals (Yadav, 2010). Plants phytochemicals if consumed can assist in the detoxification process related to antioxidants (Lobo et al., 2010). Life is impossible without some metallic ions as these are important parts of enzymes which carry reactions for life. In literature terms, like “trace metals”, “micro elements”, “trace inorganics” and heavy metals are used as synonyms. The heavy metals are added to the environment from agricultural sources, atmospheric sources, domestic effluent, industrial sources and natural sources (Nagajyoti et al., 2010). The concentration of heavy metals in environment is from natural origin as well as anthropogenic activities. Therefore, the presence of heavy metals in the environment of certain countries is high or low depending upon the activities. For example, Cd and Zn concentrations are high in China (Herawati et al., 2000), Pb, Ni, Zn are found in higher concentration in Greece (Christophoridis et al., 2009). The monitoring of twenty one sites for two years from tributary of Rawal lake Pakistan showed higher accumulation of Ni, Mn and Pb during post monsoon seasons. However, during the pre-monsoon season, the concentration of Zn was high and the concentration of Li was low (Zahra et al., 2014). The levels of heavy metals are low in residential area than high traffic area. Similarly, lower concentrations of heavy metals has been observed in the areas occupied by the dense population of plants have low concentrations of heavy metals (Chibuike and Obiora, 2014). Moreover, some plants have the higher capacity to take more heavy metals as compared to the other species (Chibuike and Obiora, 2014). The accumulation of heavy metal in same species also depends on the environmental conditions of the area. Our work was focused on exploring new potent plant species with low heavy metal contaminations, yet effective against cancer.
Materials and Methods
Medicinal plants were collected in the remote area of Peshawar. The samples were washed to remove dirt, air dried and ground to powder by a grinding machine. Wet digestion method was adopted for the preparation of samples for heavy metals analysis by atomic absorption spectrophotometer (Meena et al., 2010). Approximately, 0.5 g to 1.0 g of the each plants sample was weighed and digested with 15 ml of 20 % sulphuric acid solution in test tube. The test tube containing sample was left for 24 h, so that the sample can be fully dissolved in the solution. For efficient digestion, each test tube was heated for 10 to 15 minute. The solution of each test tube was then filtered through Whatman filter paper. Distilled water was finally added to each filtrate to make the final volume of 30 ml. Flame atomic absorption spectrophotometer was used to measure the concentration of lead, mercury, arsenic, cadmium and zinc in each extract of plant sample.
Results
Lead Concentration
The Lead concentration was different in selected species but almost all were in nontoxic level. In Saxifraga flagellaris, Moringa oleifera, and Fegonia cretica, no lead was detected, while Withania coagulans and Valeriana jatamansi had lowest concentrations of lead, i.e. 0.044±0.026 µg/g and 0.048±0.022 µg/g, respectively. Albizia lebbeck (0.204±0.11 µg/g) and Hedera helix (1.25±0.07 µg/g) had moderate concentrations of lead and the highest concentration was found in Melia azedarach 3.72±0.25 µg/g (Fig. 1).
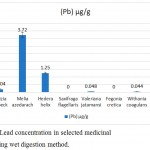 |
Figure 1: Lead concentration in selected medicinal plants, using wet digestion method.
|
Arsenic Concentration
Arsenic concentration in Saxifraga flagellaris was 0.195±0.135 µg/g. The medicinal plant species, Moringa oleifera had 0.208±0.151 µg/g, Fegonia cretica had 0.375±0.171 µg/g, Withania coagulans had 0.542±0.17 µg/g, Valeriana jatamansi 0.552±0.14 µg/g and Hedera helix 1.30±0.06 µg/g arsenic. Moreover, Albizia lebbeck had no detectable arsenic, whereas highest accumulation of arsenic was found in Melia azedarach, i.e. 3.74±0.24 µg/g (Fig. 2).
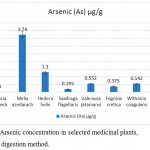 |
Figure 2: Arsenic concentration in selected medicinal plants, using wet digestion method.
|
Cadmium concentration
The availability of cadmium was nil in Saxifraga flagellaris, Withania coagulans and Valeriana jatamansi. The concentration of cadmium in Moringa oleifera was 0.060±0.021 µg/g, Fegonia cretica was 0.041±0.001 µg/g, Albizia lebbeck was 0.021±0.002 µg/g and Hedera helix was 0.41±0.02 µg/g. Among the tested medicinal plants, Melia azedarach (1.84±0.13 µg/g) had the highest amounts of heavy metal cadmium (Fig. 3).
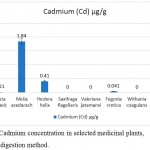 |
Figure 3: Cadmium concentration in selected medicinal plants, using wet digestion method.
|
Mercury Concentration
The highest mercury concentration was confirmed in Melia azedarach (2.39±0.18 µg/g), followed by Hedera helix (0.26±0.02 µg/g), Saxifraga flagellaris (0.051±0.031 µg/g) and Albizia lebbeck (0.041±0.01 µg/g). The tested medicinal plants, Fegonia cretica, Valeriana jatamansi, Withania coagulans and Moringa oleifera were found to have no mercury (Fig. 4).
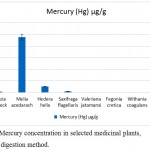 |
Figure 4: Mercury concentration in selected medicinal plants, using wet digestion method.
|
Zinc concentration
The medicinal plant, Melia azedarach (1.76±0.15 µg/g) had the highest concentration of zinc, followed by Hedera helix (0.49±0.08 µg/g), Fegonia cretica (0.395±0.15 µg/g), Moringa oleifera (0.360±0.18 µg/g), Withania coagulans (0.287±0.16 µg/g), Valeriana jatamansi (0.203±0.15 µg/g) and Albizia lebbeck (0.13±0.002 µg/g). The least concentration of zinc was found in Saxifraga flagellaris (0.108±0.007 µg/g) (Fig. 5).
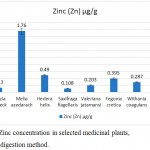 |
Figure 5: Zinc concentration in selected medicinal plants, using wet digestion method.
|
Discussion
The medicines, which are acquired from plants, are considered less toxic than Allopathic drugs, But if these plants contain heavy metals then in spite of it that these shows biological activities are rather unfit to be used as medicines. Metal noxiousness has extraordinary influence and significance concerning plants and ultimately the entire ecosystem is affected with it. Apart from the accumulation, these toxic heavy metals retard growth, lower productivity of ecosystem and alter metabolism of the whole ecosystem in large. Which effect the physiological and biochemical characters of these plants and the consumers of ecosystem as well (Nagajyoti et al., 2010). There are plants, which are “hyper accumulator” regarding their ability to adopt to metalliferous soils. There are three factors, which differentiate the “hyper accumulator” form other non- hyper accumulators plants one factor is that these plants have elevated tendency for heavy metal uptake, second that have faster translocation and third these plants have the ability to detoxify heavy metals (Rascio and Navari-Izzo, 2011). The damage by heavy metal to human beings in special and other living being in general is increasing day by day. Among the ninety two (92) elements thirty (30) elements are toxic to human health, which are Al, As, B, Be, Co, Cr, Cu, Li, Mn, Ni, Se, Ti, V, Sb, Te, Cs, Au, Hg, Pb, Sr, Mo, Pd, Bi, Ba, W, Pt, Sn, Ag and Cd. The metallic elements which contain higher atomic weight than 40.04 (the atomic mass of Ca) are known as heavy metals (Morais et al., 2012; Yu and Tsunoda, 2004; Otte, 2006). Among the metals arsenic (As), Lead (Pb), mercury (Hg), and cadmium (Cd), are present in every environment. These elements have no benefit to human health (Vieira et al., 2011). Even at low concentrations, they have very adverse effects on the human health and cause cancer (Carlin et al., 2015). Lead is brought to surface of earth by humans and is more toxic. In oceans the concentration of lead is 0.01-0.02 μg/L, but near surface the ocean water contain as much as ca. 0.3 μg/L (Castro-González and Méndez-Armenta, 2008). Plants with lead accumulated may triggers food chain with toxic effects. These toxic effects are ultimately faced by human being as man is the part of ecosystem. Humans when inhale lead, 50% of that becomes the part of the body. Especially bones and teeth contain more than 90% of lead (Yu and Tsunoda, 2004). The effect of lead on children growth is very severe and it has very bad effect on their mental health (Castro-González and Méndez-Armenta, 2008).
Children are particularly sensitive to this metal because of their more rapid growth rate and metabolism, with critical effects in the developing nervous system (ATSDR, 2007; Castro-González & Méndez-Armenta, 2008). The Joint FAO/ World Health Organization Expert Committee on Food Additives (JECFA) established a provisional tolerable weekly intake (PTWI) for lead as 0.025 mg/kg body weight (bw) (JECFA, 2004). The WHO provisional guideline of 0.01 mg/L has been adopted as the standard for drinking water (WHO, 2004).
Cadmium use is not very old to humans. The advancement in technology increased the use of Cadmium. It is now seriously considered as pollutant. It is present naturally in water, Air and soil naturally as well. The era of industrialization led to increase its concentration in environment than ever before (Fytianos et al., 2001). Tobacco contain highest amount of cadmium and its absorption via lungs is greater than gastrointestinal tract. It is present in the form of inorganic salts, and the organic compounds, which contain Cadmium, are very unsteady. Plants easily absorb it in the form of ions, and these ions are present all parts like roots, seeds and leaves of the plants. Some researcher found that the Kernel of wheat and Rice contain more cadmium than other parts (Figueroa et al., 2008). The safe mentioned slandered by FAO/WHO has the PTWI as 0.007 mg/kg for cadmium (JECFA, 2004). The EPA maximum pollutant level for cadmium in drinking water is 0.005 mg/L while the WHO accepted the conditional recommendation of 0.003 mg/L (WHO, 2004). The most toxic heavy metal in environment is Mercury. It is added into environment by man from farming industry (fungicides, seed stabilizers), by drugs industry, paper industry, and batteries (Zhang and Wong, 2007). Exposure to it can damage central nervous system and other health hazards are brought by it to humans. Majority of food contain up to 50 μg/kg (Jaishankar et al., 2014). The revised JECFA PTWI scale for toxic level of mercury is 3.3 mg/kg b.w./week PTWI of 1.6 mg/kg body weight (JECFA, 2004). As the element is extreme toxic to human health the current standard for drinkable fresh water by EPA and WHO are 0.002 mg/L and 0.001 mg/L, correspondingly (JECFA, 2004). Arsenic is rarely found in usual environment, but present as arsenides in the ore of sulphur.it is more abundant in aquatic environment in oxidation states than land, but may be abundant in those areas where arsenical insecticides are frequently used Inorganic arsenic is cancer-causing and specially cause cancer in lungs and skin (Smith et al., 2003). The JECFA established a PTWI for inorganic arsenic as 0.015 mg/kg body weight and arsenic consumptions is 0.05 mg/kg body weight/day (JECFA, 2004). Zinc is vital element for fitness and growth specially plants but in excess amount may cause severe health problems and toxicity. If the quantity of it exceeds than 225 µg/g then it is toxic. Though it is important co-factor for many nucleic acid synthesizing enzymes, but still its higher concentration may cause pyrexia, nausea and laziness. The taken amount of zinc should not exceed than 10,000 and 20,000 µg/day (Bhutta et al., 1999).
Conclusion
Medicinal plants can provide protection against life threatening ailments, like cancer. The South East Asia is blessed with potent plant species that are used as medicine and food supplement. Though, medicinal plants have benefits, but consumption of such plants in higher amounts might be harmful due to the presence of heavy metals. The heavy metals are useful when used in optimum quantities, but get toxic at higher amounts. From the results of our study, we conclude that the presence of heavy metals was in very low quantities and these plant species can be used freely to treat life threatening cancer.
Conflict of Interest
There is no conflict of interest.
Acknowledgements
The authors are thankful to the lab members of Abdul WaIi Khan Univrsity Mardan for technical support.
Funding Source
This research was supported by the Plant-Microbes Interaction lab, Abdul Wali Khan University Mardan.
References
- Fergusson J. E. Heavy elements: chemistry, environmental impact and health effects. Pergamon, Oxford. 1990;614.
- Tchounwou P. B., Yedjou C. G., Patlolla A. K and Sutton D. J. Heavy metal toxicity and the environment. Springer. 2012;133-164.
CrossRef - Kabata-Pendias A. Trace elements in soils and plants. CRC press, Boca Raton, FL. 2010;507.
CrossRef - Verkleji J. The effects of heavy metals stress on higher plants and their use as bio monitors. 1993. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 1993;8:199-216.
- Wang S and Shi X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001;222:3-9.
CrossRef - Hambidge K. M and Krebs N. F. Zinc deficiency: a special challenge. J. Nutri. 2007;137:1101-1105.
CrossRef - Shahid M., Pourrut B., Dumat C., Nadeem M., Aslam M and Pinelli E. Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Springer. 2014.
- Yadav S. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010;76:167-179.
CrossRef - Lobo V., Patil A., Phatak A and Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118.
CrossRef - Nagajyoti P., Lee K and Sreekanth T. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010;8: 199-216.
CrossRef - Herawati N., Suzuki S., Hayashi K., Rivai I and Koyama H. Cadmium, copper and zinc levels in rice and soil of Japan, Indonesia and China by soil type. Bull. Environ. Contam. Toxicol. 2000;64:33-39.
CrossRef - Christophoridis C., Dedepsidis D and Fytianos K. Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. J. Hazard. Mater. 2009;168:1082-1091.
CrossRef - Zahra A., Hashmi M. Z., Malik R. N and Ahmed Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014;470:925-933.
CrossRef - Chibuike G. U and Obiora S. C. Heavy metal polluted soils: effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014;2014:
- Meena A. K., Bansal P., Kumar S., Rao M and Garg V. Estimation of heavy metals in commonly used medicinal plants: a market basket survey. Environ. Monit. Assess. 2010;170: 657-660.
- Rascio N and Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 2011;180:169-181.
CrossRef - Morais S. e., Costa F. G and de Lourdes Pereira M. Heavy metals and human health. Intech Open, Rijeka, Croatia. 2012;227-246.
- Yu M. H and Tsunoda H. Environmental toxicology: biological and health effects of pollutants. CRC Press, Boca Raton. 2004;364.
CrossRef - Otte M. L. Environmental Toxicology–Biological and Health Effects of Pollutants by Ming‐Ho Yu. Geographical J. 2006;172: 180-180.
CrossRef - Vieira C., Morais S., Ramos S., Delerue-Matos C and Oliveira M. Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: intra-and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 2011;49:923-932.
CrossRef - Carlin D. J., Naujokas M. F., Bradham K. D., Cowden J., Heacock M., Henry H. F., Lee J. S., Thomas D. J., Thompson C and Tokar E. J. Arsenic and environmental health: state of the science and future research opportunities. Environ. Health Perspect. 2015;124:890-899.
CrossRef - Castro-González M and Méndez-Armenta M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008;26:263-271.
CrossRef - JECFA. Sixty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization & Food and Agriculture Organization of the United Nations, Rome, Italy. 2004;176.
- WHO. The World health report: 2004: changing history. World Health Organization, Geneva, Switzerland. 2004;169.
- Fytianos K., Katsianis G., Triantafyllou P and Zachariadis G. Accumulation of heavy metals in vegetables grown in an industrial area in relation to soil. Bull. Environ. Contam. Toxicol. 2001;67:0423-0430.
- Figueroa J. A. L., Wrobel K., Afton S., Caruso J. A., Corona J. F. G and Wrobel K. Effect of some heavy metals and soil humic substances on the phytochelatin production in wild plants from silver mine areas of Guanajuato, Mexico. Chemosphere. 2008;70:2084-2091.
CrossRef - Zhang L and Wong M. Environmental mercury contamination in China: sources and impacts. Environ. Int. 2007;33:108-121.
CrossRef - Jaishankar M., Tseten T., Anbalagan N., Mathew B. B and Beeregowda K. N. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary toxicology. 2014;7:60-72.
CrossRef - Smith R. A., Saslow D., Sawyer K. A., Burke W., Costanza M. E., Evans W. P., Foster R. S., Hendrick E., Eyre H. J and Sener S. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J. Clin. 2003;53:141-169.
CrossRef - Bhutta Z., Black R. E., Brown K., Gardner J. M., Gore S., Hidayat A., Khatun F., Martorell R., Ninh N and Penny M. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. The J. Pediatr. 1999;135:689-697.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





