How to Cite | Publication History | PlumX Article Matrix
Histochemical and Shoot Induction Studies of Basella Rubra L. (Basellaceae)
M. N. Abubacker*, G. Ganapathy, L. Maria Sebastin and K. Suresh Mondal
Department of Biotechnology, National College, Tiruchirapalli, Tamilnadu 620001, India.
Corresponding Author E-mail: abubacker_nct@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2736
ABSTRACT: The optimum concentrations of the plant hormones for in vitro regeneration and subsequent effect of auxins on rooting (in vitro and ex vitro) of shoots of Basella rubra L. have been investigated in present study. Nodal shoot segments were used as explants to initiate the cultures. Histochemical studies of in vitro and in vivo plants revealed alkaloids, polyphenols and terpenoids concentrations were higher in in vivo plants. Ascorbic acid and tannin concentration have shown no difference in the content in in vitro and in vivo plants. MS medium supplemented with IBA 2.0 mg/l + NAA 0.5 mg/l + GA3 0.5 mg/l induced shoot regeneration MS+IBA 3.0 mg/l induced roots and the plant regeneration was achieved in MS + IBA 0.5 mg/l + GA3 1.5 mg/l + IAA 1.0 mg/l. SEM – EDX elemental analysis of in vitro plants have shown absorption of sodium, magnesium, silica, phosphorus, sulphur, chlorine and potassium from the medium. The in vivo plants have shown absorption of magnesium, silica, chlorine, potassium, calcium and iron.
KEYWORDS: Basella Rubra; SEM-EDX; Shoot Induction
Download this article as:| Copy the following to cite this article: Abubacker M. N, Ganapathy G, Sebastin L. M, Mondal K. S. Histochemical and Shoot Induction Studies of Basella Rubra L. (Basellaceae). Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Abubacker M. N, Ganapathy G, Sebastin L. M, Mondal K. S. Histochemical and Shoot Induction Studies of Basella Rubra L. (Basellaceae). Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2Is2lBC |
Introduction
Basella rubra L. (family Basellaceae) commonly known as Indian Spinach, is mostly cultivated as leafy vegetable. It is extremely heat tolerant plant, native of tropical Asia and originated from India (Bamidele et al. 2010). It is a succulent-branched climber and the tender shoots are used as a cool season vegetable.
The fruits are the potential source of natural colourant. Anthocyanins are found responsible for different colours present in the body parts of B.rubra (Maisuthisakul and Ritthiruangdej 2008). The natural maroon colour obtained from the ripened fruit’s saps of B. rubra used in dyeing industry as natural dye in India (Ozela et al. 2007).
The dye isolated from the fruit pulp of this plant is used as an alternative to crystal violet or safranin for the staining of microbes (Mundo et al. 1995). The purple colored extract from the fruit of Basella is also used in coloring of food items.
Basella contains some unique compounds like, basella saponins, flavonoid kaempherol, ß-carotene and betacyanin betalain. (Yang et al. 2008). The leaf extract is reported to possess vital vitamins A, C, E, K, folic acid, riboflavin, niacin, thiamine and minerals like calcium, magnesium and iron. The mucilage from B.rubra has strong suspending ability along with high viscosity, and used as a good thickening agent in pharmaceutical and cosmetic industries. The gel obtained from the mucilage used as medicines for skin problems due to its antioxidant activity (Toshiyuki et al. 2001). Histochemical staining method was performed to locate phytochemicals such as alkaloid, ascorbic acid, polyphenols, tannins and terpenoids synthesized by B.rubra plant.
Basella rubra is now cultivated for its industrial purposes and naturally propagated by the stem cuttings (Cyunel 1989), this method causes the carryover of disease causing pathogens from one generation to the next (Sankar et al. 2011). The propagation through seeds has some limitations due some special requirements in germination of seeds and proper flowering in this plant (Larkcom 1991). Plant tissue culture methods can be used for rapid multiplication of improved varieties and to produce disease free and quality planting materials (Kartha and Gamborg 1975). Some reports are available on the tissue culture and anthocyanin production using B.rubra cells (Cyunel 1989; Guo and Bin 2001). However, efficient in vitro propagation protocol for this plant species is largely deficit in the literature. The histochemical and shoot induction studies by using plant growth regulators were tested and conditions were optimized for efficient shoots, roots and plant regeneration in B.rubra during present investigation of in vitro and in vivo plants and SEM-EDX elemental analysis of roots of in vitro and in vivo plants.
Material and Methods
Collection of Plant Material and Preparation of Explants
Basella rubra plants were collected from the green house, National college, Tiruchirapalli, Tamilnadu, India. Fresh shoot sprouts were used as explants. The stems were cut into 3–4 cm long segments with 1–2 nodes. The explants were first treated with 0.1 % (w/v) Bavistin (a systemic fungicide) for 5 min, and surface sterilized with 0.1 % HgCl2 (w/v) for 3 min with alternate shaking followed by washing with autoclaved distilled water for 3 times under aseptic conditions in a laminar air flow cabinet.
Establishment of culture
The sterilized nodal segment were inoculated on agar gelled (0.8 % agar) MS medium (Murashige and Skoog 1962) supplemented with IAA (Indole 3 acetic acid) ranging from 0.5 to 3.0 mg/l, NAA (α-Naphthalene acetic acid) ranging from 0.5 to 2.0 mg/l and GA3 (Gibberellic acid) ranging from 0.5 to 2.0 mg/l for shoot regeneration. MS + IBA (Indole -3- butyric acid) ranging from 0.5 to 3.0 mg/l for root induction, MS + IBA ranging from 0.5 to 3.0 mg/l and GA3 ranging from 0.5 to 3.0 + IAA 0.5 to 3.0 mg/l for plant regeneration. Explants with single node were inoculated vertically in each culture tubes for bud breaking from the nodal meristem. Cultures were incubated at 1500 lux light with 12:12 hr light: dark photo period at 25 ± 2°C.
Multiplication of shoots in cultures
The shoots were multiplied by two methods (i) the original explants were repetitively transferred to fresh medium for 2–3 passages after harvesting in vitro raised shoots, and (ii) the in vitro produced shoots were cut into 2cm long segments (each with one node) and subcultured on fresh MS medium. The culture medium used for shoot multiplication was incorporated with various concentrations and combinations of IAA + IBA + NAA + GA3. The multiple shoots were regularly subcultured on fresh medium after an interval of 4–5 weeks.
Effect of auxins on rooting of shoots (in vitro)
2cm shoots were harvested individually and transferred to MS medium containing different concentrations of IBA + BAP. The cultures were incubated (1–2 days) under diffused light (800 lux) conditions and after that under the normal conditions (at 1500 lux).
Plantlet regeneration (in vitro)
The rooted shoots were transferred to MS + IBA + GA3 + IAA at various concentrations, incubated at 1500 lux light and plantlets were established in in vitro and were selected for hardening process after 45 days of culture.
Hardening of regenerated plantlets
The regenerated (in vitro) plantlets were rinsed with water, kept on a moist filter paper and transplanted immediately to the containers containing autoclaved soilrite. These plantlets were moistened with solution of one-fourth MS basal salt and shifted to greenhouse for the hardening of plantlets. After 30–35 days, the plantlets were transferred to nursery polybags and pots having perforation at in the bottom, containing potting mixture of sand, soilrite, garden soil and organic manure (1:1:1:1).
Comparative Histochemical studies of stem anatomy
Histochemical studies were conducted to compare the in vivo and in vitro plant stem anatomy for the phytochemicals like alkaloids, ascorbic acid, phenolic compounds, tannin and terpenoids. The thin hand sections were stained in different staining agents as per the procedure (Shanmugam et.al 2010). Photomicrographs were taken in the digital camera mounted on the Microscope Olympus, Magcam DC 10 with magnification 40X.
Results and Discussion
The Table-1 reveals the comparative histochemical studies of B.rubra stem anatomy of in vivo and in vitro plants.
The histochemical assay revealed some difference in the phytochemical constitutions between in vivo and in vitro plants. The presence of alkaloids is higher in in vivo plants, especially higher in epidermis hypodermis external cortex and vascular region. Ascorbic acid has shown no difference between in vivo and in vitro histochemistry. Polyphenols are higher in in vivo plant and located in external cortex and vascular region. The concentrations of tannin are identical in in vivo and in vitro plants. Terpenoids are present in higher concentration in in vivo plants especially in hypodermis and vascular regions (Fig-1 and 2). These results are comparable with the previous work of Toshiyuki et.al (2001), Ozela et.al (2007), Adenegan and Akinnubi (2015), Kumar et.al (2015a) and Kumar et.al (2015b).
The present work is aimed at the in vitro shoot induction studies of B.rubra. It has been proved that the surface sterilization of explants is very essential for establishment, pathogen free as well as optimum induction of callus and organogenesis in vitro. In the present study mercuric chloride was used to sterilize the explants. The appropriate concentration and time for surface sterilization is 1.0% mercuric chloride for 3 minutes for the stem.
Table 1: Phytochemical analysis of in vivo and in vitro B.rubra.L plant stem (anatomy) by histochemical studies.
| Histochemical parameters | In vivo analysis | In vitro analysis |
| TBO (control) | ||
| Epidermis | Light blue | Light blue |
| Hypodermis | Greenish purple | Greenish purple |
| External cortex | Blue | Blue |
| Vascular region | Purplish blue | Purplish blue |
| Pith | Light blue | Light blue |
| Alkaloid | ||
| Epidermis | Orange | Orange |
| Hypodermis | Brown | Red |
| External cortex | Orange | Red |
| Vascular region | Grey – Brown | Brown |
| Pith | Black | Yellow |
| Result | Golden yellow /Brown | Golden yellow /Brown |
| Ascorbic acid | ||
| Epidermis | Light blue | Black |
| Hypodermis | Brown | Pinkish red |
| External cortex | Blue | Golden yellow |
| Vascular region | Dark blue | Golden yellow |
| Pith | Grey– Brown | Grey / Violet |
| Result | Black silver / Red brown | Black silver / Red brown |
| Polyphenols | ||
| Epidermis | Grey | Yellow |
| Hypodermis | Green | Green |
| External cortex | Reddish brown | Green |
| Vascular region | Grey brown | Green |
| Pith | Whitish grey | Grey |
| Result | Cherry red | Cherry red |
| Tannin | ||
| Epidermis | Pink | Pink |
| Hypodermis | Green | Green |
| External cortex | Grey | Grey |
| Vascular region | Grayish – Green | Grayish – Green |
| Pith | Grey | Grey |
| Result | Blue or Blue green | Blue or Blue green |
| Terpenoids | ||
| Epidermis | Pink | Pink |
| Hypodermis | Brown – Green | Orange |
| External cortex | Pink | Pink |
| Vascular region | Grey – Green | Orange – Green |
| Pith | Grey | Green – Grey |
| Result | Orange | Orange |
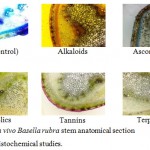 |
Figure 1: In vivo Basella rubra stem anatomical section used for Histochemical studies.
|
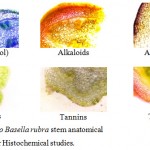 |
Figure 2: In vitro Basella rubra stem anatomical section used for Histochemical studies.
|
Shoot Regeneration
In the present study following media concentration and combination were used for root and shoot induction. The following concentration and combination of hormones are used:
MS + IBA 1.0 mg/l + NAA 0.5 mg/l + IAA 1.0 mg/l
MS + IBA 2.0 mg/l + NAA 0.5 mg/l + GA3 0.5 mg/l
MS + IBA 3.0 mg/l + IAA 1.0 mg/l
Shoot induction are initiated in 10 days the other hormones like IAA, NAA have shown but much organogenesis result after inoculation on MS medium supplemented with different concentration and combination of above mentioned hormones and shoot regeneration in 20 days further growth in 30, 40 and 60 days. Among the various combinations, 100% shoot regeneration in concentration of hormones such as MS + BA (1.0 mg/l) produced multiple shoot, in addition to this Kin (0.5, 1.0 mg/l) in the presence of BA induced more number of multiple growths in Basella rubra. The present study reveals that MS+BAP (1.0 mg/l), NAA (0.5 mg/l), IAA (1.0 mg/l) as well as MS+BAP (1.0 mg/l), NAA (0.5mg/l) and GA3 (0.5 mg/l) produced shoot regeneration in Basella rubra. The number of shoots produced in this study was on par with earlier reports by Guo and Bin (2001), Pumchaosuan and Wongroung (2009) with reference to B.alba and B.rubra.
Root Regeneration
In the present study following media concentration and combination were used for root induction:
MS + IBA 1.0 mg/l, MS + BAP 1.0 mg/l
MS + IBA 3.0 mg/l
Root induction are formed in 15 days after inoculation on MS medium supplemented with different concentration and combination of above mentioned hormones and further growth occurred in 30, 40, 60 days (Fig-4 a to d). Among the different combination MS + IBA 1.0 mg/l induced root induction in B.rubra. The worked carried out by Guo and Bin (2001), Pumchaosuan and Wongroung (2009), Shekhawat and Manokari 2016 support the present findings.
Plantlet Regeneration
In the present study following media concentration and combination were used for plant regeneration induction:
MS + IBA 0.5 mg/l + GA3 0.5 mg/l + IAA 0.5 mg/l,
MS + IBA 0.5 mg/l + GA3 1.0 mg/l + IAA 1.0 mg/l and
MS + IBA 0.5 mg/l + GA3 1.5 mg/l + IAA 1.0 mg/l.
Plant regeneration is maximum in the concentration MS + IBA 0.5mg/l + GA3 1.5 mg/l + IAA 1.0 mg/l (Fig-3) According to Hong et.al (2003) propagation of B.rubra by stem cutting into MS + BAP 1.0 mg/l + NAA 0.5 mg/l resulted good response the present work reveals that MS + IBA 1.0 mg/l combination has better response to plant regeneration in B.rubra These results are comparable with the work of Guo and Bin (2001), Tanikan Pumchaosuan and Sasitorn Wongroung (2009) and Shekhawat and Manokari (2016).
Protocol for Basella Rubra Stem Explants In Vitro Propagation
The selected stem explants were brought to the laboratory and kept in refrigerator for two days.
Surface sterilization with 75% alcohol for 50 seconds and 0.1% HgCl2 for 3 minutes.
Washed thrice in double distilled water.
Shoot induction with MS + IBA 1.0 mg/l + NAA 0.5 mg/l + IAA 1.0 mg/l.
(Or)
MS + BAP 1.0 mg/l + NAA 0.5 mg/l + GA3 0.5 mg/l.
Root induction with MS IBA 1.0 mg/l + IBA 2.0 mg/l.
Plant regeneration with MS + IBA 0.5 mg/l + GA3 1.5 mg/l +IAA 1.0mg/l
Incubated in light 1500 Lux at 25°C ± 2°C.
 |
Figure 3: Basella rubra stem explants in vitro propagation.
|
Nutrient Uptake Studies by In Vivo and In Vitro Same Plants by SEM- EDX Analysis
The SEM-EDX elemental analysis of in vivo from B.rubra have shown the elements carbon, oxygen, sodium, magnesium, silica, phosphorus, sulphur, chlorine and potassium. The concentration of potassium was 5.82%. The in vitro plants have shown carbon, oxygen, magnesium, silica, chlorine, potassium, calcium and iron (Table – 2). The result has shown that there is a significance difference in the absorption of nutrients. The in vivo plant absorbs sodium, phosphorus and sulphur. Whereas the in vitro plants are capable of absorb calcium and iron from the medium (Fig-4). The present study of nutrient uptake by SEM-EDX analyses is a tool to identify selective absorption of minerals by in vitro and in vivo plants as reported by Abubacker and Sathya (2017).
Table 2
| S.No | Element | Weight % | Atomic % |
| 1 | C K | 52.16 | 61.64 |
| 2 | O K | 39.48 | 35.02 |
| 3 | Na K | 0.39 | 0.24 |
| 4 | Mg K | 0.25 | 0.15 |
| 5 | Si K | 0.58 | 0.29 |
| 6 | P K | 0.25 | 0.11 |
| 7 | S K | 0.17 | 0.08 |
| 8 | Cl K | 0.90 | 0.36 |
| 9 | K K | 5.82 | 2.11 |
| 10 | Totals | 100.00 |
| S.No | Element | Weight % | Atomic % |
| 1 | C K | 64.23 | 71.01 |
| 2 | O K | 34.22 | 28.40 |
| 3 | Mg K | 0.18 | 0.10 |
| 4 | Si K | 0.26 | 0.12 |
| 5 | Cl K | 0.14 | 0.05 |
| 6 | K K | 0.59 | 0.20 |
| 7 | Ca K | 0.24 | 0.08 |
| 8 | Fe K | 0.14 | 0.03 |
| 9 | Totals | 100.00 |
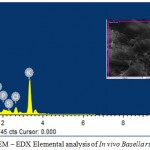 |
Figure 4: SEM – EDX Elemental analysis of In vitro Basella rubra L.
|
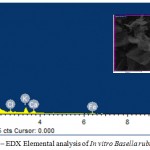 |
Figure 5: SEM–EDX Elemental analysis of In vitro Basella rubra L.
|
Conclusion
Due to over exploitation of plant source from natural environment for medical uses, the standardization and optimization of shoot regeneration protocol for B.rubra because imminent. The present histochemical and shoot induction studies would facilitate the phytochemical constituents and conservation of this important plant species.
Acknowledgements
The authors express thanks to Padmavibhushan Dr.V. Krishnamurthy, President, Sri K.Ragunathan, Secretary, Dr. K. Anbarasu, Director and Dr. R. Sundararaman Principal, National College, Tiruchirapalli for providing the infrastructure facilities, supports and encouragement given to PG and Research Department of Biotechnology to carryover the research work.
Conflict of Interest
There is no conflict of interest.
References
- Abubacker M. N and Sathya C. In vitro Bioaccumulation of Heavy Metals by Water Lettuce Pistia stratiotes Engl (Araceae) and its Combustion Process as Manure Value by SEM-EDX Analysis. Biolife. 2017;5:38–43.
- Adenegan T. A. A and Akinnubi M. F. Some anatomical features of Basella L. their adaptive significance to water stress. Res Plant Biol. 2015;5:14–22.
- Bamidele O., Akinnuga A. M., Olorunfemi J. O., Odetola O. A., Oparaji C. K and Ezeigbo N. Effects of aqueous extract of Basella alba leaves on haematological and biochemical parameters in albino rats. African Journal of Biotechnology. 2010;9:6952–6955.
- Cyunel E. Basella alba L. In vitro culture and the production of betalains. In: Bajaj Y. P. S (ed). Biotechnology in agriculture and forestry. Medicinal and aromatic plants II. Springer. Berlin, New York. 1989;7:47–68.
- Guo D and Bin X. Tissue culture and plantlet regeneration of Basella alba L. Acta Agric Zhejiang. 2001;13:16–18.
- Kartha K. K and Gamborg O. L. Elimination of cassava mosaic disease by meristem culture. Phytopathol. 1975;65:826–828.
CrossRef - Kumar S. S., Manoj P and Giridhar P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced anti-oxidant potential under fermentation. Journal of Food Science and Technology. 2015;52:3037-3043.
CrossRef - Kumar S. S., Manoj P., Giridhar P., Shrivastava R and Bharadwaj M. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. Journal of Functional Food. 2015;15:509-515.
CrossRef - Larkcom J. Oriental vegetables: the complete guide for garden and kitchen. Kodansha International, USA. 1991.
- Maisuthisakul P., Ritthiruangdej P. S. Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Compost Anal. 2008;21:229–240.
CrossRef - Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497.
CrossRef - Mundo L., Gorospe K. J., Lorilla L., Serquina A. K and Torres E. The Feasibility of Basella rubra L. as a biological stain. Bato Balani Sophomore. 1995;14:16–18.
- Ozela E. F., Stringhet P. C and Chauca M. C. Stability of anthocyanin in spinach vine (Basella rubra) fruits. Cien Investig Agrar. 2007;34:115–120.
- Sankar N. R., Sreeramulu A., Gopal D. S and Bagyanarayana G. First report of leaf blight of Basella alba caused by Alternaria alternata in India. Plant Dis. 2011;95-1476.
CrossRef - Shekhawat M. S and Manokari M. Optimization of in vitro and ex vitro regeneration and micromorphological studies in Basella alba L. Physiol Mol Biol Plants. 2016;123:22:(4):605–612.
- Shanmugam S., Kumar T. S and Selvam K. P. Laboratory Handbook On Biochemistry PHI learning private ltd, New Delhi. 2010.
- Pumchaosuan T and Wongroung S. In vitro propagation of ceylon spinach (Basella rubra L.). As. J. Food Ag-Ind. 2009;S31-S36.
- Toshiyuki M., Kazuhiro H and Masayuki Y. Structures of new oleanane-type triterpene oligoglycosides, Basella saponins A, B, C, and D, from the fresh aerial parts of Basella rubra L. Chemical and Pharmaceutical Bulletin. 2001;49:776 – 779.
CrossRef - Yang R. Y., Lin S and Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac J Clin Nutr. 2008;17:275–279.

This work is licensed under a Creative Commons Attribution 4.0 International License.





