Manuscript accepted on : 11-April-2019
Published online on: 30-03-2019
Plagiarism Check: Yes
Isolation and Charecterisation of Seaweed Endophytic Fungi as an Efficient Phosphate Solubiizers
Noorjahan A, B. Aiyamperumal and P. Anantharaman
CAS in Marine Biology, Faculty of Marine sciences, Annamalai University, Parangipettai-608502, Tamil Nadu, India.
Corresponding Author E-mail: noorbiotek@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2718
ABSTRACT: Phosphate-solubilizing fungi (PSF) generally enhance the availablility of phosphorus (P) released from soil, which contributes to plants' P requirement, especially in P-limiting regions. In this study we isolated endophytic fungi from seaweeds and screened for phosphate solubilizng in both solid and liquid culture and estimated the solubilizing index and enzyme activity. Six fungus of Penicillium oxalicum, P.citrinums and Aspergillus sp. shows maximum phosphate solubilizing activity. Hence Seaweed endophytic fungus isolated from chlorophyceae express as an alternate source to replace chemical fertilizer.
KEYWORDS: Chlorophyceae; Penicillium Oxalicum; Aspergillus sp.
Download this article as:| Copy the following to cite this article: Noorjahan A, Aiyamperumal B. Anantharaman P. Isolation and Charecterisation of Seaweed Endophytic Fungi as an Efficient Phosphate Solubiizers. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Noorjahan A, Aiyamperumal B. Anantharaman P. Isolation and Charecterisation of Seaweed Endophytic Fungi as an Efficient Phosphate Solubiizers. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2XhRPRR |
Introduction
Phosphorus (P) is the next important nutrients after nitrogen which have an impact on plant growth and its metabolic processes (Widawati S & Suliasih D, 2006). Phosphorus is essential to promote photosynthesis, energy regulation ,sugar production, synthesis of nucleic acid, and enhancing N2 fixation in leguminous plants (Saber et al.,2005). It also plays an important role in strengthening straw of the cereal plant and quality of the crop, boost flower formation and increase the fruit production, stimulates root development and essential for seed formation, maturity, and also to develop resistance against diseases (Sharma et al., 2011, Richardson, 2007).
Generally, the mobility of phosphate ions in the soil is very low due to high retention. Stevenson and Holford reported in 1986 that the plants utilize P from soil in the rate of about 10-30% (Stevenson,1986) and the remaining 70-90% of Phosphate is accumulated in the soil as immobilize form or it is bounded with Al or Fe in acid soils or Ca and Mg in alkaline soils (Prochnow et al., 2006; Yang et al.,2010).
This insoluble form of Phosphorus is converted to soluble form by the microorganisms which play a key role in dissolving both free and bound phosphate in the soil that is environmentally friendly and sustainable (Khan et al.,2007). Microorganisms including fungi, bacteria and actinomycetes are known to be efficient as fixed P solubilizes (Sundara et al., 2002).
Seaweeds are considered as a well-endowed determinant for nutrients that ultimately leads to high competition between different microbial communities (Burgess et al.1999; Armstrong et al. 2001; Penesyan et al. 2009). The second largest diverse assemblage of marine fungi is from seaweeds (Bugni and Ireland 2004; Schulz et al.2008; Suryanarayanan et al. 2010; Godinho et al. 2013). Seaweeds encompassing genera belonging to Phaeophyceae, Rhodophyceae and Chlorophyceae have been broadly studied worldwide for their fungal associations and the study revealed that the dominant species of fungal endosymbionts were ascomycetes and anarmorphic fungi (Schulz et al. 2008; Zuccaro et al. 2008; Suryanarayanan 2012; Godinho et al. 2013;). The fungi associated with seaweeds are mostly parasites, saprobes or asymptomatic fungal endosymbionts (Bugni and Ireland 2004; Loque et al.2010; Suryanarayanan et al. 2010). The diverse fungal population in Red and Brown seaweeds were comparatively high compared to that of Green seaweed (Suryanarayanan et al. 2010). This is due to short life cycle of Green seaweeds and characteristically slow growth of the endosymbionts could together be responsible for low fungal diversity (Zuccaro and Mitchell 2005). Fungal genera of Acremonium, Alternaria, Arthrinium, Aspergillus, Cladosporium, Fusarium, Geomyces, Penicillium, and Phoma (Zuccaro et al. 2003; Loque et al. 2010; Suryanarayanan et al. 2010; Flewelling et al. 2013; Godinho et al. 2013; Furbino et al.2014) were the most common fungal endosymbionts.
On comparing with bacteria fungi have greater ability to solubilize insouble phosphate(Nahas, 1996). Endophytic fungi from Marine plants and Marine algae are gaining interest because of their existence in an ecosystem renowed by resource inconstancy such as temperature, salinity, osmotic stress, light vailabity (Debbab et al., 2011; Oliveira et al., 2012).
Therefore, the objective of this study is to search for the seaweed in order to select the endophytic fungi which can solubilze phosphate. The results may provide insights into potential fertilizer in acidic soils and P-deficit soils. Microbial phosphate solubilization has an impact on plant growth promotion. There are several reports regarding plant growth promotion due to inoculation of phosphate solubilizing microorganisms. Screening and characterization of phosphate solubilizing microorganisms are important for proper utilization of their beneficial effects to increase the crop production and sustain agricultural productivity of the country without contaminating environments.
Materials and Method
Collection of Sample
Seaweeds were collected from Rameswarem coastal region. Abundant seaweeds are selected for the isolation of endophytic fungus. Cauerapa racemosa, Halimeda macrooba (Chlorophyceae), Turbinaria conoides, Sargassum sp, Padina sp (Phaeophyceae), Gracilaria sp., Portieria sp. (Rhodophyceae). Seaweeds were collected in sterile bags and processed in laboratory. Freshly collected plant materials were utilized for the isolation of the endophytic fungi to reduce the chance of contamination.
Isolation of Endophytic Fungi from Seaweeds
Healthy thallus of the seaweeds were thoroughly washed in seawater followed by running tap water, then surface sterilized by a modified method of [Raviraja, 2005]. The selected thallus segments were immersed in 95% ethanol for 30 sec, 4% sodium hypochlorite solution for 3 min and 95% ethanol for 30 sec followed by rinsing with sterile distilled water three times and allowed to surface dry under sterile conditions. After drying, each thallus segment was cut into approximately 0.5 cm and placed on petri plates containing potato dextrose agar medium (PDA) supplemented with Chloramphenicol (100 mg/L) to suppress the bacterial growth. Petri plates were sealed with parafilm and incubated at 30°C in a light chamber for up to one week. They were monitored every day for growth of endophytic fungal colonies. Fungi growing out from the samples were subsequently transferred onto fresh PDA plates to isolate pure colonies.
Identification by Morphological Characteristics of Endophytic Fungi
Sporulating fungi were identified based on colony morphology, conidiospore and conidiophore characteristics. The microscopic identification of the isolates was carried out by lacto phenol staining technique [Nagamani et al., 2006].
Screening of Phosphate Solubilizing in Solid Medium
Phosphate solubilizing ability of the isolates was confirmed by incubating them on Pikovskaya’s agar medium incorporated with 5% tri-calcium phosphate at 28 ± 2°C for 15 days. Diameter of clearance zone was measured successively after 24 h, up to 15 days. The Solubilization Index (SI) is the ratio of total diameter i.e. clearance zone including fungal growth and the colony diameter. All the observations were recorded in triplicate. Pikovskaya, 1948.
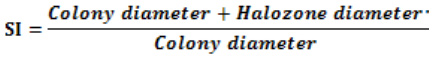
P-solubilizing Capacity under Different pH Conditions
After preparation of the conidial suspensions of the fungal strains, was inoculated into 50-mL flasks containing 30 mL of Pikovskaya’s liquid media, were added to a concentration of 5 g/L, respectively. The pH of the Pikovskaya’s liquid media was adjusted to 3.5, 4.5, 5.5, and 6.5 with 2 mol/L hydrochloric acid, respectively, before addition of the P sources the medium was sterilized at 121°C for 20 min. Then, 0.5 mL of the test fungal spore suspension was inoculated into 50 mL triangular flasks at various pH and incubated at 25°C and 120 rpm for 8 d on a shaker. The control comprised flask with uninoculated medium. All treatments were centrifuged at 10,000 ×g for 10 min, and the supernatant was used for the measurements of soluble P and pH. The content of soluble P in the supernatant was determined by the colorimetric molybdate blue method (Olsen & Sommers, 1982). All experiments were performed in triplicate.
Estimation of Phosphatase Activity
Phosphtates were estimated at weekly intervals about 30 days. The test fungal isolates were fermented in Potato Dextrose broth at 21°C for 4 weeks. The fungal broth was filtered through Whatman no. 42 filter paper and homogenized in a pestle and mortar at 4°C using 0.02 M Tris buffer (1:1 w/v, pH 7.5). The macerate was centrifuged at 16,000g for 20 min and the supernatant was collected and estimated for Alkaline and Acid phosphates.
For assaying alkaline phosphatase, to 0.1 mL the enzyme extract (the supernatant) 0.5 mL of tris citrate buffer (pH 8.5/5.5 mM) was added followed by 0.1 mL of MgCl2 (0.1 M) and then 1.0 mL of p-Nitrophenol phosphate (1 mg/mL). The mixture was incubated for 30 min at 21°C and Read at 405 nm. Same procedure was followed for estimating acid phosphatase assay but an acetate buffer (pH 4.5/0.1 M) was used in place of tris citrate buffer. The tubes were incubated at 21°C for 30 min. After incubation, 5 mL of 0.5 M NaOH was added and the release of p-Nitrophenol was measured at 405 nm. The values were converted to micromoles of p-Nitrophenol with reference to the standard curve. One enzyme unit was defined as the amount of enzyme that catalysed the formation of 1 lmol of end product (p-Nitrophenol) in 1 min under experimental conditions (Tabatabai and Bremner 1969).
Results and Discussions
Seven seaweeds belonging to Chlrophyceae, Rhodophyceae and Phaeophyceae were selected for the isolation of endophytic fungi. Totally 18 endophytic fungi were isolated and screened for Phosphate solubilization. Of that six species shows better result which is discussed in this paper.
Phosphate Solubilization Solid Medium
Endophytic fungal isolates showed phosphate Solubilizing activities as detected in Pikovskaya’s agar amended with tri-calcium phosphate in the medium by the appearance of halos around the inoculum on the medium. Aspergillus sp, P. oxalicum, P. citrinum, superior solubilization index (PSI) 5.7,5.2 and 3.2 respectively in 15 days incubation.
Table 1
| S.No | Fungal Isolates | Phosphate Solubilizng Index (PSI) mm | ||
| 7th Day | 14thday | 21st day | ||
| 1 | Pencillium oxallicum | 3.5 | 5.2 | 7.1 |
| 2 | P. Purpurogenum | 2.5 | 3 | 3.5 |
| 3 | P.citrinum | 2.6 | 3.2 | 3.7 |
| 4 | P.aurantio-grieseum | 1.3 | 2.4 | 2.9 |
| 5 | Aspergillus niger. | 1.7 | 2.6 | 3.1 |
| 6 | Aspergillus sp. | 3.2 | 5.7 | 7.9 |
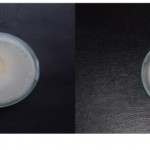 |
Figure 1: solubilizing index.
|
Phosphatase Solubilization in Liquid Culture
Phosphate solubilization by six species of fungal strains was carried out at, The fungal broth were weekly withdrawn to estimate solubilization index and the corresponding changes in the pH of the broth are presented in Fig. 3.
Phosphate solubilization was estimated on the 7th day giving the values of Penicilium oxallicum 220 µg mL–1 with the pH 3.9 ,by pH 3.3 it solubilize 110 µg mL–1 P. aurantio-griseum, 230 µg mL–1 by P.citrinum with an pH 3.7, 210 µg mL–1 by P. purpurogenum pH 3.7 and Aspergilus niger with the pH 4 it solubilize phosphate 190 µg mL–1 and Aspergillus sp. by 195 µg mL–1 with the pH 3.5.
There is a increase in solubilization rate and decrease in the pH at the 15th day of incubation. The values were recorded as pH 3.3 with 310 µg mL–1 P. oxalicum and 425 µg mL–1 with the pH 3.1 P. citrinum and at pH 3.1 P. purpurogenum solubilizes 391 µg mL–1 for Aspergillus niger at 3.9 pH it solubilizes 410 µg mL–1 and Aspergillus sp. with pH 3.5 solubilizes 325 µg mL–1 If the strains attained the maximum solubilization rate at 400 µg mL–1 decline values were recorded at 21st day. P.oxalicum attain its maximum solubilization at 30th day of incubation.
Decrease in pH of the broth coinciding with increase in phosphate solubilization estimations which indicates the production of organic acids in the broth, recorded in all the strains were noticed . Various mechanisms have been reported for phosphate solubilization, the most recognized one is through the production of organic acids (Nahas,1996). Production of organic acids, like citric, gluconic and oxalic acid, have been recognized for phosphate solubilization by several species of Penicillium, namely, P. bilaii, P. radicum, P. rugulosum, P. variabile (Asea et al., 1988; Cunningham and Kuiack 1992; Vassilev et al. 1996). Omar (1998) reported phosphate solubilization activity by Aspergillus niger and Penicillium citrinum causing remarkable drop in pH of liquid culture.
 |
Figure 2: Phosphate soubilization in pH of broth at 21°C estimated upto day 30 days at Weekly intervals.
|
Quantitative Estimation Enzyme Phosphatase
The acidic and alkaline phosphatase activity exhibited by six fungal stains were incubated at 21°C for 30 days and estimated at weekly interval is presented in Fig. 4. All the species showed acidic phosphatase activity higher (1.5–2.0 times) in comparison to the alkaline phosphatase. The maximum phosphatase activity 21.23 (Aspergillus sp.), 23.37(P. oxalicum) and 19.13 (Aspergillus niger), 16.32 (P. aurantio-griseum) was recorded at the week. After week 2 and 28.83 (P. citrinum), 24.32 (Aspergillus sp.), 24.67 (P. oxallicum), 21.34 (P. purpurogenum) and 22.41 (Aspergillus niger) after week 3 of growth. Alkaline phosphatase activity ranged between 9.67 (Aspergilus sp.) and 11.21 (P. citrinum) after week 3 of incubation. Tarafdar et al. (2003), reported the efficiency of seven fungal phosphatase producing fungi and in which the acid phosphatase was three times higher than the alkaline phosphatase. Naturally occurring phosphate solubilizing microorganisms have been recognized as a source of P fertilizer. Amongst fungi, maximum Penicillium species have been reported for various properties of biotechnological applications.
 |
Figure 3: Acid and Alkaine phosphatase activity of Fungal species estimated weekly intervals upto 4 weeks.
|
Conclusion
Therefore, these strains can be a good candidate and exploited as bio fertilizers through further evaluation and optimization test to increase agricultural productivity. Other strains such as Aspergillus niger, P. aurantio-griseum, P. purpurogenum were positive for phosphate solubilization efficiency. P. oxalicum was the superior among the isolated fungi in solubilizing index 5.3 after further evaluation on invitro test, green house and field trials as bio fertilizer. The rise in the cost of chemical fertilizer, the lack of fertilizer industries in developing countries and the growing environmental issue and biodiversity loss using chemical fertilizer timely important concern using alternative ecofriendly bio fertilizer to increase yield and productivity.
Fungus from marine ecosystems are distinct from those of terrestrial environments. The most efficient phosphate solubilizing strains penicillium sp and Aspergillus sp. were identified from green seaweed and to solubilise inorganic phosphate to organic form. Phosphate solubilizing fungus can effectively replace chemical fertilizer.
Acknowledgements
We greatly thankful to DBT-BIRAC for funding the project entitiled “Development of integrated product with plant growth and defence potential through end to end utilization of marine biological resources”. Ref BT/SBIRI1394/31/16.
References
- Armstrong E., Yan L., Boyd K. G., Wright P. C., Burgess J. G. The symbiotic role of marine microbes on living surfaces. Hydrobiologia. 2001;461:37–40.
CrossRef - Asea P. E. A., Kucey R. M. N., Stewart J. W. B. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol Biochem 20:450–464 bilaii. Appl Environ Microbiol. 1988;58:1451–1458.
- Bugni T. S., Ireland C. M. Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep. 2004;21:143–163.
CrossRef - Burgess J. G., Jordan E. M., Bregu M., Mearns-Spragg A., Boyd K. G. Microbial antagonism: a neglected avenue of natural products research.J Biotechnol. 1999;70:27–32.
CrossRef - Debbab A., Aly A. H and Proksch P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011;49:1–12.
CrossRef - Flewelling A. J., Johnson J. A., Gray C. A. Isolation and bioassay screening of fungal endophytes from North Atlantic marine macroalgae. Bot Mar. 2013;56:287–297.
CrossRef - Furbino L. E., Godinho V. M., Santiago I. F., Pellizari F. M., Alves T. M. A., Zani C. L., Junior P. A. S., Romanha A. J., Carvalho A. J. O., Gil L. H. V. G., Rosa C. A., Minnis A. M., Rosa L. H. Diversity patterns; ecology and biological activities of fungal communities associated with the endemic macroalgae across theAntarctic peninsula. Microb Ecol. 2014;67:775–787.
CrossRef - Godinho V. M., Furbino L. E., Santiago I. F., Pellizzari F. M., Yokoya N. S., Pupo D., Alves T. M., Junior P. A., Romanha A. J., Zani C. L., Cantrell C. L., Rosa C. A., Rosa L. H. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013;7:1434–1451.
CrossRef - Khan M. S., Zaidi A., Wani P. A. Role of phosphate-solubilizing microorganisms in sustainable agriculture A review. Agronomy and Sustainable Development. 2007;27:29-43.
CrossRef - Loque C. P., Medeiros A. O., Pellizzari F. M., Oliveira E. C., Rosa C. A., Rosa L. H. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33:641–648.
CrossRef - Nagamani A., Kunwar I. K., Manoharachary C. A Hand Book of Soil Fungi. New Delhi: I K international. 2006.
- Nahas E. Factors determining rock phosphate solubilization by microorganisms isolated from soil. World J Microbiol Biotech. 1996;12:567-572.
CrossRef - Oliveira A. L. L. d., Felício R. D and Debonsi H. M. Marine natural products: chemical and biological potential of seaweeds and their endophytic fungi. Rev. Bras. Farmacogn. 2012;22:906–920.
CrossRef - Olsen S. R., Sommers L. E. Phosphorus. In: Page A. L., Miller R. H., Dennis R. K (Eds). Methods of Soil Analysis. Madison: American Society of Agronomy. 1982;403-430.
- Omar S. A. The role of rock-phosphate-solubilizing fungi and vesicular-arbuscular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J Microbiol Biotechnol. 1998;14(2):211–218.
CrossRef - Pikovskaya R. I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologia. 1948;17:362-370.
- Prochnow L. I., Fernando J., Quispe S., Artur E., Francisco B., et al. Effectiveness of phosphate fertilizers of different water solubility’s in relation to soil phosphorus adsorption. 2006;65:1333-1340.
- Raviraja N. S. Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. J Basic Microbiol. 2005;45:230-235.
CrossRef - Richardson A. E. Making microorganisms mobilize soil phosphorus. In: Velazquez E., Rodriguez-Barrueco C (eds.), First International Meeting on Microbial Phosphate Solubilization. 2007;85-90.
CrossRef - Saber K. L., Nahla A. D., Chedly A. Effect of P on nodule formation and N fixation in bean. Agron Sustain Dev. 2005;25:389-393.
CrossRef - Schulz B., Draeger S., Del Cruz T. E., Rheinheimer J., Siems K., Loesgen S., Bitzer J., Schloerke O., Zeek A., Kock I., Hussain H., Dai J., Krohn K. Screening strategies for obtaining novel; biologically active; fungal secondary metabolites from marine habitats. Bot Mar. 2008;51:219–234.
CrossRef - Sharma S., Vijay K., Tripathi R. B. Isolation of Phosphate Solubilizing Microorganism (PSMs) from soil. J Microbiololgy Biotechnology Research. 2011;1:90-95.
- Stevenson F. J. Cycles of soil carbon, nitrogen, phosphorus, sulphur and micronutrients. Wiley, New York, USA. 1986;201.
- Sundara B., Natarajan V., Hari K. Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugar cane and sugar yields. Field Crops Research. 2002;77:43-49.
CrossRef - Suryanarayanan T. S., Thirunavukkarasu N., Govindarajulu M. B., Gopalan V. Fungal endophytes: an untapped source of biocatalysts. Fungal Divers. 2012;54:19–30.
CrossRef - Suryanarayanan T. S., Venkatachalam A., Thirunavukkarasu N., Ravishankar J. P., Doble M., Geetha V. Internal mycobiota of marine macroalgae from the Tamilnadu coast: distribution; diversity and biotechnological potential. Bot Mar. 2010;53:457–468.
CrossRef - Tarafdar J. C., Bareja M., Panwar J. Efficiency of some phosphatase producing soil-fungi. Indian J Microbiol. 2003;43:27–32.
- Vassilev N., Fenice M., Federici F. Rock phosphate solubilization with gluconic acid produced by immobilized Penicillium variabile P16. Biotechnol Tech. 1996;10:585–588.
CrossRef - Vazquez P., Holguin G., Puente M. E., Lopez C. A., Bashan Y. Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils. 2000;30:460-468.
CrossRef - Widawati S., Suliasih D. Augmentation of potential phosphate solubilizing bacteria (PSB) stimulate growth of green mustard (Brasica caventis Oed) in marginal soil. J Biodiversity. 2006;7: 10-14.
CrossRef - Yang M., Ding G., Shi L., Feng J., Xu F., et al. Quantitative trait loci for root morphology in response to low phosphorus stress in Brassica napus. Theor Appl Genet. 2010;121:181-193.
CrossRef - Zuccaro A., Schoch C. L., Spatafora J. W., Kohlmeyer J., Draeger S., Mitchell J. I. Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl Environ Microbiol. 2008;74:931–941.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





