Manuscript accepted on : 28-Mar-2019
Published online on: 30-03-2019
Plagiarism Check: Yes
Millena Cristiane De Medeiros Bezerra Jácome1,2, João Maria Martins Jácome1, Carlos Eduardo De Araújo Padilha1, Murilo Ricardo Do Nascimento Arrais1, Francisco Canindé De Sousa Júnior1,3, Camila Gambini Pereira2 and Everaldo Silvino Dos Santos*1
1Laboratory of Biochemical Engineering, Chemical Engineering Department, Federal University of Rio Grande do Norte (UFRN), Natal-RN, Brazil.
2Laboratory of Food Separation Processes, Chemical Engineering Department, Federal University of Rio Grande do Norte (UFRN), Natal-RN, Brazil.
3Laboratory of Bromatology, Department of Pharmacy, Federal University of Rio Grande do Norte (UFRN), Natal-RN, Brazil.
Corresponding Author E-mail: everaldo@eq.ufrn.br
DOI : http://dx.doi.org/10.13005/bbra/2719
ABSTRACT: This study investigates the mangaba residue (Hancornia speciosa Gomes) and used as a substrate for the production of enzymes by semi-solid fermentation (SSF) using Aspergillus niger. The physical-chemical and bioactive attributes of mangaba bagasse and its extracts were addressed. Polyphenols concentrations of 564.1 ± 0.047 mg of gallic acid / g of oil and Total Antioxidant Activity - TCA of 95.46 ± 1.58 mg ascorbic acid / g ethanolic extract were found. In addition to the higher enzymatic production of lipase (50.11 ± 0.02 U / g) and pectinase (0.45 ± 0.01 U / g) through SSF. In general, in comparison with other similar residues and other works the results presented by the mangaba residue are relevant because it is an inexpensive raw material with potential added value to be exploited for the production of enzymes by fermentation in solid substrate using A Niger and extraction of antioxidant compounds.
KEYWORDS: Agro-Industrial Waste; Lipase; Pectinase; Semi-Solid Fermentation
Download this article as:| Copy the following to cite this article: Jácome M. C. D. M. B, Jácome J. M. M, Padilha C. E. D. A, Arrais M. R. D. N, Júnior F. C. D. S, Pereira C. G, Santos E. S. D. Mangaba Residue (Hancornia Speciosa GOMES) Potentially used for Producing Antioxidants and Lignocellulosic Enzymes. Orient J Chem 2019;35(Special Issue 1 Spectroscopy March 2019). |
| Copy the following to cite this URL: Jácome M. C. D. M. B, Jácome J. M. M, Padilha C. E. D. A, Arrais M. R. D. N, Júnior F. C. D. S, Pereira C. G, Santos E. S. D. Mangaba Residue (Hancornia Speciosa GOMES) Potentially used for Producing Antioxidants and Lignocellulosic Enzymes. Orient J Chem 2019;35(Special Issue 1 Spectroscopy March 2019). Available from: https://bit.ly/2G1bEph |
Introduction
In world fruit production, Brazil stands out as the third largest producer with around 5.0% of world fruit production. Of note is that they are annually more than 43 million tons, according to the Brazilian Association of Producers Exporters of Fruits and Derivatives1 (Abrafrutas, 2019). Because of the large production and growing fruit industrialization and consumption, an increase in the number of agro-industries installed in the country has been occurring, generating a strong growth in the production of agroindustrial residues of vegetal origin2 (LOUSADA JUNIOR et al., 2005). Therefore, the deeper knowledge and added value for these by-products is of fundamental importance to minimize the environmental impacts, besides allowing a greater economic interest of them3 (SOUSA et al., 2011). In this way the use of agroindustrial residues has been increasing, especially those of vegetal origin, such as residues of fruit pulp, coffee leaves, cassava bagasse, soybean meal, sugar cane bagasse, etc. Thus, several biotechnological processes have been developed to use these materials in the production of enzymes, alcohol and organic acids, generating products of great economic and environmental value4 (ISRAEL, 2005).
The characterization of the residues produced by the agroindustries is important because there are variations in their composition, which depend on the type of fruit to be processed, and this by-product may consist of shells, seeds, lumps and even pulp5 (MATIAS et al. 2005). The use of agroindustrial residues does not only consist in the reuse of these as animal feed or organic fertilizer, these are currently the main applications given to these by-products. As an example of an agroindustrial residue of vegetable origin with great biotechnological potential, it is possible to mention the residue of mangaba that is the fruit of the mangabeira. The mangabeira (Hancornia speciosa Gomes) is a fruit tree belonging to the family Apocynaceae, native to Brazil and occurs in the Central-West, Southeast, North and Northeast regions of the cerrado and caatinga6 (VENTURINI FILHO, 2010). According to the Brazilian Institute of Geography and Statistics7 (IBGE, 2017) the production of mangaba (fruit) in Brazil in 2017 was about 1022 tons, this production can cause a great environmental impact, because of this total, approximately 77.0% is destined to the processing, generating a great volume of residue that is not used in human food. In addition, approximately 67.63 kg / h of residue is produced during fruit processing8 (PERFEITO et al., 2015). The mangaba has the potential to increase its production due to the climatic conditions of the country and prospects for the industrialization of new products of this fruit9 (CLERICI & SILVA, 2011). The residue derived from the mangaba processing is rich in nutrients such as proteins, fibers and fatty acids10 (BEZERRA JÁCOME, 2014). Studies have shown that solid-state fermentation (SSF) allows the use of these residues for the production of biomolecules of interest, mainly enzymes11,12 (RAHARDJO et al., 2005; ALBANO, 2012). Defined as the microbial development process on the surface of solid materials SSF is a suitable and inexpensive alternative to producing enzymes such as cellulases, xylanases and pectinases13,14 (SANTOS., 2007, El-Bakry et al, 2015) and other biomolecules such as organic acids15 (RODRIGUES et al., 2007); aromas16 (UENOJO, 2007), vitamins, ethanol and bioherbicides17 (MORAES et al., 2007). Thus, agricultural residues and those derived from biorefineries offer a wide range of possibilities as components of culture media in the production of enzymes and other higher value-added and low-cost biomolecules18,19 (SORENSEN et al., 2011; BORIN et al., 2015). The residues derived from the processing of mangaba, largely seeds, are rich in nutrients such as proteins, fibers, minerals and fatty acids20,21 (SOUZA & AQUINO, 2012; DO NASCIMENTO et al., 2015). Some studies reported in the literature to use this residue for protein enrichment for animal feed23 (VIEIRA, 2007) as well as the use of mangaba seeds for the production of bio-oil24 (SANTOS, 2014).
In this context, the present study aims to evaluate the potential of the mangaba (Hancornia speciosa Gomes) processing residue for the production of phenolic compounds and antioxidants, from its phenolic extract, as well as to evaluate the production of lipases and pectinases by the fungus Aspergillus niger IOC 4003 in solid state fermentation (SSF) using this residue as substrate. It should be noted that the oil present in this residue was extracted, characterized by fatty acid profile and antioxidant power (phenolic and total antioxidants).
Material and Methods
The mangaba processing residue (Hancornia speciosa Gomes) used was donated in two distinct lots by Sterbom Indústria e Comércio Ltda (Fruit pulp) located in Macaíba (RN) in the northeastern region of Brazil and one of them was used only for the extraction of the oil and its characterization. Upon receipt, the residue was washed and oven dried at 70°C for 48 hours. Then they were ground in a knife mill (Willye type mill, TE-680, Tecnal, Brazil), sieved at 48 mesh strainer and stored in a plastic container at room temperature (25°C). Figure 1 below illustrates the proposed scheme for the use of the Mangaba residue (Hancornia speciosa Gomes) in the present study.
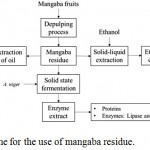 |
Figure 1: Proposed scheme for the use of mangaba residue.
|
Physical-Chemical Characterization of Mangaba Residue
The mangaba residue was characterized in terms of cellulose, hemicellulose, lignin, ash, moisture, carbohydrates and extractives according to NREL24,25 (National Renewable Energy Laboratory – USA) (SLUITER et al., 2008, SLUITER et al., 2012). In addition to these analyzes, the residue was also evaluated for Kjeldahl method26 (AOAC, 2005) and fibers using the gravimetric-enzymatic method (using ANKOM extractor).
Extraction Stages
The mangaba residue was submitted to oil extraction26 (AOAC, 2005) and ethanolic extraction27 (PADILHA, et al., 2018).
Extraction of Oil From Mangaba Residue
To extract the oil from the mangaba residue, a Soxhlet extractor was used and the solvent hexane (70ºC for 7 hours). The solvent was evaporated in a rotary evaporator (Solab, model SL-126) to obtain the final oil. Afterwards, acidity, peroxide, refraction and saponification analyzes were performed, as well as the moisture content and free fatty acids present in the oil.
Physical-Chemical Analysis in Oil
In the oil of the mangaba residue, acidity and free fatty acids were analyzed according to MORETTO et al. (1998)28; peroxides, refraction and saponification according to the official methods of the American Oil Chemists’ Society (AOCS, 2011),29 as well as the moisture content according to the Official methods of analysis of the Association of the Analytical chemists (AOAC, 2005).26
Phenolic Components of Oil
The total phenolic compound concentration of the oil was determined using the methodology described by SINGLETON & ROSSI (1965).30 Thus, approximately 1.0 mL of the extract and 1.0 mL of Folin-Ciocalteau reagent were mixed. After 5 minutes 1.0 ml of a saturated solution of Na2CO3 (35% solution in methanol) was added and the sample was then diluted to a final volume of 25 ml with methanol. The mixture was protected from light for 90 min before reading the absorbance at 725 nm using a spectrophotometer (SP-220 Bioespectro, Brazil). A calibration curve was generated using the gallic acid standard diluted in methanol at concentrations of 0.02 to 0.2 g / mL with 1 mL of Folin-Ciocalteau reagent. Calculation of the total phenolic content was expressed as mg of gallic acid / g of the sample.
Antioxidant Activity
The antioxidant activity of the oil was determined using the methodology used by HERRERO et al. (2004).31 In this method, free radicals of DPPH (2,2-diphenyl-1-picrylhydrazyl) were neutralized with antioxidants in the extracts resulting in a decrease in absorbance at 516 nm. To effect the reaction, a solution of 0.0214 ± 0.0005 mg / mL DPPH in methanol was prepared and 3.9 mL of that DPPH solution was mixed with 0.1 mL of extract (sample / methanol) solutions with concentrations of 10 mg / mL to 0.5 mg / mL. The total volume of the reaction was 4 mL. The reaction was allowed to proceed for 4 hours at room temperature (~ 25°C). The absorbance was then determined at 516 nm using a spectrophotometer (Bioespectro SP-220, Brazil). The degree of discoloration indicates the scavenging efficacy of the extract, was calculated in terms of percent inhibition using Equation (1):
AA% = 100 – {[(Abs sample – Abs control) x 100] / Abs DPPH} (1)
Where AA% = Percentage of antioxidant activity; Abs sample= Sample Absorbance; Abs control = Control Absorbance and Abs DPPH = Absorbance of DPPH solution.
Profile of Total Fatty Acids
The identification of the fatty acid profile of the oil extracted from the mangaba residue was performed by gas chromatography (GC) using the SHIMADZU GC – 2010 Plus AF chromatograph, which features a flame ionization detector and 100% dimethyl polysiloxane capillary column model RTX. The programming of the analysis was determined for a column temperature starts at 110 ° C ranging from 8°C per minute to 220°C. The temperatures used on the injector and detector were 220 and 240°C, respectively. The samples were injected in the volume of 1μL, adopting the split ratio of 1:50. The trawl gas used was nitrogen with a linear velocity of 22.9 cm / s. The fatty acids present in the sample were identified by comparing the retention times of each component for the retention time of pure standards of fatty acid methyl esters injected into the chromatograph.
Ethanolic Extraction and Antioxidant Compounds from Mangaba Residue
In order to recover antioxidant compounds from the mangaba residue, a previous ethanolic extraction was performed. Thus, 50.0g of the mangaba residue was weighed into a beaker and immersed in 500 mL of solution (1: 1) ethanol and water for 60 minutes at 60°C in a thermostatic bath. Agitating every 15 minutes. After this period the extract was filtered and stored at -10°C for further analysis of antioxidant compounds27 (Padilha, et al., 2018).
Solid State Fermentation (SSF)
After characterization of the residue, it was used as substrate for the production of lipase and pectinase enzymes through SSF. The microorganism used was a filamentous fungus Aspergillus niger IOC 4003 belonging to the collection of the Osvaldo Cruz Institute and assigned to the Laboratory of Biochemical Engineering of the Federal University of Rio Grande do Norte (Natal-Brazil). The fungus was kept in test tubes with threaded lids contained sterile alone and stored at -18 ° C. The conidia were activated differently for the production of each enzyme. The activation of the microorganism for the production of pectinase occurred using a basic medium proposed by COURI (1993)32 composed of: citrus pectin (10.0 g/L), NaNO3 (3.0 g/L), KH2PO4 (1.0 g/L), MgSO4 (0.5 g/L), KCl (0.5 g/L), FeSO4.7H2O (0.01 g/L) e Agar-agar (20.0 g/L). For the production of lipase, the microorganism was activated using the medium proposed by COURI & FARIAS (1995) composed of: NaNO3 (3.0 g/L), MgSO4 (0.5 g/L), KCl (0.5 g/L), FeSO4.7H2O (0.01 g/L), KH2PO4 (1.0 g/L), Agar-ágar (30.0 g/L) e Óleo de oliva (20.0 mL/L). They were transferred with a platinum loop and incubated for five days in an oven at 30°C.
The cultivation was carried out in 250 ml Erlenmeyers, where 5.0 g of the mangaba residue (with a diameter of less than 48 mesh) was added to the moisture-corrected flasks, by adding supplementation of the substrate with the nitrogen source, adding 1% ammonium sulfate, 60% average humidity and 30 ° C temperature. A suspension of Aspergillus niger IOC 4003 spores of 1.0 x 106 spores / g of solid medium was used as inoculum for SSF. SSF was performed at 30 ° C for 120 h (5 days) in BOD. The cultivation conditions for lipase production were the same as those optimized by SOUZA & AQUINO (2012)20 and for the production of pectinases were reported by COURI (1993).32
Quantification of Enzymatic Activity and Total Proteins
The quantification of the enzyme was performed according to the following methods. For lipase the dosage of the hydrolytic activity was used, according to PAULA (2011)33 from Equation (2):
![]()
Where Vb is the volume of the blank (L); Va is the volume of the sample (L); M is the molarity of the HCl solution, t is the incubation time (min) and m is the mass of the enzyme extract added (g). One unit of activity (U) was defined as the amount of enzyme that released 1 μmol of fatty acid per minute under the assay conditions.
For pectinase activity was determined using 1.0% citrus pectin as substrate, according to MINJARES et al. (1997)34 with the following modifications. The reaction mixture (1.3 mL) containing 0.8 mL of substrate (1.0%) and 0.5 mL of enzymatic extract diluted adequately in acetate buffer (0.5 M, pH 4.3) was incubated at 50 ° C for 30 minutes in a water bath. After incubation, 0.5 mL of DNS solution was added and the tubes were kept in boiling water for 10 minutes. After cooling, the developed color was read at 575 nm using a spectrophotometer (Termo Spectonic, Genesys 10 uv). One unit of pectinolytic activity corresponds to the amount of enzyme that liberates 1.0 μmol of galacturonic acid per minute of reaction.
The total protein concentration estimated by the Bradford method (1976).35 The total protein content was quantified from a standard curve of BSA (bovine serum albumin) at concentrations in the range of 0.1 to 1 mg / ml at 0.1 mg / ml intervals. It used 0.5 ml samples of the defined concentrations was added 0.5 ml of Bradford reagent. The contents of all the tubes were shaken, immediately after stirring at room temperature the absorbance reading was carried out in a spectrophotometer at 595 nm. A standard curve correlating the absorbance values to the protein content was used to quantify the total protein concentration.
Statistical Analysis
Statistical analysis were performed by using Statistica 7.0 software (Statsoft, USA)36 using the Tukey test for three independent samples at 5% level of significance (p < 0.05).
Results and Discussion
Physical-Chemical Characterization of Mangaba Residue
In the present study, the characterization of the mangaba residue (Hancornia speciosa) was carried out, as shown in Tables 1 and 2. It is observed that the agroindustrial residue evaluated presents in its constitution lignocellulosic characteristics, that is, they have in their chemical composition predominance of cellulose (44.31%), hemicellulose (approximately 18%) and lignin (approximately 22.0%). When compared to the other lignocellulosic residues, the largest amount of cellulose present in the mangaba residue is found, which is interesting for the production of cellulases or glucose for the production of second-generation ethanol, within the context of a biorefinery. It was also observed a significant difference when compared with the results reported by Santos (2014),23 such differences may be due to the variety of the fruit, influence of the cultivation site and, mainly, of the processing conditions to which the mangaba residue in the present study was submitted.
Table 1: Composition (percentage dry weight) of the different residue.
| Residue | C | H | TL | A | E | M | Author |
| Residue Mangaba | 44.31± 0.93 | 17.57 ±0.33 | 21.82 ±0.86 | 2.28 ±0.17 | 0.74 ±0.017 | 6.90 ±0.3 | This study |
| Residue Mangaba | 17.07 | 22.57 | 10.16 | 1,87±0,06 | – | 7,78±0,03 | Santos, (2014)23 |
| Moringa | 12.02 ± 0.81 | 14.61 ± 0.43 | 5.37 ± 0.98 | 3.60 ± 0.02 | 69.94 ± 1.40 | – | Magalhaes et al. (2018)64 |
| Carnauba | 23.96 ± 0.75 | 11.84 ± 0.82 | 32.79 ± 0.91 | 7.69 ± 0.05 | 11.62 ± 0.45 | – | Silva et al. (2018)65 |
| Cashew apple bagasse | 21.02 ± 0.31 | 11.50 ± 1.13 | 45.84 ± 1.28 | 9.50 ± 0.30 | 10.75 ± 0.86 | 1.04 ± 0.02 | Oliveira et al. (2018)61 |
C – Cellulose; H – Hemicellulose; TL – Total Lignin; A – Ash; E – Extractable; M – Moisture.
With respect to total protein content were found at residue mangaba 10.54 ± 0.03% it relates to the potential for degradation of the substrate, according to Teixeira et al. (2011)42 the low protein content means that the biomass has a great potential for degradation, whereas, high concentrations of nitrogen limit the degradation of the lignin present in the material. For example, Melzer et al. (2013)36 related the proteins to the content of nitrogen compounds present in the bio-oil as nitriles and amides. It was found by ARAÚJO et al. (2011)37 11.43% of total protein for mangaba seed meal and only values from 0.62 to 1.2% of total protein for the pulp mangaba second CLERICI et al. (2011).9 The results of chemical composition the residue mangaba this study are presented in Table 2.
Table 2: Centesimal composition of mangaba residue.
| Content (%) | Residue of Mangaba |
| Moisture | 6.90 ± 0.30 |
| Proteins | 10.54 ± 0.03 |
| Lipids | 22.30 ± 0.44 |
| Ashes | 1.50 ± 0.42 |
| Fibers | 47.02 ± 0.91 |
| Carbohydrates | 12.20 ± 0.52 |
It is observed that the major nutrients were the fibers (approximately 47.0%), followed by lipids (approximately 22.0%). In terms of lipids, the values obtained for the mangaba seeds were very close to those found in the literature. For example, Souza & Aquino (2012)20 obtained 23.0% of lipids in the mangaba seed meal and Vieira (2007)22 quantified 24.68% of lipids in the seeds of this fruit. Santos (2014)23 reported about 27.0% lipid, protein of approximately 12.0% and approximately 12.0% fiber to the residue mangaba. As mentioned above, different values are due to the different processes and the residue obtained beyond the cultivation and management in which the fruits were subjected mangaba.
With respect to carbohydrate content found in the pulped waste mangaba (12.2 ± 0.5%) was lower than that reported by Souza & Aquino (2012),20 reported that the content of 58.66%. This difference is because the amount of carbohydrates has been calculated in total terms, including the alimentary fibers. Thus, when considering the fibers and carbohydrates (47.02 ± 0.91% of fibers + 12.2 ± 0.5% of carbohydrates = 59.22 ± 1.41%), the results obtained in the present study are consistent with the results already mentioned in [20].
In relation to the ash content in mangaba seeds (1.5 ± 0.4%), the results found were slightly lower than those reported by Vieira (2007),22 which was 2.13% and Souza & Aquino (2012),20 which was 2.09%. It is noteworthy that this result is interesting for food purposes, because the organism limits its absorption.
Extraction of Oil and its Characterization
The oil extracted from the mangaba residue showed a yield of 22.3 ± 0.4%. This result is shown in the literature to seeds of fruits such as pomegranates (20.0%), orange (15.0%) and guava (12.3%)38,42 (KOBORI & JORGE, 2005; TEIXEIRA et al. 2011). In Table 3 are presented the results of the physicochemical analyzes the oil of mangaba residue.
Table 3: Mean values of the physicochemical characteristics of the oil.
| Analyze | Oil of mangaba residue (seeds) |
| Acidity level | 20.2 ± 0.4 |
| Peroxide Index (meq / 1000g) | 10.6 ± 0.2 |
| Refractive index | 1.4653 |
| Saponification Index (mg KOH / g) | 498.7 ± 0.4 |
| Moisture (%) | 4.6 ± 0.2 |
| Free Fatty Acids (%) | 11.10 ± 0.05 |
We did not identify in the literature studies on physical chemical analysis of the oil of the mangaba residue for comparison with the results obtained in this work. From the values presented in Table 2, it can be observed that the acid value found for the oil of the mangaba residue was 20.2 ± 0.4. Only for comparison of order of magnitude Pereira (2009)39 obtained 16.2 ± 0.2, studying the physical-chemical characteristics of the crude oil of jatropha extracted with the same solvent (hexane).
In relation to the peroxide index, in Brazil there is no value established by the National Agency of Sanitary Surveillance (ANVISA, BRASIL 2005)40 for crude vegetable oils; however, for refined oils and fats the maximum peroxide value is 10 meq / kg. The value found for the oil of the mangaba residue was 10.6 ± 0.2 meq / kg. This shows that even without the refining process the peroxide content of the oil studied was very close to the legislation.
The observed result for the refractive index in the oil of the mangaba residue was 1.4653 at a temperature of 40 ° C. The refractive index is widely used as the criterion of quality and identity of the oils. This index can be used to control the hydrogenation process. The refraction of the oils is related to the degree of unsaturation being affected by factors such as free fatty acid content, oxidation and heat treatment41 (CECCHI, 2003). The literature presents the refractive index for refined soybean oil in a range of 1,466-1,47040 (BRAZIL, 2005), so the refractive index the oil of the mangaba residue is very close to the refractive index of common edible oil.
The result found for the saponification index in the studied oil was 489.7 ± 0.4 mg KOH / g. According to the Technical Regulation for determination of identity and quality of vegetable oils and fats40 (BRASIL, 2005), soybean and coconut oil have a saponification index ranging from (189-195 and 248-265 mg KOH / g), respectively. The saponification index is indicative of the length of the fatty acid chain that make up the oil, thus being specific to each oil. According to MORETTO et al. (1998)28 the lower the molecular weight of the fatty acid, the higher the saponification index. In terms of food, the higher the saponification rate the better the oil for food. Therefore, the high values of this index presented in the studied oil, makes it interesting for food purposes.
The oil of the mangaba residue had a humidity of 4.6%. This value is high when compared to refined vegetable oils, which tend to have a zero moisture value. The refining process can remove the moisture.
Chromatographic Analysis of Oil
Figure 2 shows the chromatogram and Table 4 the Fatty acid composition of the oil of the mangaba residue (seed) (Hancornia speciosa). It is observed that the total amount of unsaturated fatty acids found for oil of the mangaba residue was 78.5%. Among the unsaturated fatty acids, the predominant acid in the oil of the mangaba residue was oleic acid (61.13 ± 0.12%) as shown in Table 4.
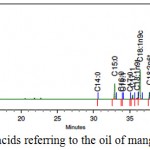 |
Figure 2: Profile of fatty acids referring to the oil of mangaba residue.
|
Table 4: Composition in terms of fatty acids to the oil of the mangaba residue.
|
Fatty Acid |
Percentage (%) | |
| Oil of the mangaba residue (seed). | ||
| Myristic acid – | C14 | 0.07 ± 0.17 |
| Pentadecyl acid – | C15 | 19.61 ± 0.83 |
| Palmitic acid – | C16 | 0.15 ± 0.01 |
| Palmitolico acid – | C16:1 | 0.13 ± 0.02 |
| Marginic acid – | C17 | 0.04 ± 0.01 |
| Cis-10-heptadecanoic acid- | C17:1 | 6.36 ± 0.05 |
| Elaidic acid – | C18:1n9t | 3.43 ± 0.13 |
| Oleic acid – | C18:1n9c | 61.13 ± 0.12 |
| Linolelaic acid – | C18:2n6t | 7.42 ± 0.11 |
| Gamma-linolenic acid – | C18:3n6 | 0.15 ± 0.01 |
| Alpha-linolenic acid – | C18:3n3 | 0.33 ± 0.01 |
| Heneicosanoic acid- | C21 | 1.6 ± 0.02 |
| Arachidonic acid – | C20:4n6 | 0.07 ± 0.01 |
| Nervonic acid – | C24:1 | 0.11 ± 0.05 |
| Saturated Fatty Acids | % | 21.48 |
| Unsaturated fatty acids | % | 78.5 |
The quality and digestibility of edible oils are determined by the amount and composition of unsaturated fatty acids43 (JORGE & LUIZA, 2012). The greater the amount of unsaturated fatty acids, the better the oil quality and digestibility. In this sense, it is interesting to observe the content of unsaturated fatty acids as well as their configuration in the molecule.
The polyunsaturated fatty acids are represented by the series omega 3 and omega 6, depending on where the double bond closest to the methyl end is positioned. Since the human body is unable to synthesize these fatty acids, they are called essential and must be obtained through diet44 (MOURA, 2008). The alpha-linonenic or omega-3 fatty acid stands out because it performs several biological functions in the human body45 (MARQUES, 2008). The oil of the mangaba residue (seeds) presented 0.33% of omega 3. Thus, taking into account the percentage of this fatty acid in common oil such as sunflower 0.3%46 (CODEX ALIMENTARIUM COMMISSION, 2008) the oil of the mangaba residue (seeds) presented a quantity similar to that found in common edible oils such as sunflower this being a considerable aspect for the use of these oils for food purposes. Santos (2014)23 reported the composition of fatty acids in the seed oil mangaba by GC / MS analysis where the fatty acids with greater abundance in the sample were octadecenoic acid (oleic) and hexadecanoic (palmitic). In this present study, the major fatty acids are oleic (61.1 ± 0.1%) and pentadecylic (19.6 ± 0.8%). Another author, Almeida (2008),47 characterized the mangaba seed oil found that the major fatty acids contained 10 to 24 carbons and thus oleic acid (C18) and palmitic acid (C16). This fact is important because it is an essential fatty acid (omega 9), which participates in our metabolism, playing a fundamental role in the synthesis of hormones. The content of oleic acid present in different oils according to RAMALHO & SUAREZ, (2013)48 as soybean oil (30.0%), grape seeds (28.0%), coconut oil or fat (10.0%) and peanut (72.0%) are less than the oil content of the mangaba residue (seeds). This information is relevant to justify the study of this new oil.
Total Phenolic Compounds (CFT)
The result obtained for the total content of phenolic compounds determined in the oil of the mangaba residue was 564.1 ± 0.047 mg of gallic acid / g of oil. It is noteworthy that Soong & Brarlow (2004)49 found a content of 117 mg of gallic acid / g in mango seed extract. According to PERFEITO (2015),8 the mangaba pulp presented phenolic compounds content of 115.84 ± 0.075 mg gallic acid / 100 g fresh fruit. In the processing of the mangaba pulp the peels are incorporated into the pulp. According to Rodrigues et al. (2006)50 the hydrophilic extract of Hancornia speciosa hulls constitutes an extremely complex mixture, being verified the presence of isoprenoid polymers (latex) and a large amount of condensed tannins. As related by Rodrigues (2007),51 the tannins, the chlorogenic acid and the catechins found in the bark helped to form the highest index of phenolic compounds in the pulp. The values of phenolic compounds found in the present study are higher than those found for similar fruit seed extract and the mangaba fruit itself. Indicating that the oil of the mangaba residue (seeds) may be an interesting possibility of application for food purposes. Since functional activity from phenolic compounds is desirable in foods.
Antioxidant Activity of Seed Oil
There is currently no single method for determining the antioxidant activity in food of plant origin and its by-products, considering the various antioxidant mechanisms that may occur, as well as the diversity of bioactive compounds. Among the methodologies that have been used, the ones that use DPPH radicals (2,2-diphenyl-1-picryl-hydrazyl) are the most outstanding.
The ability to sequester the DPPH moiety was evaluated in of the oil of the mangaba residue at concentrations ranging from 0.5 to 10 mg / mL and were calculated in percentages. The color change in the solution is an important indicator of the presence of antioxidant activity in the reaction medium, as shown in Figure 3.
 |
Figure 3: Antioxidant activity of the oil of the mangaba residue (seeds), where a) oil of the mangaba residue (seed), b) DPPH solution.
|
According to Figure 3, the standard solution has intense purple coloration (Figure 3b). After the reaction, the solution containing oil of the mangaba residue showed orange coloration (Figure 3a), indicating the antioxidant activity. Although the antioxidant activity of the oils has been observed visually, it is important to quantify this antioxidant activity effectively. The results obtained from the quantification of the antioxidant activity of the oil showed that in all concentrations studied there was antioxidant activity. The results indicate an antioxidant activity above 70% for all concentrations ranging from 72-86%. The content of phenolic compounds present in the oil of the mangaba residue was (564.1 mg / g oil). The antioxidant potential in relation to the DPPH radical and phenolic compound content were evaluated by Perfect (2015),8 which reported values of 91.99 (μM TE / 100 g) and 46.06 (mg of acic / 100 g).
Antioxidant Properties of Ethanolic Extract
The industrial processing of mangaba pulp as well as other fruits includes several steps, such as mechanical extraction (maceration), filtration and freezing. The processing steps may result in oxidation, degradation, leaching or other events that reduce the content of bioactive compounds in processed fruits,52-54 (NORA et al., 2014, TOMAS et al., 2015, BRANCO et al., 2016). For the present study, the extract obtained from the unfermented mangaba residue showed results of capture of the DPPH radical of (3.55 ± 0.16 μmol TE/g of sample) and the capture of the hydroxyl radical 7.42%, as well as Total Antioxidant Activity (TCA) of 95.46 mg. ascorbic acid / g sample. These values are interesting because due to the processing suffered by the residue there is an expected decrease of the antioxidant activity. Agroindustrial residues such as grape marc have been the subject of studies that showed results of antioxidant activity (AA) of (1.12 ± 0.04 mmol TEg -1 dry residue) as reported by Tournour et al. (2015).55 As well as Machado et al. (2015)56 who studied the residue of blackberry (Rubus fruticosus L.) reporting antioxidant activity of 76.03 μmol TE / g of fresh residue. As these are waste, these values are interesting from the point of view of reuse and use of cheap raw material. Some studies were carried out with the mangaba; Dutra (2017)57 obtained for the frozen pulp an antioxidant activity of (1907.56 ± 102.43 μmol TEAC 100 g – 1) in the DPPH assay. For the value of total antioxidant activity (TCA), Lima et al. 2015(a)58 reported that mangaba fruit showed the value of 182.58 mg / 100g. Thus, the value of AA present in the mangaba residue is promising when compared to the pulp of the fruit allowing to verify that even though it is submitted to several processes, from the pulp to the residue, the antioxidant activity, although smaller, still persists.
Semi-Solid Fermentation and Enzyme Extract
Lignocellulosic biomass, such as agricultural residues and forest residues, has been recognized as a potential sustainable source of sugars for biotransformation in value-added biological products such as enzymes59,60 (HIMMEL et al., 2007; LI et al., 2008). Therefore, the reutilization of the mangaba residue as a substrate for the production of pectinase and lipase by semi-solid fermentation using Aspergillus niger. The extract obtained was analyzed for the activity of each enzyme (AE) as well as total proteins as exposed Table 5.
Table 5: Enzyme production and protein quantification.
| Enzyme (Enzyme Extracts) | AE (U/g) | Total Proteins (mg / mL) |
| Lipase | 50.01 ± 0.02 | 0.548 ± 0.01 |
| Pectinase | 0.45 ± 0.01 | 0.255 ± 0.15 |
In the present study, 0.45 ± 0.01 U / g and 50.01 ± 0.02 U / g were obtained for pectinase and lipase respectively. The concentration of total proteins in the enzymatic extracts in the best condition were 0.255 ± 0.15 mg / mL in the pectinolytic extract and 0.548 ± 0.01 mg / mL in the lipolytic extract. Oliveira et al. (2018)61 studied the use of lignocellulosic residues as a substrate for the production of enzymes through SSF and presented values of 4.18 U / g and 0.62 U / g for CMCase and FPase, respectively. Reinehr et al. (2014)62 produced lipase by SSF using 85% soybean meal and 15% soybean husk as substrate and Aspergillus niger fungus and obtained maximal lipolytic activity of 45.49 U / g similar to that found in the present study. Similar work done by Damaso and coworkers63 using corn bagasse as a substrate as inducer for 48 h of SSF yielded a lipase activity of 62.7 U/g.
Conclusion
The lignocellulosic residue of mangaba after extraction and characterization presented several possibilities of use with characteristics of industrial interest, being these physical-chemical of the oil, as a peroxide index, saponification and refraction that showed results close to those found for edible oils. The high concentration of total phenolics and important antioxidant activity present in the oil of the mangaba residue (seeds) indicate the great potential and the possible application for food in a functional way. Finally, it has potential to be used as a substrate for the production of enzymes such as pectinase and lipase by Aspergillus niger in SSF.
Acknowledgements
The authors thank Brazilian National Council for Research (CNPq) for financial support.
References
- Abrafrutas – Brazilian Association of Producers of Fruits and Derivatives: www.abrafrutas.org (2019). Access: January 08, 2019.
- Junior J. E. L.,Neiva J. N. N.,Rodriguez N. M.,Pimentel J. C. M. P.,Lôbo R. N. B. Consumption and Digestibility of Fruit Processing Subproducts in Sheep. Revista Brasileira de Zootecnia. 2005;34(2):659-669.
- Sousa M. S. B.,Vieira L. M.,Lima A. Total phenolics and in vitro antioxidant capacity of tropical fruit pulp residues. Brazilian Journal of Food Technology. 2011;14(3):202-210.
- Israel M. C. Use of Residue from Palmiter Processing for the Production of Hydrolytic Enzymes by Fungi of Polyporus Genus. 135 f. Dissertation (Master in Environmental Engineering), Regional University of Blumenau, Blumenau. 2005.
- Matias M. F. O.,Oliveira E. L.,Gertrudes E.,Magalhâes M. A. Use of fibres obtained from the cashew (Anacardium ocidentale L) and guava (Psidium guayava) fruits for enrichment of food products. Brazilian Archives of Biology and Technology. 2005;48:143-150.
- Filho W. G. V. Alcoholic beverages – Science and technology. São Paulo: Blucher.2010;461.
- Ibge. Brazilian Institute Of Geography And Statistics. Production of Vegetal Extraction and Forestry 2017. Available at: https://cidades.ibge.gov.br/brasil/rn/pesquisa/16/12705. Access: January 08. 2019.
- Perfeito D. G. A., Carvalho N.,Lopes M. C. M.,Schmidt F. L. Caracterização de frutos de mangabas (Hancornia speciosa Gomes) e estudo de processos de extração da polpa. Revista de Agricultura Neotropical. 2015;2(3):1–7.
- Clerici M. T. P. S.,Silva L. B. C. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Research International. 2011;44:1658-1670 .
- Jácome M. C. B. M. de. Characterization and evaluation of the oils extracted from the seeds of yellow melon (Cucumis melo L.) and mangaba (Hancornia speciosa): possibility of food use. 73f. Dissertation (Master degree in Chemical Engineering) Federal University of Rio Grande do Norte. 2014.
- Rahardjo Y. S. P., Tramper J.,Rinzema A. Modeling conversion and transport phenomena in solid-state fermentation: a review and perspectives. Biotechnology Advances. 2005;24(2):161–179.
- Albano M. Comparison of cellulase and xylanase production by filamentous fungi in submerged and solid state fermentation. Dissertation – Postgraduate Program in Microbiology. Paulista State University. São José do Rio Preto. 2012.
- Santos S. F. M. Study of the production of pectinases by solid state fermentation using cashew peduncle as substrate. Thesis – Graduate Program in Chemical Engineering. Federal University of Rio Grande do Norte. Natal/RN Brazil. 2007.
- El-Bakry M., Abraham J., Cerda A., Barrena R., Ponsá S., Gea T., Sánchez A. From wastes to high value added products: novel aspects of SSF in the production of enzymes. Crit. Rev. Environ. Sci. Technol. 2015;45(18):1999–2042.
- Rodrigues C.,Vandenberghe L. P. S.,Boza A. P. O., Teordoro J.,Miayoka M.,Soccol C. R. Optimization of citric acid production by solid state fermentation using citric pulp as substrate. In: SIMPÓSIO NACIONAL DE BIOPROCESSOS, 16, 2007, Curitiba. Anais, Curitiba. 2007.
- Uenojo M. Production and characterization of fruit flavors by pectinolytic microorganisms using agroindustrial residues. Dissertation – Post-graduation program in Food Engineering, State University of Campinas. .
- Moraes I. O.,Arruda R. O. M.,Moraes R. O. Bioproducts for organic agriculture. In: National simpósio de bioprocessos, 16, Curitiba, 2007. Anais. 2007.
- Sørensen A.,Lübeck P. S.,Lübeck M.,Teller P. J.,Ahring B. K. β-Glucosidases from a new Aspergillus species can substitute commercial β-glucosidases for saccharification of lignocellulosic biomass. Canadian Journal of Microbiology. 2011;57(8):638–650. https://doi.org/10.1139/w11-052.
- Borin G. P., Sanchez C. C.,De Souza A. P., De Santana E. S.,De Souza A. T., Leme A. F. P., et al. Comparative Secretome Analysis of Trichoderma reesei and Aspergillus niger during Growth on Sugarcane Biomass. PLoS ONE. 2015;10(6):1-20. 0129275. doi:10.1371/journal.pone. 0129275.
- Souza F. M., Aquino L. C. L. Potential of mangaba seed meal for the lipase production of Aspergillus niger: Influence of temperature and humidity in the process. Scientia Plena. 2012;8:12.
- Do Filho W. B. N.,Franco C. R. Evaluation of the potential of waste produced through agro-industrial processing in Brazil. Rev. Virtual Quim. 2015;7(6):1968-1987.
- Vieira G. S. Development and characterization of cereal bars with mango. Monography (Completion of Course in Food Engineering) – Federal University of Sergipe, UFS, Sergipe. 2007.
- Santos R. M. Production and characterization of bio-oil from agroindustrial residue of mangaba seeds. São Cristóvão, 2014. 82p. Dissertation (Master in Chemistry) – Federal University of Sergipe. 2014.
- Sluiter A., Hames B., Hyman D., Payne C., Ruiz R., Scarlata C., Sluiter J., Templeton D., Nrel J. W. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. 2008;9. doi:NREL/TP-510-42621
- Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J., Templeton D., Crocker D. Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP). Nrel/Tp-510-42618; doi: NREL/TP-510-42618.
- Association Of Official Analytical Chemists (Aoac). Official methods of analysis of the Association of the Analytical chemists. 18th ed. Whashington. 2012;15.
- Padilha C. E. A., Dantas P. V. F.,Nogueira C. C.,Leitão A. L. S.,Almeida H. N., Souza D. F. S.,Oliveira J. A.,Macedo G. R., Santos E. S. Enhancing the recovery and concentration of polyphenols from camu-camu (Myrciaria dubia H.B.K. McVaugh) by aqueous two-phase flotation and scale-up process. Separation Science and Technology. 2018;53(13):2126-2135. DOI: 10.1080 / 01496395.2018.1442865.
- Moretto E.,Fett R. Technology of Vegetable Oils and Fats in the Food Industry, 1st. Ed., Varela: São Paulo. 1998.
- AOCS Official methods and recommended practices (6th ed.).American Oil Chemists’ Society: AOCS Press. 2011.
- Singleton V. L.,Rossi J. A. J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16(3):144-158.
- Herrero M., Ibañez E.,Señorans F. J.,Cifuentes A. J. Pressurized liquid extracts from Spirulina platensis microalga: Determination of their antioxidant activity and preliminary analysis by micellar electrokinetic chromatography. Journal of Chromatography A. 2004;1047(2):195–203.
- Couri S. Effect of cations on aggregate morphology and the production of polygalacturonase by Aspergillus niger mutant 3T 5B8. 198 f. Thesis (Doctorate in Sciences). Post-graduation in Biochemical Process Technology. Federal University of Rio de Janeiro. Rio de Janeiro. 1993.
- Paula A. V. Restructuring of milk fat by enzymatic interesterification using immobilized lipase: optimization of reactional and operational conditions. Thesis (PhD in Sciences – Graduate Program in Industrial Biotechnology in the area of Applied Microbiology) – School of Engineering of Lorena, University of São Paulo. 2011;212.
- Minjares-Cassanco A., Trejor-Aguillas B. A.,Aguilar G. Physiological comparison between pectinase producing mutants of Aspergillus niger adopted either to solid state fermentation or submerged fermentation. Enzyme Microb. Technol. 1997;21:26-27.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1–2):248–254.
- Melzer M.,Blin J.,Bensakhriac A.,Valette J., Broust F. Pyrolysis of extractive rich agroindustrial residues. Journal of Analytical and Applied Pyrolysis. 2013;104:448–460.
- Araújo K. B.,Santos R. C. A., Souza F. M., Aquino L. C. L. Protein enrichment of mangaba seed meal with Rhizopus oryzae: optimization using surface response methodology. Technology & Agricultural Science. 2011;5(4):45-50.
- Kobori C. N.,Jorge N. Characterization of the oils of some fruit seeds as use of industrial waste. Ciênc. Agrotec. 2005;29(5):1008-1014.
- Pereira C. S. S. Evaluation of different technologies in the extraction of Jatropha curcas L. Seropédica, RJ. 2009;86. Dissertation – (Master in Chemical Engineering), Institute of Technology, Federal Rural University of Rio de Janeiro. UFRRJ.
- Brazil. Ministry of Health. National Agency of Sanitary Surveillance (ANVISA). 2005. Resolution RDC No. 270 of September 22, 2005. Approves the Technical Regulation: “Identity and Quality Fixation of Vegetable Oils and Fats”. Available at: (http://www.anvisa.gov.br). Accessed on 15 Jan. 2019.
- Cecchi H. M. Theoretical and practical foundations in food analysis. Publisher of Unicamp: 2nd Ed. Rev.- Campinas / SP, publisher of UNICAMP. 2003;20.
- Teixeira A. Z.,Nascimento M. M. F.,Rodrigues S. Á .,Trindade J. L. F. Physical-chemical characterization, bio-conversion of agroindustrial residues and comparison of inoculation methods. Revista Brasileira de Tecnologia Agroindustrial. 2011;5:450-457.
- Jorge N., Luiza D. M. M. Characterization of the oils of the seeds of Pachira aquatica Aublet for food use. Acta Amazonica. 2012;42:149-156.
- MOURA C. M. Physical-chemical, nutritional and sensory characteristics of bread with flaxseed addition. 2008. [Doctoral Thesis in Food Science and Technology]. São Paulo: Luiz de Queiroz College of Agriculture, University of São Paulo, Piracicaba. 2008.
- Marques A. C. Functional properties of linseed (Linum usitatissimum L.) under different conditions of preparation and use in food. [Masters dissertation]. Santa Maria, RS: Rural Sciences Center of the Federal University of Santa Maria, Rio Grande do Sul. 2008.
- Codex Alimentarius Commission. Codex Stan 210: codex standards for named vegetable Oils. Rome: Codex Alimentarius. 2008.
- Almeida S. P., De Costa T., Da S. A., Silva J. A. da Cerrado Native Fruits: Physical-chemical characterization and potential nutrient source. in: CERRADO ecology and flora. ed. technicians Sueli Matiko Sano, Semiramís Pedrosa de Almeida and José Felipe Ribeiro. 1(06):353-381. Embrapa Cerrado. Brasília, DF: Embrapa Information Technology. 2008;2:1279.
- Ramalho H. F.,Suarez P. A. Z. A. Chemistry of Oils and Fats and their Extraction and Refining Processes. Rev. Virtual Quim. 2013;5(1):2-15.
- Soong Y. Y.,Barlow P. J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry. 2004;88:411- 417.
- Rodrigues C. M.,Brito A. R. M. S.,Hiruma-Lima C. A., Vilegas W. Chemical constituents of the barks of Hancornia speciosa Gomes (Apocynaceae). 29th Annual Meeting of the Brazilian Chemical Society, 2006. Available at: http://sec.sbq.org.br/cd29ra/resumos/T1814-2.pdf. Accessed on: August 15. 2018.
- Rodrigues C. M. Qualitative and quantitative characterization of secondary metabolites in plant extracts. Thesis (Doctorate in Chemistry) – Institute of Chemistry, Paulista State University, Araraquara. 2007;199.
- Nora C. D.,Müller C. D.,Bona G. S., Rios A. O.,Hertz P. F., Jablonski A.,Jong E. V.,Flôres S. H. Effect of processing on the stability of bioactive compounds from red guava (Psidium cattleyanum Sabine) and guabiju (Myrcianthes pungens). Journal of Food Composition and Analysis. 2014;34(1):18-25.
- Tomas M.,Toydemir G.,Boyacioglu D.,Hall R.,Beekwilder J. C. E. The effects of juice processing on black mulberry antioxidants. Food Chemistry. 2015;186:277-284.
- Branco I. G.,Moraes I. C.,Madron G. S. A.,Santos C.,Ruz A. L., Carvalho J. E.,Haminuik C. W. I. Influence of pasteurization on antioxidant and in vitro anti-proliferative effects of jambolan (Syzygium cumini (L.) Skeels) fruit pulp. Industrial Crops and Products. 2016;89:225-230.
- Tournour H.,Segundo M., Magalhaes L.,Barreiros L.,Queiroz J.,Cunha L. M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties November.Industrial Crops and Products. 2015;74:397-406. DOI: 10.1016/j.indcrop. 2015.05.055.
- Machado A. P. F., Pasquel-Reátegui J. L.,Barbero G. F.,Martínez J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: a comparison with conventional methods. Food Research International. 2015;77(3):675-683.
- Dutra R. L. T. Evaluation of the in vitro bioaccessibility of phenolic compounds in mangaba (Hancornia speciosa), seriguela (Spondias purpurea) and umbu-cajá (Spondias spp.). Dissertation (Master in Food Science and Technology), Post-Graduate Program in Food Science and Technology, Technology Center, Federal University of Paraíba. 2017.
- Lima J. P.,Fante C. A., Pires C. R. F.,Nunes E. E.,Alves R. R.,Elias H. H. S., Nunes C. A.,Boas E. V. V., De B. The antioxidative potential and volatile constituents of mangaba fruit over the storage period. Sci. Hortic. 2015;194:1–6.
- Himmel M. E.,Ding S. Y., Johnson D. K., Andey W. S., Nimlos M. R., Brady J. W., Foust T. D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807.
- Li C.,Wang Q., Zhao Z. K. Acid in ionic liquid: An efficient system for hydrolysis of lignocellulose. Green Chem. 2008;10:177–182.
- Oliveira S. D.,Padilha C. E. De A.,Azevedo E. A., Pimentel V. C.,Araújo F. R.,Macedo G. R.,Santos E. S. Utilization of agroindustrial residues for producing cellulases by Aspergillus fumigatus on Semi-Solid Fermentation. Journal of Environmental Chemical Engineering. 2018;6:937–944.
- Reinehr C. O., Rizzardi J.,Silva M. F.,Oliveira D., Treichel H., Colla L. M. Production of lipases from Aspergillus niger and Aspergillus fumigatus by solid state fermentation, evaluation of substrate specificity and its use in esterification and alcohololysis reactions. Quim. Nova. 2014;37(3):454-460.
- Damaso M. C. T., Passianoto M. A., Freitas S. C., Freire D. M. G., Lago R. C. A And Couri S. “Utilization of agroindustrial residues for lipase production by solid-state fermentations”. Braz J Microb. 2008;39:676-681.
- Stat Soft Inc: Stat Soft, http://www.statsoft.com/. 2005.
- Magalhães E. R. B., Silva F. L.,Sousa M. A., Dos S. B.,Santos E. S. Use of different agro-industrial wastes and produced water for biosurfactant production Biosci. Biotech. Res. Asia. 2018;15(1):17-26.
- Silva F. L.,De Campos O. A.,Dos Santos D. A.,De Júnior S. D. O., De Padilha C. E. A., De Junior F. C. S., De Macedo G. R., Dos Santos E. S. Pretreatments of Carnauba (Copernicia prunifera) straw residue for production of cellulolytic enzymes by Trichorderma reesei CCT-2768 by solid state fermentation. Renew. Energy. 2018;116:299–308.

This work is licensed under a Creative Commons Attribution 4.0 International License.





