How to Cite | Publication History | PlumX Article Matrix
Mycobiota and Mycotoxins Contaminating Rice Grains in El-Minia, Governorate, Egypt
Moharram A. M1, Yasser M. M*2, Sayed M. A2, Omar O. A3 and Idres M. M. M2
1Department of Botany and Microbiology, Faculty of Science, Assiut University, Egypt.
2Department of Botany and Microbiology, Faculty of Science, Beni-Suef University, Egypt.
3Department of Agricultural Microbiology, Faculty of Agriculture, Minia University, Egypt.
Corresponding Author E-mail: manal_yaser2006@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2734
ABSTRACT: The mycological analysis of 51 samples of rice grains collected from different localities in El-Minia Governorate revealed the isolation of 54 species of fungi belonging to 21 genera. Most common mycobiota (genera) were Aspergillus and Penicillium being isolated from 96.07% and 54.9% of samples contributing 63.08% and 21.89% of total fungal counts. The prevalent species were represented by Aspergillus flavus, A. candidus, A. niger, Penicillium chrysogenum, P. islandicum especially on Dichloran Rose Bengal Chloramphenicol Agar medium (DRBC). These species in addition to some osmophilic fungi including A. chevalieri, A. montevidensis, A. rubrum were also common when Dichloran Glycerol agar (DG18) was used for the culturing of rice samples. About 12.5% of samples analysed for natural occurrence of mycotoxins were contaminated either with Aflatoxin – B1 (100-200 µg/ kg), ochratoxin –A (50-100 µg/ kg) or sterigmatocystin (10-20 µg/ kg). The majority of fungal strains tested for their mycotoxin production in liquid cultures were able to produce variable levels of aflatoxin B1, Aflatoxin G1 , Ochratoxin –A , terrein , gliotoxin and fumagillin
KEYWORDS: Aflatoxin B1; B2 and G1; Mycotoxins; Rice
Download this article as:| Copy the following to cite this article: Moharram A. M, Yasser M. M, Sayed M. A, Omar O. A, Idres M. M. M. Mycobiota and Mycotoxins Contaminating Rice Grains in El-Minia, Governorate, Egypt. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Moharram A. M, Yasser M. M, Sayed M. A, Omar O. A, Idres M. M. M. Mycobiota and Mycotoxins Contaminating Rice Grains in El-Minia, Governorate, Egypt. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2VKiczh |
Introduction
Rice is one of the most famous and important cereals worldwide. According to1 the cultivated area was estimated as 156 million hectars producing about 721 million metric tons (MMT) of rice. The most important world rice producers are China (202.3 MMT), and India (154.5 MMT). Rice is one of the most important commercial crops planted in Egypt. It is a privileged source of carbohydrates and proteins. It is used for different food and nonfood products. The foods include cooked rice, rice flour, breakfast cereals, and desserts. The inedible rice hull is used as fertilizer, fuel, and others, while the bran is a source of cooking oil. Straw from the stems and leaves is used as feeding or bedding for animals and for making roofs, bricks, hats, sandals and baskets. Rice bran and straws are also used as suitable substrates in mushroom cultivation.2
Fungal contamination of cereals is an important issue for grain quality and from consumer’s health point of view. Rice is one of the famous cereals which favor mycotoxin contamination.3 In recent years, there have been many studies from various countries on the occurrence of high levels of aflatoxins by fungal rice contamination.4,5,6 In 2001, Reddy and Sathyanarayana7 listed 143 fungal species from rice. Reddy et al.8 published a review containing the important groups of mycotoxins including aflatoxins (AFS), fumonisins, ochratoxin A(OTA), deoxynivlenol, and zearalenone (ZEN), which contaminated rice in different countries. Sempere & Santamarina9 confirmed that the majority of mycotoxins were produced by Aspergillus, Penicillium, and Fusarium, Ferre10 further found that AFS, citrinin, deoxynivalenol, fumonisins, sterigmatocystin, ZEN, cyclopiazonicacid, gliotoxin, patulin and some trichothecenes are the main mycotoxins that have been identified in rice with a high variable of contaminated varieties and at different infected levels.
In Egypt, several studies on fungi and mycotoxins have been focused on food grains including rice since rice is the main food of most people in the world, the presence of mycotoxin contamination can be a serious health risk, corn, soybean and peanuts.11,12 Therefore, the present study was carried out for identification of species of fungi contaminating rice grains as well as for evaluation of mycotoxin levels in the tested samples and in cultures of toxinogenic fungi.
Materials and Methods
Collection of Rice Samples
A total of 51 rice samples were collected from the market at different localities of El-Minia governorate covering El-Edwa, Maghagha, Bni Mazar, Mattay, Samalott, Minia, Abu-Qurqas, Mallawi, Deir Mawas between March 2015 and March 2016 (Fig.1). Collected samples kept in plastic bags were transported immediately to laboratory and kept at 5-7°C in the refridgerator till mycological and mycotoxin analysis were carried out on them.
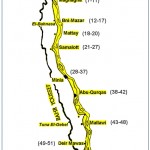 |
Figure 1: Map of El-Minia Governorate showing different places from which rice samples were collected.
|
Isolation of Fungi
The method of seed-plate (direct plating) was utilized to determine the seed borne fungi on the rice grains. The grains were then plated on a suitable isolation media at a plating rate of 5 rice grains per plate and four replicates for each rice sample.13
A general purpose enumeration agar medium, Dichloran Rose Bengal Chloramphenicol Agar: DRBC, which contained (g/l of distilled water): glucose 10, peptone 5, potassium dihydrogen phosphate 1, magnesium sulphate 0.5, dichloran 0.002 (0.2% in ethanol 1ml), rose bengal 0.025, chloramphenicol 0.1, agar 15, pH 5.6.
A selective isolation medium, Dichloran 18 % Glycerol agar, (DG18) was used to detect and isolate xerophilic fungi, the medium DG18 containing (g/l of distilled water): peptone 5, glycerol 220, chloramphenicol 0.1, glucose 10, potassium dihydrogen phosphate 1, magnesium sulphate 0.5, dichloran 0.002 (0.2% in ethanol 1 ml), agar 15, final pH 5.6. Both DRBC and DG18 media were prepared as described by 13.
All plates were inoculated with rice grains (5 grains/plate). Cultures in quadriplicates were incubated for 7-8 days at 30°C but plates containing DG18 were incubated for 14 days to allow growth of slow growing fungi.
Identification of Isolated Fungi
Fungi isolated from rice grain samples were transferred to fresh Czapek`s Dox medium in Petri dishes and slant media bottles for identification. Morphological and cultural characteristics of the growing fungi were evaluated for preliminary identification. Then fungal colonies were subjected to microscopic identification according to.14-20
Analysis of Mycotoxins
The isolated fungi were screened for mycotoxin production by growing on Potato Dextrose Broth (PDB). Erlenmeyer flasks (250 ml) containing 50 ml aliquots of Potato Dextrose Broth were autoclaved, inoculated with fungi, and incubated for 7 days at 30°C. After incubation period, the extraction of mycotoxins was carried out according to.21-23 The flask contents were blended using surface sterilized hand free blender and 50 ml of chloroform was added to flasks which were shaken for 24 hours. Cultures were filtered using what man NO.1 filter paper. Fifty ml of this filtrate was shaken with an equal volume of chloroform for 30 min. The chloroform layer was separated using a separating funnel and filtered again over a bed of anhydrous sodium sulfate. Porcelain chips were added to flasks containing filtrates and were steam evaporated.
Estimation of Mycotoxins
Thin layer chromatography (TLC) was used for detection of mycotoxins.24 About 50 µl of chloroform extract of the mycotoxin was applied on silica gel plates together with specific standards developed with mobile phase methanol: chloroform (4:96) and observed under long wave length UV light(365 nm) in a UV chamber CN-15. LC Vilber Lourmat, France. Qualitative detection of mycotoxins was done on the basis of their fluorescence and retention factor (RF) values.25
Mycotoxin Detection in Rice Samples
Twenty four samples of rice grains were chosen for this part of study. Samples were selected on the basis of their content of potentially toxinogenic fungi.
One hundred grams of rice grains and 150 ml chloroform were mixed in 250 ml Erlenmeyer flasks. Flasks were shaken for 24 hours then filtered through What man NO.1 filter paper. Detection of mycotoxins in the rice extract was done as mentioned above using TLC plates.
Results
Using two isolation media (DRBC and DG18) it was possible to isolate and identify 54 fungal species attributed to 21 genera from the tested rice samples.
It was observed that on the on DRBC medium as shown in Table 2 the total gross fungal population reached 653 colonies per 20 grains in all samples. The mycological analysis of 51 samples revealed the isolation of 52 species related to 20 genera of fungi. Aspergillus was the most dominant genus, being isolated from 49 samples matching 96.07% of rice samples and 63.08% of total fungal population. Nineghteen species of Aspergillus were isolated of which A. flavus was the most dominant species (62.74% of samples). Two species of Aspergillus occurred in moderate incidence and these were A. candidus and A. niger (35.29% and 45.09% of samples matching 8.57% and13.93% of total fungal population respectively). A. terreus was isolated in low frequency being recovered from 17.64% of rice samples accounting for 2.14% of total fungal count.
Table 1: Total count (colonies/20 grains) and number of species (NS) of fungal species isolated from rice grains.
| Sample no | Grade | Locality | Level of break | DRBC | DG18 | ||
| TC | NS | TC | NS | ||||
| 1 | 3 | El-Edwa | High | 13 | 4 | 20 | 9 |
| 2 | 2 | El-Edwa | Moderate | 23 | 9 | 9 | 8 |
| 3 | 3 | El-Edwa | High | 4 | 11 | 27 | 11 |
| 4 | 3 | El-Edwa | High | 18 | 7 | 15 | 7 |
| 5 | 3 | El-Edwa | High | 30 | 7 | 49 | 7 |
| 6 | 3 | El-Edwa | High | 9 | 6 | 32 | 9 |
| 7 | 1 | Maghagha | Low | 25 | 6 | 15 | 4 |
| 8 | 3 | Maghagha | High | 13 | 6 | 34 | 8 |
| 9 | 2 | Maghagha | Moderate | 11 | 6 | 10 | 10 |
| 10 | 3 | Maghagha | High | 22 | 6 | 23 | 7 |
| 11 | 2 | Maghagha | Moderate | 10 | 5 | 1 | 5 |
| 12 | 2 | Bni-Mazar | Moderate | 29 | 7 | 32 | 7 |
| 13 | 2 | Bni-Mazar | Moderate | 18 | 4 | 18 | 5 |
| 14 | 1 | Bni-Mazar | Low | 7 | 11 | 12 | 5 |
| 15 | 1 | Bni-Mazar | Low | 1 | 7 | 1 | 7 |
| 16 | 3 | Bni-Mazar | High | 14 | 2 | 13 | 2 |
| 17 | 1 | Bni-Mazar | Low | 14 | 4 | 6 | 5 |
| 18 | 3 | Mattay | High | 22 | 6 | 23 | 3 |
| 19 | 3 | Mattay | High+ Rice weevil | 15 | 2 | 7 | 4 |
| 20 | 1 | Mattay | Low | 10 | 4 | 36 | 8 |
| 21 | 3 | Samalott | High | 27 | 2 | 28 | 5 |
| 22 | 3 | Samalott | High + Rice weevil | 2 | 2 | 9 | 6 |
| 23 | 3 | Samalott | High | 2 | 2 | 25 | 3 |
| 24 | 1 | Samalott | Low | 1 | 1 | – | – |
| 25 | 3 | Samalott | High | 9 | 1 | 35 | 1 |
| 26 | 2 | Samalott | Moderate | 16 | 7 | 11 | 5 |
| 27 | 3 | Samalott | High | 21 | 7 | 7 | 11 |
| 28 | 3 | El- Minia | High | 38 | 8 | 17 | 8 |
| 29 | 3 | El- Minia | High | 18 | 4 | 30 | 10 |
| 30 | 3 | El- Minia | High | 3 | 2 | 9 | 9 |
| 31 | 2 | El- Minia | Moderate | 9 | 4 | 11 | 10 |
| 32 | 3 | El- Minia | High | 2 | 5 | 22 | 11 |
| 33 | 3 | El- Minia | High | 12 | 1 | 33 | 7 |
| 34 | 1 | El- Minia | Low | 9 | 2 | 14 | 6 |
| 35 | 3 | El- Minia | High | 2 | 1 | 32 | 6 |
| 36 | 3 | El- Minia | High | 7 | 4 | 42 | 3 |
| 37 | 1 | El- Minia | Low | 11 | 4 | 10 | 7 |
| 38 | 3 | Abu-Qurqas | High | 10 | 3 | 27 | 12 |
| 39 | 1 | Abu-Qurqas | Low | 16 | 3 | 9 | 5 |
| 40 | 2 | Abu-Qurqas | Moderate | 22 | 8 | 12 | 4 |
| 41 | 3 | Abu-Qurqas | High | 17 | 8 | 15 | 1 |
| 42 | 3 | Abu-Qurqas | High | 3 | 4 | 32 | 11 |
| 43 | 3 | Mallawi | High | 15 | 3 | 5 | 7 |
| 44 | 2 | Mallawi | Moderate | 6 | 3 | 12 | 6 |
| 45 | 2 | Mallawi | Moderate | 16 | 2 | 24 | 13 |
| 46 | 3 | Mallawi | High | 9 | 1 | 13 | 12 |
| 47 | 1 | Mallawi | Low | 3 | 3 | 9 | 11 |
| 48 | 3 | Mallawi | High | 3 | 4 | 39 | 9 |
| 49 | 3 | Deir Mawas | High | 8 | 3 | 22 | 9 |
| 50 | 1 | Deir Mawas | Low | 20 | 6 | 6 | 5 |
| 51 | 2 | Deir Mawas | Moderate | 8 | 1 | 23 | 14 |
Grade 1= less than 5%, grade 2 = from 5% to 10% and grade 3 = more than 10%.
The genus Penicillium appeared in high incidence (54.90% of samples) giving rise to 21.89% of total fungi. Eleven species of Penicillium were recovered, among which P. chrysogenum and P. islandicum were of low incidence (17.64% and 19.60% of rice samples respectively).
Each of Alternaria and Cladosporium were found to contaminate low number of rice samples (15.68% and 21.56%) participating in fungal population with 2.45% and 3.98% respectively.
On DG18
A total of 47 species of fungi belonging to 16 genera were isolated. Aspergillus was the most dominant genus (49 samples matching 96.07% of rice samples) representing 65.51% of total fungal counts. It was represented by 19 species, of which A. candidus and A. niger prevaled in 52.94% and 50.98% of samples matching 8.28% and 6.93% of total fungal counts respectively as shown in Table (2).
Table 2: Total count (TC), percentage total count (%TC), Incidence (I) out of 51 samples and percentage incidence (%I) of fungal species recovered from rice samples on DRBC and DG18 Media.
| Fungal species | Types of media | |||||||
| DRBC | DG18 | |||||||
| TC | %TC | I | %I | TC | %TC | I | %I | |
| Lichtheimia corymbifera | 15 | 2.29 | 4 | 7.84 | 18 | 1.86 | 8 | 15.68 |
| Alternaria alternata | 16 | 2.45 | 8 | 15.68 | 34 | 3.51 | 13 | 25.49 |
| Aspergillus | 412 | 63.08 | 49 | 96.07 | 633 | 65.51 | 49 | 96.07 |
| A. aegyptiacus | 2 | 0.3 | 1 | 1.96 | – | – | – | – |
| A. amstelodami | 5 | 0.76 | 5 | 9.8 | 2 | 0.2 | 2 | 3.92 |
| A. candidus | 56 | 8.57 | 18 | 35.29 | 80 | 8.28 | 27 | 52.94 |
| A. chevalieri | 17 | 2.6 | 3 | 5.88 | 44 | 4.55 | 16 | 31.37 |
| A. clavatus | 11 | 1.68 | 4 | 7.84 | 15 | 1.55 | 8 | 15.68 |
| A. flavipes | 7 | 1.07 | 3 | 5.88 | 10 | 1.03 | 5 | 9.8 |
| A. flavus | 137 | 20..98 | 32 | 62.74 | 48 | 4.96 | 19 | 37.25 |
| A. flavus var. columnaris | 8 | 1.22 | 3 | 5.88 | 61 | 6.31 | 13 | 25.49 |
| A. fumigatus | 9 | 1.37 | 4 | 7.84 | 16 | 1.65 | 8 | 15.68 |
| A. montevidensis | 5 | 0.76 | 4 | 7.84 | 57 | 5.9 | 23 | 45.09 |
| A. niger | 91 | 13.93 | 23 | 45.09 | 67 | 6.93 | 26 | 50.98 |
| A. ochraceus | 5 | 0.76 | 5 | 9.8 | 15 | 1.55 | 8 | 15.68 |
| A. oryzae | – | – | – | – | 9 | 0.93 | 8 | 15.68 |
| A. parasiticus | 2 | 0.3 | 2 | 3.92 | 6 | 0.62 | 5 | 9.8 |
| A. rubrum | 10 | 1.53 | 3 | 5.88 | 84 | 8.69 | 17 | 33.33 |
| A. sydowii | 1 | 0.15 | 1 | 1.96 | 11 | 1.13 | 5 | 9.8 |
| A. tamarii | 1 | 0.15 | 1 | 1.96 | 6 | 0.62 | 4 | 7.84 |
| A. terreus | 14 | 2.14 | 9 | 17.64 | 16 | 1.65 | 9 | 17.64 |
| A. versicolor | 29 | 4.44 | 5 | 9.8 | 85 | 8.79 | 16 | 31.37 |
| A. wentii | 2 | 0.3 | 2 | 3.92 | 1 | 0.1 | 1 | 1.96 |
| Cladosporium | 26 | 3.98 | 11 | 21.56 | 39 | 4.03 | 14 | 27.45 |
| C. cladosporioides | 8 | 1.22 | 4 | 7.84 | 19 | 1.96 | 6 | 11.76 |
| C. herbarum | 10 | 1.53 | 5 | 9.8 | 12 | 1.24 | 6 | 11.76 |
| C. sphaerospermum | 8 | 1.22 | 4 | 7.84 | 8 | 0.82 | 4 | 7.84 |
| Cochliobolus | 2 | 0.3 | 2 | 3.92 | – | – | – | – |
| C. lunatus | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| C. spicifer | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| Fusarium | 10 | 1.53 | 5 | 9.8 | 24 | 2.48 | 8 | 15.68 |
| F. semitectum | 4 | 0.61 | 3 | 5.88 | 22 | 2.27 | 8 | 15.68 |
| F. verticillioides | 6 | 0.91 | 2 | 3.92 | 2 | 0.2 | 2 | 3.92 |
| Geosmithia sp. | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| Geotrichum candidum | 5 | 0.76 | 3 | 5.88 | 1 | 0.1 | 1 | 1.96 |
| Gliocladium roseum | 1 | 0.15 | 1 | 1.96 | 4 | 0.41 | 3 | 5.88 |
| Hyalodendron sp. | – | – | – | – | 1 | 0.1 | 1 | 1.96 |
| Mucor circinelloides | 8 | 1.22 | 3 | 5.88 | 8 | 0.82 | 3 | 5.88 |
| Nigrospora oryzae | 2 | 0.3 | 2 | 3.92 | 11 | 1.13 | 6 | 11.76 |
| Paecilomyces sp. | 1 | 0.15 | 1 | 1.96 | 4 | 0.41 | 3 | 5.88 |
| Penicillium | 143 | 21.89 | 28 | 54.9 | 178 | 18.42 | 32 | 62.74 |
| P. aurantiogriseum | 6 | 0.91 | 4 | 7.84 | 22 | 2.27 | 7 | 13.72 |
| P. chrysogenum | 12 | 1.83 | 9 | 17.64 | 20 | 2.07 | 9 | 17.64 |
| P. citrinum | 21 | 3.21 | 5 | 9.8 | 30 | 3.1 | 11 | 21.56 |
| P. corylophilum | 2 | 0.3 | 1 | 1.96 | 3 | 0.31 | 3 | 5.88 |
| P. crustosum | 17 | 2.6 | 6 | 11.76 | 15 | 1.55 | 6 | 11.76 |
| P. duclauxii | 3 | 0.45 | 2 | 3.92 | 13 | 1.34 | 8 | 15.68 |
| P. glabrum | 10 | 1.53 | 5 | 9.8 | 14 | 1.44 | 7 | 13.72 |
| P. islandicum | 38 | 5.81 | 10 | 19.6 | 23 | 2.38 | 8 | 15.68 |
| P. oxalicum | 7 | 1.07 | 3 | 5.88 | 12 | 1.24 | 8 | 15.68 |
| P. pinophilum | 1 | 0.15 | 1 | 1.96 | 1 | 0.1 | 1 | 1.96 |
| P. thomii | 26 | 3.98 | 4 | 7.84 | 25 | 2.58 | 3 | 5.88 |
| Rhizopus oryzae | 4 | 0.61 | 2 | 3.92 | 6 | 0.62 | 2 | 3.92 |
| Scopulariopsis koningii | 1 | 0.15 | 1 | 1.96 | 1 | 0.1 | 1 | 1.96 |
| Quambalaria cyanescens | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| Trichoderma harzianum | 2 | 0.3 | 2 | 3.92 | 3 | 0.31 | 1 | 1.96 |
| Trichurus spiralis | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| Ulocladium chartarum | 1 | 0.15 | 1 | 1.96 | 1 | 0.1 | 1 | 1.96 |
| Wallemia sebi | 1 | 0.15 | 1 | 1.96 | – | – | – | – |
| Total fungal counts | 653 | – | – | – | 966 | – | – | – |
High incidence= 50-100%.
Moderate =25-< 50%.
Low=13-< 25%.
Rare=1-12.
Six species of Aspergillus appeared in moderate incidence and these were A. chevalieri, A. flavus, A. flavus var. columnaris, A. montevidensis, A. rubrum and A. versicolor (31.37%, 37.25%, 25.49%, 45.09%, 33.33% and 31.37% respectively). The following 5 species of Aspergillus (A. clavatus, A. fumigatus, A. ochraceus, A. oryzae and A. terreus) were found to contaminate low number of rice samples (15.68%, 15.68%, 15.68%, 15.68% and 17.64%) participating in fungal population with 1.55%, 1.65%, 1.55%, 0.93% and 1.65% respectively.
The genus Penicillium appeared in high incidence (62.74% of samples) giving rise to 18.42% of total fungi. Eleven species of Penicillium were recovered, among which, P. aurantiogriseum, P. chrysogenum, P. citrinum, P. duclauxii, P. glabrum, P. islandicum and P. oxalicum were of low incidence (13.72%, 17.64%, 21.56%, 15.68%, 13.72%, 15.68% and 15.68% respectively).
Each of Alternaria and Cladosporium occurred in moderate incidence (25.49 % and 27.45%) participating in fungal population with 3.51% and 4.03% respectively. Lichtheimia corymbifera was found to contaminate low number of rice samples (15.68%) accounting for 1.86% of total fungal count.
The majority of fungi isolated on DRBC were also recovered on DG18 but the following differences were observed:
Each of A. chevalieri, A. montevidensis and A. rubrum were isolated in moderate frequency on DG18 but they were of rare incidence on DRBC. This is often due to the osmophilic character of these species.
Aspergillus niger which occurred in high frequency (50.95%) on DG18 , was found to be of moderate incidence on DRBC (45.09%). On the other hand A. flavus occurred in high incidence on DRBC (62.74%) but was moderately found on DG18 (37.25%).
Each of A. clavatus, A. fumigatus and A. ochraceus were of low frequency on DG18 but they were rare on DRBC. Aspergillus oryzae occurred in low incidence on DG18 (15.68%), while it was absent on DRBC.
Cladosporium genus occurred in low incidence on DRBC (21.56%) but was moderately found on DG18 (27.45%).
Each of Penicillium aurantiogriseum, P. citrinum, P. duclauxii, P. glabrum and P. oxalicum, were isolated in low frequency on DG18 but they were of rare incidence on DRBC.
Fusarium genus and F. semitectum which occurred in low incidence on DG18 (15.68%) was found to be of rare frequency on DRBC (9.80% and 5.88%).
Lichtheimia corymbifera appeared in low frequency on DG18 (15.68%) but was rarely found on DRBC (7.84%), Alternaria alternata was isolated in moderate frequency on DG18 (25.49%) but it was of low incidence on DRBC (15.68%).
Natural Occurrence of Mycotoxins
With reference to Table (3), twenty four samples of rice were randomly selected and analyzed for natural occurrence of mycotoxins, only 3 samples were found contaminated with 3 different types of mycotoxins namely streigmatocystin (10-20 µg/ kg), ochratoxin-A (50-100 µg/ kg ) and aflatoxin B1(100-200 µg/ kg) ranging from 10 to 200 µg/ kg. These samples were collected from El- Minia City (NO.33), Mattay (NO.19) and Samalott (NO.22), respectively as shown in Table 3. Other tested rice samples were free from any mycotoxin contamination. All of the toxin contaminated samples were of grade 3 where more than 10% of grains were broken. Moreover, samples 19 and 22 were infested with rice weevil. The presence of A. flavus is of main concern because the fungus is a potent aflatoxin producer.
Table 3: Natural occurrence of mycotoxins in rice grain samples.
| Rice samples (Locality) | Mycotoxins | Level(ug/ kg) |
| Sample 2 (El-Edwa) | -ve | -ve |
| Sample 5 (El-Edwa) | -ve | -ve |
| Sample 6 (El-Edwa) | -ve | -ve |
| Sample 7 (Maghagha) | -ve | -ve |
| Sample 8 (Maghagha) | -ve | -ve |
| Sample 10 (Maghagha) | -ve | -ve |
| Sample 13 (Benimazar) | -ve | -ve |
| Sample 14 (Benimazar) | -ve | -ve |
| Sample 16 (Benimazar) | -ve | -ve |
| Sample 19 (Mattay) | Ochratoxin-A | 50– 100 |
| Sample 21 (Samalott) | -ve | -ve |
| Sample 22 (Samalott) | Aflatoxin B1 | 100-200 |
| Sample 23 (Samalott) | -ve | -ve |
| Sample 25 (Samalott) | -ve | -ve |
| Sample 29 (EL-Minia) | -ve | -ve |
| Sample 30 (EL-Minia) | -ve | -ve |
| Sample 33 (EL-Minia) | Sterigmatocystin | 20-Oct |
| Sample 38 (Abu-Qurqas) | -ve | -ve |
| Sample 41 (Abu-Qurqas) | -ve | -ve |
| Sample 42 (Abu-Qurqas) | -ve | -ve |
| Sample 43 (Mallawi) | -ve | -ve |
| Sample 45 (Mallawi) | -ve | -ve |
| Sample 46 (Mallawi) | -ve | -ve |
| Sample 49 (Deir Mawas) | -ve | -ve |
Mycotoxins Produced by Fungal Strains
Twenty eight fungal isolates belonging to A. flavus (21 isolates), A. ochraceus (3), A. parasiticus (1), A. fumigatus (1) and A. terreus (2) were analyzed for mycotoxin production using TLC technique. as general these strains were able to produce aflatoxins, Ochratoxin-A, fumagillin, Gliotoxin and terrein, respectively (Table 4).
All isolates of A. flavus produced aflatoxins in variable degrees as shown in Table 4. Levels of aflatoxins (B1, B2, G1, and G2) at levels ranging from 10 to 300 ug/L. A. parasiticus produced B1 and G1 (10-20 ug/L). A. terreus (2 isolates) were able to elaborate terrein (10 -300 ug/L). Ochratoxin-A (20 -200 ug/L) was produced by 3 isolates of A. ochraceus. Finally, A. fumigatus produced Gliotoxin, fumagillin (10-20 ug/L for each).
Table 4: Mycotoxins produced by fungal strains (positive strains).
| Fungal species | Strain No. and Locality | Mycotoxins detected | Level (ug/L) |
| A. flavus | AUMC no. 11399 | Aflatoxin B1 | 20-Oct |
| El-Edwa | Aflatoxin G1 | 20-Oct | |
| A. flavus | -7 | AflatoxinB1 | 20-Oct |
| Maghagha | AflatoxinG1 | 20-Oct | |
| A. flavus | AUMC no.11394 Maghagha | AflatoxinB1 | 200-300 |
| Aflatoxin B2 | 200-300 | ||
| AflatoxinG1 | 20-Oct | ||
| AflatoxinG2 | 20-Oct | ||
| A. flavus | -13 | Aflatoxin B1 | 20-50 |
| Bni-Mazar | Aflatoxin G1 | 20-50 | |
| A. flavus | -14 | AflatoxinB1 | 50-100 |
| Bni-Mazar | Aflatoxin B2 | 200-300 | |
| AflatoxinG1 | 20-Oct | ||
| AflatoxinG2 | 20-Oct | ||
| A. flavus | -18 | Aflatoxin B1 | 20-Oct |
| Mattay | Aflatoxin G1 | 20-Oct | |
| A. flavus | AUMC no. 11395 | Aflatoxin B1 | 200-300 |
| Mattay | Aflatoxin B2 | 200-300 | |
| AflatoxinG1 | 20-Oct | ||
| AflatoxinG2 | 20-Oct | ||
| A. flavus | AUMC no. 11400 Mattay | Aflatoxin B1 | 20-Oct |
| A.flavus | AUMC no. 11396 Samalott | AflatoxinB1 | 50-100 |
| Aflatoxin B2 | 50-100 | ||
| AflatoxinG1 | 20-Oct | ||
| A. flavus | -25 | 50-100 | |
| Samalott | Aflatoxin B1 | ||
| A. flavus | (29) El-Minia | AflatoxinB1 | 200-300 |
| Aflatoxin B2 | 200-300 | ||
| AflatoxinG1 | 20-50 | ||
| AflatoxinG2 | 20-50 | ||
| A. flavus | (30) El-Minia | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. flavus | AUMC no.11398 El-Minia | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. flavus | (33) El-Minia | AflatoxinB1 | 200-300 |
| Aflatoxin B2 | 200-300 | ||
| AflatoxinG1 | 20-Oct | ||
| AflatoxinG2 | 20-Oct | ||
| A. flavus | (34) El-Minia | Aflatoxin B1 | 20-50 |
| Aflatoxin G1 | 20-Oct | ||
| A. flavus | (37) El-Minia | Aflatoxin B1 | 50-100 |
| A. flavus | (38) Abu-Qurqas | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 10-20 | ||
| A. flavus | -42 | 50-100 | |
| Abu-Qurqas | Aflatoxin B1 | ||
| A. flavus | (46) Mallawi | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. flavus | (50) Deir Mawas | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. flavus | (51) Deir Mawas | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. fumigatus | AUMC no.11372 El-Minia | Gliotoxin | 20-Oct |
| Fumagillin | 20-Oct | ||
| A. ochraceus | -4 | OchratoxinA | 20-50 |
| El-Edwa | |||
| A. ochraceus | AUMC no. 11382 El-Minia | OchratoxinA | 20-50 |
| A. ochraceus | -34 | OchratoxinA | 100-200 |
| El-Minia | |||
| A. parasiticus | (8) Maghagha | Aflatoxin B1 | 20-Oct |
| Aflatoxin G1 | 20-Oct | ||
| A. terreus | -2 | Terrein | 200-300 |
| El-Edwa | |||
| A. terreus | -12 | Terrein | 20-Oct |
| Bni-Mazar |
AUMC, Assiut university mycological centre.
Discussions
These results showed that the total fungal counts which were prduced on two medium types were revealed the most predominant genus were Aspergillus, Penicillium, Alternaria, cladosporium and Fusarium respectively.
This finding corresponding with previous studies recorded by26,27 who investigated the mycobiota of rice which were grown on two isolation media including DRBC (dichloran rose- bengal chloramphenicol agar) and DG18 (dichloran 18% glycerol agar) media and he reported that a total of sixty two species related to 34 genera. The broadest species spectrum were from the genera Aspergillus, Penicillium, Eurotium followed by Fusarium, Cladosporium and Cochliobolus. Rice grains were mostly contaminated by Aspergillus candidus, A. flavus, A. niger, A. amstelodami, A. rubrum, Penicillium citrinum, P. oxalicum and Talaromyces spp.
In Swed, Fredlund et al.28 collected 99 rice samples from the Swedish retail market. Aspergillus was the most common fungal genus identified but also Penicillium, Eurotium, Cladosporium, Wallemia, Alternaria, Epicoccum and Trichotecium were isolated. A. flavus presented in 21% of the samples.
Abdel-Hafez et al.29 sampled Egyptian paddy rice and isolated Aspergillus flavus, A. sydowii, A. terreus, A. fumigatus and A. ochraceus) and Penicillium species (P. chrysogenum and P. corylophilum) along with Fusarium oxysporum, Alternaria alternata, Cladosporium cladosporioides, Trichoderma viride and Mucor racemosus. Abd-Allah & Ezzat11 Sampled Paddy rice from EI-Sharkia, E1-Dakahlia , EI-Gharbia, and Kafr E1-Shekh governorates and recorded an average of 6.79 x 104 fungal spores per gram rice. The fungal isolates were 47 species belonging to twenty eight genera. Aspergillus, Cladosporium and Penicillium were the most predominant genera. Aspergilli were represented by 22 species, Aspergillus niger and A. flavus had the highest occurrence. Previous studies on Egyptian rice, reported by El-Shanshoury et al.12 who surveyed the incidence and load of fungi and aflatoxins in maize, wheat and peanut, collected from some markets in central Delta provinces, Egypt.
Other studies on the contamination of storage rice in many regions as compared to this study have been reported.30,31,32,33,2,28,34,35,36,37 Sales and Yoshizawa38,39 studied occurrence of Aspergillus section Flavi in rice from Philippines. Toman et al.40 collected 60 samples of white and parboiled rice purchased from the Czech food market. It was reported that Ochratoxin-A (OTA) analysis showed that 58 samples (96.7%) were found to be positive. OTA levels in white and parboiled rice fluctuated from 0.05 to 0.17 ug/g. These all finding are almost in agreement with our result in this part of the study.
The natural occurrence of mycotoxins in rice has been studied in different countries of the world: In Nigeria,2 studied fungi and some mycotoxins contaminating rice., high levels of AFB1 contamination in rice have also been reported at 200.19± 320.98µg/ kg2 and 37.2±14.0µg/ kg.41 Also,42 determined the mycobiota associated with rice (Oryza sativa), maize (Zea mays), and millet (Pennisetum typhoiodes) in storage.
While43 screened samples of rice, maize, cocoa and cocoa-based powder beverage collected from different markets and stores in south-western Nigeria.
In China, different studies on rice and other cereal contamination with mycotoxins have been reported;44,45,6,46,47 Other studies included those of India;48,49,50,51 Korea;52,53 Spanish;54,55,10 Turkey;56,57 Canada,3 South America.58 and Pakistan.59
In South Vietnam, Trung et al.33 screened twenty five samples of Vietnamese rice coming from the Mekong Delta for fungal contamination. Ergosterol content measurement and total fungal load determination indicated that moulds contamination was quite weak. Identification of fungal species revealed that Aspergillus was the most common genus (43.75 % of isolates) followed by Fusarium (21.8 %) and Penicillium (10.9 %). The presence of toxigenic strains such as A. flavus, A. ochraceus and P. citrinum was confirmed by cultures. Fungal strains were tested for their toxinogenic potential and the results showed that 80 % of A. flavus strains were able to synthesized cyclopiazonic acid (levels up to 32.3 ppm), all strains of A. ochraceus produced ochratoxin A (one at 178 ppm) and the studied strain of P. citrinum was moderately toxigenic for citrinin. Also two rice samples were found contaminated with high level of ochratoxin A (21.3 and 26.2 ppb). This contamination can probably be linked to unfavorable post harvest storage and climatic conditions.
Also Sani and Sheikhzadeh discussed the different methods of aflatoxin (AFT) degradation in rice. Mycotoxins are mainly present in cereal grains such as rice and are not completely destroyed during their processing and cooking.60
Detection of aflatoxins as aprimary mycotoxin in stored rice was also reported by demonstrated time to time by.61,62,48 These mycotoxins were detected on the basis of fluorescence and retention factors (R.F.) values. Presence of them was confirmed by long wave UV light.
The retention factors of all the mycotoxins produced were determined, this was done by thin layer chromatography. Konishi et al.44,63 studied aflatoxins and other mycotoxins in rice from China and Japan, respectively. Subsequently, Tanaka et al.64 who find the variation in mycotoxin contamination in rice from different regions may be due to differences in toxigenic microflora influenced by different agricultural practices and the differences in climate and also their storage conditions, Taligoola et al.65,27 morover reported toxigenic fungi and mycotoxins in rice.
Earlier, Madsen & Rasmussen66 reported the presence of AFB1 contamination in milled rice. Prasad et al.67 screened sixty five samples of stored rice and twelve were positive for aflatoxin. Levels of aflatoxins ranged between 84 to 2830 µg/ kg mycelium.
Recently, Kushiro; Toman et al.; Xiang et al.; Sani & Sheikh zadeh; Urooj et al.; Mukhtar et al.; Aingkharat et al.; Majeed et al.,68,40,69,60,70-73 were studies contamination of storage rice and other cereals by different fungal toxins.
Conclusion
Our results suggest that there is a need for proper storage of rice seed to minimize the fungal contamination and their mycotoxin production. Aflatoxins and ochratoxin are among the five most significant and abundant mycotoxins contaminating foods and food stuffs in the world74 and have also been shown in this work to be major contaminants of rice in El-Minia Government.
Acknowledgements
Authors thanks, staff members of Assiut university mycological centre (AUMC), Egypt for their support in this research.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- FAO (Food and Agriculture Organization). Rice marked monitor. Trade and markets division. 2012;15(1):1-33.
- Makun H. A., Gbodi T. A., Akanya O. H., Salako E. A., Ogbadu G. H. Fungi and some mycotoxins contaminating rice (Oryza sativa) in Niger state, Nigeria. Afr J Biotechnol. 2007;6(2):099-108.
- Bansal J., Pantazopoulos P., Tam J., Cavlovic P., Kwong K., Turcotte A. M., Lau B. P. Y., Scott P. M. Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit Contam. 2011;28(6):767-774.
- Suarez-Bonnet E., Carvajal M., Mendez-Ramirez I.,Urueta P. C., Cortes-Eslava J., Gomez-Arroyo S., Melero-Vara J. M. Aflatoxin (B1, B2, G1, and G2) contamination in rice of Mexico and Spain from local sources or imported. J Food Sci. 2013;78:1822-1829.
CrossRef - Ok H. E., Kim D. M., Kim D.,Chung S. H., Chung M., Park K. H., Chun H. S. Mycobiota and natural occurrence of aflatoxin, deoxynivalenol, nivalenol and zearalenone in rice freshly harvested in South Korea. Food Control. 2014;37:284–291.
CrossRef - Lai X. W., Liu R. C., Ruan C. Q., Zhang H., Liu C. L. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control. 2015a;50:401–404.
CrossRef - Reddy O. R., Sathyanarayana N. Seed-borne fungi of rice and quarantine significance. In: Sreenivasaprasad S., Johnson R., editors. Major fungal diseases of rice, recent advances. Dordrecht, The Netherlands: Kluwer Academic Publishers. 2001;367.
CrossRef - Reddy K. R. N., Reddy C. S., Abbas H. K., Abel C. A., Muralidharan K. Mycotoxigenic fungi, mycotoxins and management of rice grains. Toxin Rev. 2008;27:287-317.
CrossRef - Sempere F., Santamarina M. P. Efficacy of Trichoderma harzianum in suppression of Fusarium culmorum. Ann Microbiol. 2010;60:335–340.
CrossRef - Ferre F. S. Worldwide occurrence of mycotoxins in rice. Food control. 2016;62:291-298.
CrossRef - Abd-Allah E. F., Ezzat S. M. Natural occurrence of citrinin in rice grains and its biocontrol by Trichoderma hamatum. Phytoparasitica. 2005;33:73–84.
CrossRef - El-shanshoury A. R., El-sabbagh S. M., Emara H. A., Saba H. A. E. Occurrence of moulds, toxicogenic capability of Aspergillus flavus and levels of aflatoxins in maize, wheat, rice and peanut from markets in Central Delta Provinces, Egypt. Int J Curr Microbiol App Sci. 2014;3:852-865.
- Pitt J. I., Hocking A. D. Fungi and food spoilage . 3rd Edition. Springer Science and Business Media. 2009;519.
CrossRef - Domsch K. H., Gams W., Anderson T. Compendium of soil fungi. 2nd Edition. IHW Verlag. Eching Germany. 2007;672.
- Ellis M. B. Dematiaceous hyphomycetes. Commonwealth Mycological Institute. Kew, Surrey, England. 1971;608.
- Ellis M. B. More dematiaceous hyphomycetes. Commonwealth Mycological Institute, England. 1976;481.
- Leslie J. F., Summerell B. A. Fusarium laboratory manual. Blackwell publishing, John Wiley and Sons. 2006;338.
CrossRef - Moubasher A. H. Soil fungi of Qatar and other Arab countries. The Scientific and Applied Research Centre, University of Qatar. 1993;566.
- Pitt J. I. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press Inc (London) LTD. 1979;634.
- Raper K. B., Fennell D. I. The genus Aspergillus. Baltimore, Maryland: Williams and Wilkins Company. 1965;686.
- Jackson L. K., Ciegler A. Production and analysis of citrinin in corn. Appl Environ Microbiol. 1978;36:408-411.
- Trantham A. L., Wilson D. M. Fluorometric screening method for citrinin in corn, barley and peanuts . J Assoc off Anal Chem. 1984;6:37-40.
- Zohri A. A. Mycoflora and mycotoxins of some meat products. Ph.D. Thesis, Botany Department, Faculty of Science, Assiut University, Assiut, Egypt. 1990.
- El-Kady I. A., Moubasher M. H. Toxigenicity and toxin of Stachybotrys chartarum isolates from wheat straw samples in Egypt. Experimental Mycology. 1982;6:25-31.
CrossRef - Scott P. M., Lawrence J. W., Walbek W. Detection of mycotoxins by thin layer chromatography :application to screening of fungal extracts. Appl Microbiol. 1970;20:839-842.
- Taligoola H. K., Ismail M. A., Chebon S. K. Mycobiota associated with rice grains marketed in Uganda. J Biol Sci. 2004;4(1):271-278.
- Taligoola H. K., Ismail M. A., Chebon S. K. Mycobiota and aflatoxins associated with imported rice grains stored in Uganda. Czech Mycol. 2011;63:93-107.
CrossRef - Fredlund E., Thim A. M., Gidlund A., Brostedt S., Nyberg M., Olsen M. Moulds and mycotoxins in rice from the Swedish retail market. Food Addit Contam. 2009;26(04):527-533.
CrossRef - Abdel-Hafez S. I. I., El-Kady I. A., Mazen M. B., El-Maghraby O. M. Mycoflora and trichothecine toxins of paddy grains from Egypt. Mycopathologia. 1987;100:103-112.
CrossRef - Tonon S. A., Marucci R. S., Jerke G., Garcia A. Mycoflora of paddy and milled rice produced in the region of Northeastern Argentina and Southern Parguay. Int J Food Microbiol. 1997;37:231-235.
CrossRef - Butt A. R., Yaseen S. I., Javaid A. Seed-borne mycoflora of stored rice grains and its chemical control. J Anim Plant Sci. 2011;21(2):193-196.
- Shanakht H., Shahid A. A., Ali S. W. Characterization of fungal microbiota on rice grains from local markets of Lahore. Journal of Hygienic Engineering and Design. 2014;9:35-40.
- Trung T. S., Bailly J. D., Querin A., Bars P. L., Guerre P. Fungal contamination of rice from South Vietnam, mycotoxinogensis of selected strains and residued in rice. Revue Med Vet. 2001;152:555-560.
- Małgorzata P. Contamination of breakfast cereal products by fungi and mycotoxins – a potential risk for consumer’s health Biotechnol Food Sci. 2013;77(1):3-10.
- Stanciu O., Banc R., Cozma A., Filip L., Miere D., Manes J., Loghin F. Occurrence of Fusarium mycotoxins in wheat from Europe – Areview. Food Technol. 2015;19(1):35- 60.
- Christensen C. M., Kaufmann H. H. Microflora: In storage of cereal grains and their products. CM Christensen (Ed) American Association of Cereal Chemists. 1974;159-191.
- Christensen C. M. Field and storage fungi. In: Beuchat L. R., ed. Food and everage Mycology 2nd ed New York. 1987;211-232.
- Sales A. C., Yoshizawa T. Mold counts and Aspergillus section Flavi populations in rice and its byproducts from the Philippines. J Food Prot. 2005;68(1):120-125.
CrossRef - Sales A. C., Yoshizawa T. Updated profile of aflatoxin and Aspergillus flavi contamination in rice and its byproducts from the Philippines. Food Addit Contam. 2005;22(5):429–436.
CrossRef - Toman J., Malir F., Ostry V., Grosse Y., Dvorak V., Roubal T., Neuchlova L. The occurrence of ochratoxin A in white and parboiled rice. Food Chemistry and Safety Czech J Food Sci. 2016;34:32-38.
CrossRef - Makun H. A., Dutton M. F., Njobeh P. B., Mwanza M., Kabiru A. Y. Natural multi-occurrence of mycotoxins in rice from Niger State, Nigeria. Mycotoxin Res. 2011;27:97– 104.
CrossRef - Amadi J. E., Adeniyi D. O. Mycotoxin production by fungi isolated from stored grains. Afr J Biotechnol. 2009;8(7):1219- 1221.
- Egbuta M. A., Mwanza M., Njobeh P. B., Phoku J. Z., Chilaka C. A., Dutton M. F. Isolation of filamentous fungi species contaminating some Nigerian food commodities. J Food Research. 2015;4(1):38-50.
CrossRef - Liu Z., Gao J., Yu J. Aflatoxins in stored maize and rice grains in Liaoning Province, China. Journal of Stored Products Research. 2006;42:468-479.
CrossRef - Sun G., Wang S., Hu X., Su J., Zhang Y., Xie Y.,Zhang H., Tang L., Wang J. S. Cocontamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:461-470.
CrossRef - Lai X. W., Zhang H., Liu R. C., Liu C. L. Potential for aflatoxin B1 and B2 production by Aspergillus flavus strains isolated from rice samples. Saudi J Biol Sci. 2015;22:176–180.
CrossRef
- Sun X. D., Su P., Shan H. Mycotoxin contamination of rice in China. J Food Sci. 2017;82(3):573-584.
CrossRef - Reddy K. R. N., Reddy C. S., Muralidharan K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009;26:27- 31.
CrossRef - Gautam A. K., Gupta H., Soni Y. Screening of fungi and mycotoxins associated with stored rice grains in Himachel Pradesh. International J Theoretical App Sci. 2012;4(2):128-133.
- Uma V., Wesely E. G. Seed borne fungi of rice from South Tamil Nadu. J Acad Indus Res. 2013;1(10):612-614.
- Almeida M. I., Almeida N. G., Carvalho K. L., Goncalves G. A. A., Silva C. N., Santos E. A., Garcia J. C., Vargas E. A. Cooccurrence of aflatoxins B1, B 2, G 1 and G2, ochratoxin A, zearalenone, deoxynivalenol, and citreoviridin in rice in Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:694–703.
CrossRef - Park J. W., Choi S. Y., Hwang H. J., Kim Y. B. Fungal mycoflora and mycotoxins in Korean polished rice destined for humans. Int J Food Microbiol. 2005;103:305-314.
CrossRef - Oh J. Y., Sang M. K., Oh J. E.,Lee H., Ryoo M. I., Kim K. D. Microbial population, aflatoxin contamination and predominant Aspergillus species in Korean stored rice. Plant Pathol J. 2010;26(2):121-129.
CrossRef - González L., Juan C., Soriano J. M., Moltó J. C., Mañes J. Occurrence and daily intake of ochratoxin A of organic and non-organic rice and rice products. Int J Food Microbiol. 2006;107:223-227.
CrossRef - Cantalejo M. J.,Carrasco J. M., Hernandez E. Mycoflora of seeds and feeds from the Spanish market. J Basic Microbiol. 1997;37:395-402.
CrossRef - Aydin A., Aksu H., Gunsen U. Mycotoxin levels and incidence of mould in Turkish rice. Environ Monit Assess. 2011;178:271–280.
CrossRef - Buyukunal S. K., Kahraman T., Ciftcioglu G. R. Occurrence of AF, AFB1, OTA in rice commercialized in Eastern Turkey. Polish J Environ Stud. 2010;19(5):907–912.
- Morrison D. M., Chester l., Samuela C. A. N., Ledoux D. R. The determination of aflatoxins in paddy and milled fractions of rice in Guyana. Int J Biol.Biomolecular. Agric. Feed Biotechnol Engineering. 2016;10(11):725-729.
- Iqbal S. Z., Asi M. R., Hanif U., Zuber M., Jinap S. The presence of aflatoxins and ochratoxin A in rice and rice products: a survey. Food Chem. 2016;210:135-140.
CrossRef - Sani A. M., Sheikhzadeh M. Aflatoxin degradation in rice.” Nutrition and Food Sci. 2017;47(4):469-476.
- Reddy C. S., Reddy K. R. N., Kumar N. R., Laha G. S., Muralidharan K. Exploration of aflatoxin contamination and its management in rice. J Mycol Plant Pathol. 2004;34(3):816-820.
- Reddy K. R. N., Reddy C. S., Muralidharan K. Characterization of aflatoxin B1produced by Aspergillus flavus isolated from discolored rice grains. J Mycol Plant Pathol. 2005;35(3):470- 474.
- Konishi S., Nakajima M., Tabata S., Ishikuro H., Tanaka T., Norizuki H. Occurrence of aflatoxins, ochratoxin A and fumonisins in retailed foods in Japan. J Food Prot. 2006;69:1365-1370.
CrossRef - Tanaka K., Sago Y., Zheng Y., Nakagawa H., Kushiro M. Mycotoxins in rice. Int J Food Microbiol. 2007;119:59-66.
CrossRef - Taligoola H. K., Ismail M. A., Chebon S. K. Toxigenic fungi and aflatoxins associated with marketed rice grains in Uganda. J Basic Appl Mycol. 2010;1:45-52.
- Madsen B., Rasmussen G. Aflatoxin B1, B2, G1 and G2 in maize, rice, millet, buckwheat, lentils and beans etc in 1987 and 1988. Levnedsmiddelstyrelsen. 1990;200(33):9.
- Prasad T., Sinha R. K., Jeswal P. Seed mycoflora of cereals and aflatoxin contamination under storage systems. J Indian Bot Soc. 1987;66:156–160.
- Kushiro M. Historical review of researches on yellow rice and mycotoxigenic fungi adherent to rice in Japan. JSM Mycotoxins. 2015;65(1):19-23.
CrossRef - Xiang D. S., Ping S., Hong S. Mycotoxin contamination of rice in China. J Food Sci. 2017;82(3):573-584.
CrossRef - Urooj R., Achakzai K., Zaman M., Bibi A., Afreen T., Iram S. Occurrences of mycotoxins contamination in crops from Pakistan. A Review, American-Eurasian J Agric Environ Sci. 2015;15(7):1289-1297.
- Mukhtar H., Farooq Z., Manzoor M. Determination of aflatoxins in super kernel rice types consumed in different regions of Punjab, Pakistan. The J Anim Plant Sci. 2016;26(2):542-548.
- Aingkharat T., Taraporn J., Orawan A. Mycotoxins in feed and feed ingredients 2015-2016. Thai J Vet Med Suppl. 2017;47:313.
- Majeed S., De Boevre M., De Saeger S., Rauf W., Tawab A., Fazel-e-Habib., Rahman M., Iqbal M. Multiple mycotoxins in rice: Occurrence and health risk assessment in children and adults of Punjab, Pakistan.Toxins. 2018;10(77):1-30.
- Bhat R. V., Vasanthi S. Food safety in food security and food trade. Mycotoxin food safety risk in Developing Countries. Food Agric Environ. 2003. IFPRI Brief 3 of 17.

This work is licensed under a Creative Commons Attribution 4.0 International License.





