How to Cite | Publication History | PlumX Article Matrix
Phycoremediation of Heavy Metals by Botryococus Brurauni from Wastewater
Azhar Uddin and A. M. Lall*
Department of Biochemistry and Biochemical Engineering, JSBB, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, U. P. 211007 India.
Corresponding Author E-mail: alokmilton@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2730
ABSTRACT: Phycoremediation is the use of macro-algae or micro-algae for the removal or biotransformation of pollutants, including nutrients and xenobiotics from wastewater. In the present study, the phycoremediation capacities of live green algae, Botryococus brurauni changed into evaluated for poisonous heavy metals, Pb, Cd, and Cu from wastewater and artificial solution. Botryococus brurauni algae proved efficient biological vectors for heavy metallic uptake. Phycoremediation research performed on wastewater effluent revealed that 93 % Pb, 89 % Cd, and 82 % of Cu on 90 mins of remedies. Experimental consequences discovered that Botryococus brurauni have the most accumulation of Pb followed via Cd and Cu after 90 mins of exposure. Cd observed by using Pd and Cu maximally decreased the overall growth performance of the algae measured regarding Chl-a and Chl b content material accompanied via Pd and Cu. The outcomes showed that Botryococus brurauni were suitable for Pb and Cd removal and bioaccumulation of heavy metals from effluent wastewater.
KEYWORDS: Botryococus Brurauni; Cd and Cu; Heavy Metals; Phycoremediation; Pb; Wastewater
Download this article as:| Copy the following to cite this article: Uddin A, Lall A. M. Phycoremediation of Heavy Metals by Botryococus Brurauni from Wastewater. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Uddin A, Lall A. M. Phycoremediation of Heavy Metals by Botryococus Brurauni from Wastewater. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2CUV2Pu |
Introduction
Nowadays, the selection of wastewater treatment method is one of the most exciting topics among the researcher either conventional, bioremediation or advanced mode. Phycoremediation is a bioremediation technique in wastewater treatment that utilises microorganism such as microalgae. Environmental contamination by heavy metals is a severe problem due to their incremental accumulation in the food chain (Awofolu 2005). Unlike most organic wastes and the microbial load in aquatic bodies, metal contaminants are not biodegradable, tending to accumulate in living organisms, thus becoming a permanent burden on ecosystems (Sivakumar et al. 2014). Most heavy metals are transition elements with incompletely filled d-orbitals. Living organisms require trace amounts (μg L-1) of some metal ions such as lead, copper, zinc, cobalt, iron, nickel as cofactors for the enzymatic activities. However, heavy metal ion concentrations at ppm (mg L-1) level are known to be toxic to the organisms because of irreversible inhibition of many enzymes by the heavy metal ions. The process of accumulation and adsorption of metals by algae involves adsorption onto the cell surface (wall, membrane or external polysaccharides) and binding to cytoplasmic ligands, phytochelatins and metallothioneins, and other intracellular molecules.
Since metal ions in water are generally in the cationic form, they are adsorbed onto the cell surface (Crist et al. 1992; Romera et al. 2007; Singh and Kalamdhad 2012). Algal cell walls are porous and allow the free passage of molecules and ions in aqueous solutions. The constituents of the algal cell wall provide an array of ligands with different functional groups capable of binding various heavy metals. These cells can be used live or dead (Zou et al. 2014). They are generally rugged organisms with fast growth in a simple medium, and the algal biomass produced can efficiently be processed into useful biosorbents (Tuzen and Sarı 2010).
However recently it has been reported that live species exhibits higher biosorption capacity than dead biomass probably due to enzymatic reactions during intracellular uptake (Doshi, Ray, and Kothari 2009). The present study, therefore, aimed to compare the performance of Botryococus brurauni sequestering Pb, Cd, and Cu ions from wastewater and aqueous solutions. The growth performance of the Botryococus brurauni algal species regarding their Chl a and Chl b content after heavy metals accumulation was also examined. The algae were distinct from their morphology; one is unicellular while other is multicellular. In literature, no such study has been undertaken so far where a comparison is made between two morphologically distinct algae for bioremediation of heavy metals.
Material and Methods
Algal Cultures and Water Samples
The freshwater macroalgae, Botryococus brurauni algal samples from sample 1 old bridge phul- mandi Naini Allahabad (25° 25’18” N; 81° 51’4″ E), sample 2 SHUATS University Campus Forestry Department. The collected samples were subjected to microscopic identification for characterisation of species distribution and selection of source with a high number of Spirogyra species. The cultures were further maintained in Fog’s medium. Slant cultures were prepared from the pure culture for further use. One loopful of algal biomass from best growth obtained above was inoculated in a sterile 15ml test tube with an enriched medium (Bold’s Basal Media).
Wastewater was collected in bulk from the samples A; Yamuna river Address – Sangam yatra mandir Sachcha Baba Nagar Arail Ghat Naini Allahabad situated (25° 24’14” N; 81° 52′ 49″ E) Sedimentation and filtration through filters paper removed solid particles. After filtration, wastewater was stored at 4 °C in the dark until needed for the experiments.
Total ten numbers of 25ml test tubes were inoculated with isolated algae, maintained at 24°C ± 1°C and illuminated at 3500–4000 lux light intensity with a light/dark cycle of 16/8-h for ten days. After ten days, inoculated algae from the test tubes were inoculated into 250 ml Erlenmeyer flasks containing Bold’s Basal Media for another seven days. After seven days the medium inside the container appear green, these were examined under the microscope. At every 12 days, a new medium was prepared, and the algal cells were inoculated to it to continue the algal cell generation. To avoid bacterial and fungal contamination appropriate amount of antifungal and antibacterial were added to the medium.
Preparation of Heavy Metal Stock Solutions
Stock solutions of Pb(II), Cu(II) and Cr(IV) were prepared by dissolving their salts. The trace elements of Pb, Cd, and Cu were added to the culture media. Stock solutions of Pb(II), Cu(II) and Cr(IV) were prepared by dissolving their salts Pb (NO3)2, 3Cd SO4. 8H2O and CuCl2. 2H2O in the distilled water. From this stock solution, different concentration of heavy metals was prepared.
Characterisation of Wastewater Parameters
Wastewater was analysed for various chemical and physical parameters such as pH, Colour, Hardness, Alkalinity, Total Nitrogen, Nitrate (NO3-), Phosphate , Chloride, Ammonical Nitrogen, Total dissolved solids (TDS), Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD) and Test for Dissolved Oxygen (DO) estimation by standard prescribed methods in APHA (Eaton et al. 2005).
Phycoremediation Experiments
The 1, 3, and 5 mg/l concentrations of Pb+2, Cd+2 and Cu+2, were exposed to the Botryococus brurauni culture, respectively. The culture sample of Botryococus brurauni was centrifuged at 9000×g for 10 min, and the supernatant was discarded. The algal cells (biomass) were washed twice with sterile Milli-Q water and re-suspended in sterile Milli-Q water for inoculation into the growth medium. These concentrations were arranged based on preliminary research reviews (Soeprobowati and Hariyati 2014). The concentrations of Pb, Cd and Cu were measured at the initial time, minutes of 30 and minutes of 90. The heavy metals concentrations in media culture and the Botryococus brurauni were measures with AAS. A reduction of heavy metals (percentage of removal) was calculated as well as Botryococus brurauni population. BioConcentration Factor (BCF) was calculated to determine the accumulation of heavy metals in the Botryococus brurauni. BCF is a comparison between heavy metal concentrations on the Botryococus brurauni with the concentration on the aqueous environment.
BCF =Corg / Cmedium
Corg was heavy metals concentration in Spirogyra cummin or Botryococus brurauni
Cmedia was heavy metals concentration in the culture media.
Measurement of Chlorophyll
Ten ml of sample was taken and centrifuged at 6000 rpm for 15 min. Supernatants have been discarded and re-suspended in a known volume of methanol, at the same time as pellets extracted with 5 ml of 96% methanol extraction. The tubes were wrapped with aluminium foil and kept in darkish. The samples had been centrifuged again, and the supernatants were used for measuring the optical density at 663 nm and 645 nm towards 96 % methanol as a blank by spectrophotometer. After extraction chlorophyll attention was determined spectrophotometrically and calculated Chlorophyll content material (Chlorophyll a, chlorophyll b and total chlorophyll) had been computed using the following equations.
Chlorophyll-a (μg/ml) = {(15.65xA666 – 7.340xA653) x V/ 50 x W} x dilution
Chlorophyll-b (μg/ml) = {(27.05xA653 – 11.21xA666) x V/ 50 x W} x dilution
Total chlorophyll = chlorophyll-a + chlorophyll-b
Results and Discussion
Microscopic studies of cultures have shown that species contamination is present in each the cultures however the species distribution varies extensively. In the case of Botryococus brurauni cultivation, the population of contaminant species had been low till the 7 th day after which they commenced growing.
Change in Physicochemical Parameters
The wastewater contains various toxic contaminants including heavy metals as lead, cadmium, nickel, mercury, arsenic, copper etc. producing a significant poisonous impact on the aquatic environment (Siddiqui and Sharma 2009; Oyeku and Eludoyin 2010; Pandey et al. 2010). Data represented in Table 1 and Fig 1. Three indicates the changes in physicochemical parameters before and after treatment with algae Botryococus brurauni. After 90 min there was 93 % Pb, 89 % Cd, and 82 % of Cu on 90 minutes of treatments with algal biomass Fig.1.
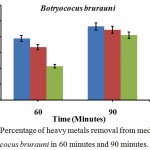 |
Figure 1: Percentage of heavy metals removal from media culture of Botryococus brurauni in 60 minutes and 90 minutes.
|
Moreover, a significant change was observed in physicochemical parameters of wastewater after 14 days of treatment with algae under investigation. The study suggests that Botryococus brurauni shows promising approach towards the purification process of wastewater at various parameters. Along with bringing the properties such as pH, Colour, Hardness, Alkalinity, Total Nitrogen, Nitrate (NO3-), Phosphate, Chloride, Ammonical Nitrogen, Total dissolved solids (TDS), Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD) and Test for Dissolved Oxygen (DO) etc showed in Table 1. Towards the desirable limit, the algae have been found quite sufficient for the removal of heavy metals also. Among heavy metals, although a significant reduction was observed in all the three metals, the algae have been found to be especially useful in the reduction of Pb, followed by Cd and Cu.
Table 1: Change in physicochemical parameters at 14 days interval with Botryococus brurauni.
| No. | Parameter | Unit | Initial value | Wastewaters + Botryococus brurauni |
| 1. | pH | 5.8 | 8.9 | |
| 2. | Colour | Light brown | pale White | |
| 3. | Hardness | 253 | 63 | |
| 4. | Alkalinity | mg/L | 187 | 233 |
| 5. | Total Nitrogen | mg/L | 200 | 156 |
| 6. | Nitrate (NO3-) | mg/L | 3.3 | 1.9 |
| 7. | Phosphate | mg/L | 3.77 | 1.2 |
| 8. | Chloride | mg/L | 837 | 469 |
| 9. | Ammonical Nitrogen | mg/L | 40 | 17 |
| 10. | Total dissolved solids (TDS) | mg/L | 2076 | 453 |
| 11. | Chemical Oxygen Demand (COD) | mg/L | 125 | 68 |
| 12. | Biological Oxygen Demand (BOD) | mg/l | 112 | 76 |
| 13. | Test for Dissolved Oxygen (DO) | mg/L | 2.7 | 3.8 |
Chl-a as an algal biomass measurement in herbal structures was very famous. Chl-b is used to calculate pigment concentrations. The total Chl-(a + b) is used to degree algal boom(Ramaraj et al. 2013). Boom device was set up out of doors conditions. Table 2 shows the chlorophyll-a concentration in microalgae determined using the standard method. Biomass measured by total chlorophyll results were average as, 6.7 μg/ mL, for Pb, 5.2 μg/ mL, for Cd, and 8.1 μg/ mL, for Cu respectively for Botryococus brurauni. Heavy metals enter algal cells using either active transport or endocytosis through chelating proteins and affect various physiological and biochemical processes of the algae.
Table 2: Chlorophylls estimation in algae strains exposure to the heavy metal.
| Parameter | Botryococus brurauni | |||
| Initial value | Pb | Cd | Cu | |
| Chl-a ( μg/mL) | 5.3 | 4.6 | 3.3 | 5.3 |
| Chl-a ( μg/mL) | 2.8 | 2.1 | 1.9 | 2.8 |
| Total Chl (a + b) ( μg/mL) | 8.1 | 6.7 | 5.2 | 8.1 |
The obtained results in this investigation concerning the tolerance of S. communis and C. pyrenoidosa to the tested heavy metal ions (Pb, Cu and Cr) were in agreement with the results reported by Foster, 1982 and Stokes, 1983 concerning the tolerance and resistance of green algal species to heavy metal ions (Cu, Cd, Pb and Zn). Also, high Cr2+ concentrations reduced cell sizes and caused a decrease in growth rate (Leborans and Novillo 1996). Nassiri et al., (1997) found no growth inhibition at Cr2+concentrations < 1mg/l, but Tetraselmis suecica had 10, 30 and 50% growth inhibition, after 4 days in solutions contained 2, 5 and 10 mg/l Cr2+ respectively (Nassiri et al. 1997). Chlorophyll content associated with heavy metal stress may be the result of inhibition of the enzymes responsible for chlorophyll biosynthesis. Cadmium and chromium were reported to affect chlorophyll biosynthesis and inhibit protochlorophyll reductase and aminolevulinic acid (ALA) synthesis (Stobart et al. 1985). The inactivation of the enzymes involved in the chlorophyll biosynthetic pathway could also contribute to the general reduction in chlorophyll content. The present results showed that lead, copper and chromium toxicity decreased the chlorophyll a content of the two algae under investigation. The highest reduction in chlorophyll content was found in algae exposed to chromium, followed by copper and lead. A large reduction in chlorophyll content due to Cr toxicity can be explained by the destruction of stomata and mesophyll cells, which decreases their efficiency of light utilisation and electron transport rates involving PS I and PS II (Munné-Bosch and Alegre 2003; Hernández et al. 2004).
In conclusion, in the present study, the bioaccumulation potential of algal biomass Botryococus brurauni has been assessed for the removal of Pb(II), Cu(II) and Cr(IV from wastewater and aqueous solution. Experiments conducted on wastewater showed a significant decrease in physicochemical parameters and heavy metal content by the algal biomass. The chlorophyll content of both algae was highly suppressed by high reduced by Cd followed by Pd and Cu levels heavy metal removal from wastewaters.
Conclusion
The present findings revealed that live biomass of Botryococus brurauni algae is better Phycoremediation tool for removal of heavy metals under optimised conditions. Further, it showed better reusability potential for removal of lead and cadmium ions from the metal contaminated aquatic system.
Acknowledgements
The authors wish to express gratitude to Dean of JSBB, SHUATS India, for work was support Grant made to AM Lall.
Conflict of Interest
There is no conflict of interest.
Reference
- Awofolu O. A survey of trace metals in vegetation, soil and lower animal along some selected major roads in metropolitan city of Lagos. Environmental monitoring and Assessment. 2005;105(1-3):431-447.
CrossRef - Crist R. H., Oberholser K., McGarrity J., Crist D. R., Johnson J. K., Brittsan J. M. Interaction of metals and protons with algae. 3. Marine algae, with emphasis on lead and aluminum. Environmental science & technology. 1992;26(3):496-502.
CrossRef - Doshi H., Ray A., Kothari I. L. Live and dead Spirulina To remove arsenic (V) from water. Int J Phytorem. 2009; 11:53–63.
CrossRef - Eaton A. D., Clesceri L. S., Greenberg A. E., Franson M. A. H. Standard methods for the examination of water and wastewater. American public health association. 2005;1015:49-51.
- Hernández I., Alegre L., Munné-Bosch S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree physiology. 2004;24(11):1303-1311.
CrossRef - Leborans G. F., Novillo A.Toxicity and bioaccumulation of cadmium in Olisthodiscus luteus (Raphidophyceae). Water Research. 1996;30(1):57-62.
CrossRef - Munné-Bosch S., Alegre L. Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiology. 2003;131(4):1816-1825.
CrossRef - Nassiri Y., Wery J., Mansot J., Ginsburger-Vogel T. Cadmium bioaccumulation in Tetraselmis suecica: an electron energy loss spectroscopy (EELS) study. Archives of environmental contamination and toxicology. 1997;33(2):156-161.
CrossRef - Oyeku O., Eludoyin A. Heavy metal contamination of groundwater resources in a Nigerian urban settlement. African Journal of Environmental Science and Technology. 2010;4(4).
- Pandey J., Shubhashish K., Pandey R., Kanethe E., Kironchi G. Heavy metal contamination of Ganga river at Varanasi in relation to atmospheric deposition. Tropical Ecology. 2010;51(2):365-373.
- Ramaraj R., Tsai D. D., Chen P. H. Chlorophyll is not accurate measurement for algal biomass. 2013.
- Romera E., González F., Ballester A., Blázquez M., Munoz J. Comparative study of biosorption of heavy metals using different types of algae. Bioresource technology. 2007;98(17):3344-3353.
CrossRef - Siddiqui W. A., Sharma R. R. Assessment of the impact of industrial effluents on groundwater quality in Okhla industrial area, New Delhi, India. Journal of Chemistry. 2009;6(S1):S41-S46.
- Singh J., Kalamdhad A. S. Concentration and speciation of heavy metals during water hyacinth composting. Bioresource technology. 2012;124:169-179.
CrossRef - Sivakumar D., Kandaswamy A., Gomathi V., Rajeshwaran R., Murugan N. Bioremediation studies on reduction of heavy metals toxicity. Pollution Research. 2014;33:553-558.
- Soeprobowati T. R., Hariyati R. Phycoremediation of Pb, Cd, Cu, and Cr by Spirulina platensis (Gomont) Geitler. American Journal of BioScience. 2014;2(4):165-170.
CrossRef - Stobart A. K., Griffiths W. T., Ameen‐Bukhari I., Sherwood R. P. The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiologia Plantarum. 1985;63(3):293-298.
CrossRef - Tuzen M., Sarı A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kinetic studies. Chemical Engineering Journal. 2010;158(2):200-206.
CrossRef - Zou H. X., Li N., Wang L. H., Yu P., Yan X. F. Equilibrium and kinetic studies of Cd2+ biosorption by the brown algae sargassum fusiforme. PloS one. 2014;9(4):e95242.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





