How to Cite | Publication History | PlumX Article Matrix
Effect of Anaerobic Co-Digestion on Microbial Community and Biogas Production
Fasosin Emmanuel Olufemi*, David Veronica and Hero Godwin
Department of Microbiology, University of Jos, Jos, Plateau State, Nigeria.
Corresponding Author E-mail: efasosin@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2754
ABSTRACT: A study was carried out to explore the biogas production potential of co-digestion using chicken droppings as inoculum and different substrate combinations. The various substrate combinations were mixed with the chicken droppings in the ratio 1:1 and subjected to anaerobic digestion using fabricated laboratory scale biodigesters, immersed in water bath and set at 37°C for a period of 30 days. The initial and final pH values of the digesters were recorded. The amount of biogas generated was measured by the method of downward displacement of water from a measuring cylinder. The bacteria and fungi associated with the production of biogas were isolated and then characterized using standard microbiological techniques. The microbial population isolated from the biodigesters include species of Bacillus, Clostridium, Escherichia, Pseudomonas, Yersinia, Methanosarcina, Methanobacterium, Penicillium and Aspergillus. It also indicated a slight shift from an alkaline medium to a slightly acidic environment in all the digesters. The result shows that all the substrate combinations demonstrated potentials for biogas production.
KEYWORDS: Biodigesters; Methanobacterium; Penicillium and Aspergillus
Download this article as:| Copy the following to cite this article: Olufemi F. E, Veronica D, Godwin H. Effect of Anaerobic Co-Digestion on Microbial Community and Biogas Production. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Olufemi F. E, Veronica D, Godwin H. Effect of Anaerobic Co-Digestion on Microbial Community and Biogas Production. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2YOzXiA |
Introduction
With growing concern about change in climate, quality of air, energy import dependence and fossil fuel depletion, the yearning for renewable fuels is increasing and biogas is one of the versatile renewable fuels (Rasi et al., 2011). Biogas is produced by anaerobic digestion of biological wastes such as poultry and sheep droppings, vegetable wastes, cattle dung, municipal solid waste, industrial waste water and land fill to give mainly methane (50-70%), carbon dioxide (20-40%) and traces of other gases such as nitrogen, hydrogen, ammonia, hydrogen sulphide, water vapour, etc (Gopinath et al., 2014). Basically, it can be used to generate energy-heat, electricity and cooking.
Compared to other solid fuels, biogas is smokeless, hygienic and more convenient to use (Gopinath et al., 2014). Wastes, more commonly known as garbage or trash consist of everyday items used and discarded, such as product packaging, grass clippings, furniture, clothing, bottles, food residues, newspapers and other appliances from hospitals, homes, schools and businesses (US EPA, 2015).
Refuse dump sites, due to poor and ineffective management has turned to a source of health hazard to people living in the vicinity of such dumps (Ogunrinola et al., 2012). These ‘mountain’ of rubbish in landfills and open waste dumps are sources of harmful microorganisms and are usually covered with flies, thus serving as breeding grounds for rodents and mosquitoes which are disease vectors (Ozturk, 2013).
In a bid to examine the link between environmental pollution arising from waste dumps and public health, the United Nations Environmental Programme (UNEP) conducted a pilot study of the Dandora Waste Dump in Kenya. The study, as tentative as it was, showed that a link exists between environmental pollution and public health (UNEP, 2010). The extensive tests carried out on the soil and water around the dump site in comparison with samples from other sites as well as medical tests carried out on humans living around the dumpsite shows evidence of infections from water, land and air pollution. The leachates generated in the landfills and open dumpsites are sources of pollution which is inimical to public health (UNEP, 2010).
The process of anaerobic digestion involves the decomposition of organic matter by a microbial consortium in an environment free of oxygen (Ward et al., 2008). Arguably, biogas is the most valuable product of anaerobic digestion. However, it is not the only useful product (Park et al., 2012). Biogas is produced in the process and also slurry- biologically known as digestate. The digestate can be applied directly on farmland and used as fertilizers due to their excellent N-content (Olufunmi, 2014). Anaerobic digestion is a process where microorganisms break down organic matter, such as waste and manure, in the absence of oxygen and it has a series of metabolic reactions (Khalid et al., 2011). The metabolic reactions produce biogas from a three-phase process namely, hydrolysis, acid-forming and methane-forming phases. Microbes and their enzymatic pathways ensure these transformations.
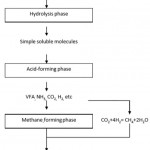 |
Scheme 1
|
Co-digestion is a method of treating waste that involves various waste being mixed and treated together (Khalid et al., 2011). This technique is mostly used to increase methane production (EPA, 2012).
Comparing single waste digestions with co- digestion of combined wastes, it is seen that co-digestion results in higher methane gas yields, with a positive impact on the quality and quantity (CH4 content) of biogas produced (Kangle et al. 2012). For instance, an optimization protocol for maximizing production of biogas by anaerobic co-digestion of several wastes was carried out by (Alvarez et al. 2015). Pig manure, tuna fish waste and biodiesel wastes were considered in order to validate the process. The results obtained showed that the highest methane production rate (16.4 L CH4/kg COD) was obtained by a mixture containing 88% pig manure, 4% fish waste and 8% bio- diesel waste as compared to single digestion. Also, in studies done by (Ozturk, 2013), co-digestion of cattle manure and cheese whey yielded a higher rate of biogas production(0.72 min-1m-3) as compared to digestion of only cattle manure (0.63 min-1m-3). (Park et al. 2012), in evaluating the effect of co-digestion on methane yield and macronutrient degradation, co-digested algae biomass residue with lipid-rich fat, oil, and grease waste (FOG). The corresponding result was an increase in methane production. Aragaw et al. (2013), asserted that anaerobic co-digestion strategies are needed to enhance biogas production when treating certain residues such as cattle/pig manure. They co-digested food waste with animal manure or other feedstock with low carbon content and found that this can improve process stability and methane production. In their study, anaerobic digestion and co-digestion of cattle manure with organic kitchen waste using rumen fluid as inoculum was experimentally tested to determine the biogas potential, and co-digestion substantially increased the biogas yields by 24 to 47% over the control (organic kitchen waste and dairy manure only). Ugwu. (2012) studied 5 batch digesters containing varying ratios of mixture of chicken droppings and cow dung for a period of 30 days at ambient temperature for biogas production. Results from this study showed that co-digestion of chicken droppings and cow dung increased biogas yield as compared to pure samples of either chicken droppings or cow dung. To crown it all up, Wei Wu in the article titled “Anaerobic Co-digestion of Biomass for Methane Production: Recent Research Achievements”, included numerous studies which demonstrates that co-digestion improves biogas yields, with special attention being paid to the anaerobic co-digestion of animal waste, crop and crop residues, municipal solid waste, as well as municipal sewage sludge. This he attributed to positive synergisms established in the digestion medium and the supply of missing nutrients by the co-substrates.
The bacteria that have been isolated in biogas production from chicken droppings include: Methanococcus spp. Methanobacterium spp, Pseudomonas aeruginosa and Bacillus subtilis. The fungi that have been isolated in biogas production from chicken droppings include: Mucor mucedo, Aspergillus niger and Penicillium notatum (Oyewole, 2010). In addition, co- digestion offers several possible ecological and economic advantages as nowadays, refuse is no longer considered as waste but rather something that must be recovered or reused as potential resources (Wei, 2007). To this effect, efficient planning for municipal solid waste management system requires accounting for the entire cycle of wastes (Emery et al., 2006). Thus, to achieve a functioning yet stable process with high production of methane, it is necessary to create and maintain a beneficial environment for the activity of bacterial consortia of suitable specie (Kankal et al., 2012). This study is therefore aimed at determining the effect of co-digestion on microbial community during biogas production with a view of comparing the biogas productivity of the different feedstock composition.
Materials and Method
Biodigester
Three 1L, laboratory-scale digesters were constructed from 1000 cm3 non-corrosive pipes with internal diameters of 6cm. 3cm screw taps were tightly fitted towards the base of the biodigesters, which served as the influent and effluent ports. Gas cylinder valves were tightly fitted at the top of each digester and a gas meter attached to the valve to measure biogas production. The gas meter was calibrated at the University of Jos Chemistry Laboratory.
Sample Collection
The inoculum (Chicken droppings) was gotten from a commercial poultry and the substrates: Energy crops (Vegetables), Fat, oil and grease [FOG] (Animal fat) and Sugar source (Sugarcane juice, Waste yoghurt and Waste fruit) were gotten from Angwan rukuba market, Farin gada market and Chobe market. The substrates were dried and sorted into the different combinations as shown below:
Combination of substrates used
Chicken droppings (Inoculum)
Vegetables
Animal fat
Sugar source [Sugarcane juice (A), Waste yoghurt (B), Waste fruit (C)]
The segregates were then crushed into fine particles using a mortar and pestle. Slurry of each segregate was prepared in the ratio 1:1:1:1 of inoculum, vegetable, animal fat and sugar source. All the samples used were collected in Jos North LGA of Plateau State in tightly sealed sterile polythene bags and isolation, identification and characterization of microorganisms was carried out at Central Diagnostic Laboratory (CDL) of National Veterinary Research Institute (NVRI), Vom.
Experimental Design
The experimental design used for the production of biogas using anaerobic co-digestion involved the use of various combinations of substrates and chicken droppings, which served as an inoculum. The design is shown in Table A.
Table A: Experimental Design for the Production of Biogas using Anaerobic Co-digestion.
| Chicken dropping (g) | Vegetable (g) | Animal fat (g) | Sugar source (g) | |
| Digester 1 | 150 | 150 | 150 | 150 (A) |
| Digester 2 | 150 | 150 | 150 | 150 (B) |
| Digester 3 | 150 | 150 | 150 | 150 (C) |
The prepared slurry was placed in each digester in the ratio of 1:1 of chicken droppings and the various substrates in a water bath set at 37°C. The biodigester was tightly fitted to prevent the entry of oxygen using a stopper. The top of the biodigester was tightly fitted with valves and a gas meter attached to it. Air tightedness was ensured by the use of gas in sealing the biodigester to prevent leakage. Stirring was done during and after feeding the biodigester to avoid the formation of scum (Usack et al., 2014). The experimental set up was left for 30 days and a record of the amount of biogas produced was taken. Polyvinyl chloride tubes (PVC tubes) was fitted to the valve of the gas meter and the unattached end was channeled to an inverted measuring cylinder in a filled water trough. The measuring cylinder was firmly held using a retort stand. The rate of downward displacement of water in the measuring cylinder served as a measure of determining the amount of biogas generated. This was set up as the gas meter was unable to give readings of the amount of biogas generated.
Isolation of Microorganisms
Bacteria was isolated using nutrient agar and modified methanogenic agar medium under anaerobic condition at 37°C for 24hours while fungi were isolated using sabauraud dextrose agar under anaerobic condition at 28°C for 3-5 days after which total plate count was done.
Procedure
1g of digestate from each of the biodigesters was inoculated in 10mls of Reinforced Clostridial Medium (RCM) and incubated for 24 hours at 37°C to enable easy isolation of strict anaerobes and 1g of digestate was also inoculated in 10mls of Brain-Heart Infusion Broth (BHIB) to isolate facultative aerobes. This served as the stock. The digestate from the Reinforced Clostridial Medium (RCM) was serially diluted to 10-4 in Reinforced Clostridium Medium (RCM) and the digestate from the Brain-Heart Infusion (BHIB) was serially diluted to 10-4 in peptone water. 0.2 ml of the diluent was inoculated on Modified Methanogenic Agar Medium (MMAM) and Nutrient Agar (NA) and incubated for 24 hours at 37°C anaerobically (in an anaerobic jar) for RCM and aerobically for BHIB and sub-cultured on MacConkey agar and Blood agar to obtain pure isolates. Fungi was inoculated on Sabauraud Dextrose Agar and incubated at room temperature for 9 days.
Development of Modified Methanogenic Agar Medium
A Modified Methanogenic Agar Medium was developed in the laboratory to stimulate the standard methanogenic agar as described by Uwaezuoke, (1998).
Identification of Isolates
The bacterial isolates were identified based on their Gram reaction and biochemical tests. Anaerobic organisms was cultured and subcultured on Modified Methanogenic Agar Medium while other organisms were cultivated on conventional Nutrient agar, Blood agar and Mac Conkey agar media. The various bacterial isolates were repeatedly subcultured and subjected to morphological, microscopic and biochemical characterization which included production of coagulase, catalase, urease, oxidase, citrate utilization test, H2S production and carbohydrate fermentation as described by Cheesbrough, (2006) and Egbere, (2008) while fungal isolates were identified based on their morphological characteristics and microscopic appearance and then compared with the fungal atlas.
Results
Table 1 shows bacteria isolated from the biodigesters. The result obtained in Table 2 and Table 3 shows a pattern of bacteria and fungi respectively isolated from the inoculum and digestate. The organisms isolated from the inoculum (chicken droppings) were Camphylobacter spp, Bacillus cereus, Staphylococcus epidermidis, Staphylococcus aureus, Rhizopus spp and Aspergillus spp while Yersinia enterocolitica, Clostridium, Methanosarcina, Methanobacterium, Aspergillus and Penicillium species were predominant in the digestate in all biodigesters. The result of the percentage occurrence of bacteria and fungi isolated from the biogas digesters is presented in Figure 1. Methanobacterium spp and Methanosarcina spp were the methanogens isolated. Methanobacterium spp had the highest frequency of occurrence with 52% while Methanosarcina spp had a frequency of occurrence of 17%. The fungi isolated were Aspergillus spp and Penicillium spp with Aspergillus spp having a frequency of occurrence of 11% and Penicillium spp having a frequency of occurrence of 8%. Other bacteria isolated had 12% frequency of occurrence. Figure 2 depicts the cumulative volumes of biogas produced from the different biodigesters. Biodigester 3 produced the highest amount of 21 cm3, biodigester 2 produced 13 cm3 and biodigester 1 produced 11 cm3. The result of the pH of the various digesters before and after biogas production is presented in Table 4. The result indicates that there was a general decrease in the pH values with digester 1 yielding the highest percentage decrease in the pH value and digester 3 yielding the lowest.
 |
Table 1: Isolation and Characterization of Bacterial Isolates from the Biogas Digesters.
|
Table 2: Bacterial Isolates in the Inoculum and Digestate.
| Isolate | ||||||||||
| Camphylobacter | ||||||||||
| spp | ||||||||||
| Bacillus | ||||||||||
| cereus | ||||||||||
| Bacillus | ||||||||||
| megaterium | ||||||||||
| Staphylococcus | ||||||||||
| epiderdimis | ||||||||||
| Staphylococcus | ||||||||||
| aureus | ||||||||||
| Escherichia coli | ||||||||||
| Pseudomonas | ||||||||||
| spp | ||||||||||
| Clostridium | ||||||||||
| spp | ||||||||||
| Yersinia | ||||||||||
| enterocolitica | ||||||||||
| Methanogens | ||||||||||
| B A | B A | B A | B A | B A | B A | B A | B A | B A | B A | |
| Digester 1 | + – | + – | + + | + – | + – | + + | – – | – + | – + | – + |
| Digester 2 | + – | + – | – – | + – | + + | + – | – – | – + | – + | – + |
| Digester 3 | + – | + – | – – | + – | + – | + – | – + | – + | – + | – + |
Key: + Present B – Before fermentation (Inoculum)
– Absent A – After fermentation (Digestate)
Table 3: Fungal Isolates in the Inoculum and Digestate.
| Isolates | Rhizopus spp | Aspergillus spp | Penicillium spp |
| B A | B A | B A | |
| Digester 1 | + – | + + | – + |
| Digester 2 | + – | + + | – + |
| Digester 3 | + – | + + | – + |
Key + Present B – Before fermentation (Inoculum).
– Absent A – After fermentation (Digestate).
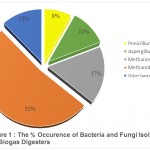 |
Figure 1 : The % Occurence of Bacteria and Fungi Isolated from the Biogas Digesters.
|
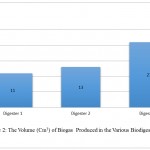 |
Figure 2: The Volume (Cm3) of Biogas Produced in the Various Biodigesters.
|
Table 4: pH of the Digesters Before and After Biogas Production.
| Digester 1 | Digester 2 | Digester 3 | |
| Duration of Fermentation (Days) | 32 | 32 | 32 |
| Initial pH | 8.67 | 8.52 | 9.14 |
| Final pH | 6.73 | 7.14 | 7.92 |
| % decrease in pH | 22.37 | 16.20 | 13.35 |
Discussion
There was a general decrease in the pH values of each digester showing a slight shift from an alkaline medium to a slightly acidic environment in all digesters. Digester 1 had the highest percentage decrease in pH (22.37%) while digester 3 had the lowest (13.35%). The decrease in pH values is obviously due to the acid production, a second stage of biodigestion (acidogenesis) in the production of biogas. The pH values fell within the optimum pH range as reported by Gashaw. (2014) for the production of methane, indicative of the presence of methanogens in the digestate. The high percentage decrease in pH in digester 1 could be attributed to the fermentation of sugarcane juice, as this was reported to lead to a reduction in pH by Pound et al. (2011) as opposed to other high sugar sources.
Digester 3 had the highest amount of biogas with 21cm3 generated compared to the other two digesters. This could be due to adequate moisture content provided by the waste fruit as Kigozi et al. (2014) reported that adequate moisture content in feedstock combination is required for excretive and other essential metabolic processes of the microorganisms, which leads to the production of more gas. It can also be attributed to the presence of indigenous microorganisms associated with waste fruit. High availability of nutrients makes microorganisms more active producing gas at a higher rate (Garba et al., 2009).
Less production in digester 1 could be due to the low final pH of 6.7, which is indicative of an acidic environment and Kangle et al. (2012) and other sources have reported that methanogens are sensitive to acid concentrations within the digester and their growth is inhibited by acidic conditions thus, in such an environment, biogas production will be at a lower rate.
Aspergillus spp and Penicillium spp isolated from the digesters are efficient physical and enzymatic degraders of lignocellulose-rich substrates and they make biogas production more efficient. Owamah et al. (2014) reported a similar result when they isolated Aspergillus spp and Penicillium spp from the co-digestion of food waste and human excreta.
Bacteria and fungi isolated from the inoculum and digestate indicates that the strict and facultative anaerobes thrived in the digestate because the pH and temperature of the digesters during biodigestion favoured their growth and as oxygen was depleted in the biodigesters, obligate aerobes that were in the inoculum died off and the methanogens utilized the resultant Co2 to generate methane.
The two groups of bacteria isolated from the digester during the anaerobic co-digestion include the acid-formers (Bacillus, Escherichia and Clostridium species) and methane formers (Methanosarcina and Methanobacterium species). These findings are in line with that of Egbere et al. (2011), Rabah et al. (2010) and Kankal et al. (2012). The presence of both groups of microorganisms determines the successful operation of anaerobic digesters for biogas production as reiterated in the study done by Owamah et al. (2014).
Conclusion
This study has shown that different segregates of municipal solid waste can be used in combination for the generation of biogas. The active microorganisms present during the biodigestion include Bacillus megaterium, Escherichia coli, Pseudomonas spp, Yersinia enterocolitica, Methanosarcina spp, Methanobacterium spp, Penicillium spp and Aspergillus spp. Digester 3 (Chicken droppings, Vegetable, Animal fat and Waste fruit) produced the highest amount of biogas from the different substrate combinations. Biogas technology will transform waste into resources, generate income and provide a pollution-free environment. The selective and strategic adoption of biogas technology in Nigeria will solve the problems of solid waste management confronting the nation presently.
This study recommends further studies should be carried out, using the various substrate combinations to produce biogas in large quantities. The methanogenic bacteria involved in biogas production should be isolated and characterized to species and strain levels and then used selectively for biogas production. Cultural techniques gives far less accuracy compared to culture-independent techniques as it is difficult to cultivate methanogens at culture level; Molecular characterization should therefore be adopted in further researches to give a true picture of the microorganisms implicated in biogas production. The health hazards associated with indiscriminate dumping of wastes around residential areas and other ecological sensitive areas such as rivers, streams and arable lands cannot be underestimated. Nigeria should therefore direct her effort towards the treatment of wastes before disposal so as to minimize the health hazards associated with dumping of wastes.
References
- Alvarez, J. A., Otero, L. and Lema, J. M. (2015). A Methodology for Optimising Feed Composition for Anaerobic Co-digestion of Agro-Industrial Wastes. Bioresourse Technology. 2(10); 1-7.
- Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J. L., Guwy, A. J., Kalyuzhnyi, S., Jenicek, P. and Van Lier, J. B. (2009). Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Science and Technology – WST. 59(5); 927-934.
- Aragaw, T., Andargie, M. and Gessesse, A. (2013). Co-digestion of Cattle Manure with Organic Kitchen Waste to Increase Biogas Production Using Rumen Fluid as Inoculums. International Journal of Physical Sciences. 8(11); 443-450.
- Asgari, A., White, E., Warton, E. M., Hararah, M. K., and Friedman, G. D. (2011). High Performances of Anaerobic Digesters Operating at Room Temperature. Bioresource Technology. 76(5); 428-443.
- Babaee, A., Shayegan, J. and Roshani, a. (2013). Anaerobic Slurry Co-digestion of Poultry Manure and Straw: Effcect of Organic Loading and Temperature. Journal of Environmental Health Science and Engineering. 11(1); 1-6.
- Bhattad, U. H. (2012). Preservation of Methanogenic Cultures. Dissertations. Paper 209; 1-171.
- Bijman Theo. (2014). Biogas from Poultry Waste – A case. Nijhuis Water Technology. 6; 1-12.
- Bitton Gabriel. (2005). Wastewater Microbiology. 3rd Ed. New Orleans. John Wiley and Sons Incorporated / Pp 345-369
- Braun, R., Wellinger, A., Holm-neilsen, J., Seadi, T., Jormanainnen, M., Jonsson, O. and Maltin, C. (2012). Potentials of Co-digestion. IEA Bioenergy. 6; 1-16.
- Breed, R. S., Murray, E. G. D., Smith, N. R. and Ninety-Four Contributors. (1957). Bergey’s Manual of Determinative Bacteriology. 7th Ed. Baltimore. The Williams and Wilkins Company / Pp 1-1130.
- Castano, J. M., Martin, J. F. and Citola, R. (2014). Performance of Small-Scale Variable Temperature Fixed Dome Digester in a Temperate Climate. Energies. 7; 5701-5716.
- Cheesbrough, M. (2005). District Lboratory Practice in Tropical Countries Part 1. 2nd Ed. New york. Cambridge University Press / Pp 62-70, 148-152.
- Cheesbrough, M. (2006). District Lboratory Practice in Tropical Countries Part 2. 2nd Ed. New york. Cambridge University Press / Pp 234-247.
- Christy, E. M., Sampson, N. M., Meyer, L. E., Okoh, A. I., Makaka, G. and Simon, M. (2013). Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. International Journal of Environmental Research and Public Health. 10(9); 4390-4417.
- Davidsson, K., Gilpin, L. C., Hart, C., Laurent, C., and Leakey, J. G. (2007). The Influence of Inorganic and Organic Nitrogen on the Trophic Dynamics of Microbial Food Webs. American Society of Limnology and Oceanography. 52(5); 2147-2163.
- Demirbas, M. F. and Balat, M. (2006). Recent Advances on the Production and Utilization Trends of Bio-fuels: A Global Perspective. Energy Conversion and Management. 47; 2371-2381.
- Dioha, I. J., Ikeme, C. H., Nafi’u, T, Soba, N. I., and Yusuf, M. B. S. (2013). Factors Affecting Biogas Production. Academic Journals. 3(7); 1-10.
- Egbere, J. O., Omogo, E. G., Henry, M. U., Henry U. I. and Chollom, P. F. (2011). Generation of Biogas from Segregates of Municipal Solid Wastes in Jos, Nigeria. Global Journal of Pure and Applied Sciences. 17; 41-45.
- Egbere, O. J. (2008). Principles and Practice of Food Microbiology. 1st Ed. Jos, Nigeria. Deka Publications, Jos / Pp 9.
- EPA (2012). United States Environmental Protection agency (EPA) (2012). Increasing Anaerobic Digester Performance with Co-digestion. Agstar. 9; 1-3.
- Fernandez, R. L., Montavez, J. P., Saenz, J., and Zorita, E. (2007). Anaerobic Digestion: Current Research and Future Research. International Energy Journal. 18(5); 87-98.
- Fezzani, M., Bencheikh, P., and Neves, O. (2007). Effect of Thermophilic Temperature on Anaerobic Co-Digestion. Research Gate. 20(9); 200-207.
- Garba, B., Usman, N. S. and Sagagi, B. S. (2009). Studies on Biogas Production from Fruits and Vegetable Waste. Bayero Journal of Pure and Applied Sciences. 2(1); 115-118.
- Gashaw, Alemayehu. (2014). Anaerobic Co-digestion of Biodegradable Municipal Solid Waste with Human Excreta for Biogas Production: A Review. American Journal of Applied Chemistry. 2(4); 55-62.
- Gelegensis, E., Johnson, K. V., Blow, F., and Kampman, P. (2007). Biogas Production by Anaerobic Co-Digestion of Casttle Slurry. Journal of Renewable and Sustainable Energy. 6(12); 124-136.
- Glass Jennifer. (2011). Characterization of Methanogenic Communities and Nickel Requirements for Methane Production from Wood Hole Marshes and Isolation of a Novel Methanogen of the Order Methanomicrcobiales from Eel Pond Mud. Microbial Diversity. 1; 1-28.
- Gomez Rebekkah. (2006). Methane Generation from Anaerobic Digesters: Considering Different Substrates. Journal of Science, 2(1); 1-11.
- Gopinah, L. R., Christy, P. M.., Mahesh, K., Bhuvaneswari, R and Divya, D. (2014). Identification and Evaluation of Effective Bacterial Consortia for Efficient Biogas Production. IOSR Journal of Environmental Science, Toxicology and Food Technology. 8(3); 80-88.
- Green, S., Maanum, B. and Brown, L. (2006). Gas Biodigester Information and Construction Manual for Rural Families. 1st Ed. Honduras. FUCOSH / Pp 1-8.
- GUA (Undated). Guyana Energy Agency. Biodigester Information and Construction Manual for Small Farmers. 1st Ed. / Pp 1-23.
- Jenangi, L and Harris, P. (2012). Producing Methane Gas from Efluent. Diploma in Agricultural Production Individual Project. 1; 1-23.
- Jepsen, S. E. (2012). Co-digestion of Animal Manure and Organic Household Waste – The Danish Experience. Ministry of Environment and Energy, Danish EPA. 1; 1-7.
- Jijai, S., Srisuwan, G., O-thong, S., Ismail, R. and Siripatana, C. (2014). Specific Methanogenic Activities (SMA) and Biogas Production of Different Granules Size and Substrates. 1st Environmental and Natural Resources International Conference. 1; 7-10.
- Kanger, K., Truu, J., Ebner, C. and Nolvak, H. (2013). Biogas Production Under Co-digestion of Food Waste with Sewage Sludge. Journal of Ecology and Earth Sciences. 1; 5-32.
- Kangle, K. M.., Kore, S. V. and Kulkarni, G. S. (2012). Recent Trends in Anaerobic Co-digestion: A Review. Universal Journal of Environmental Research and Technology. 2(4); 210-219.
- Kankal, N. C., Sharda, D. and Kumari, B. (2012). Study of Diverse Methanogenic and Non-methanogenic Bacteria Used for the Enhancement of Biogas Production. International Journal of Life Sciences Biotechnology and Pharma Research. 1(2); 176-191.
- Karlsson, A., Bjorn, A., Yekta, S. S. and Svensson, B. (2014). Improvement of the Biogas Production Process. Biogas Research Center (BRC). 2; 61-88.
- Karlsson, M., Ak, A. E. W. Hallin, S.,Vallin, L. and Schnurer, A. (2011). Slaughterhouse Waste Co-digestion – Experiences from 15 Years of Full-Scale Operation. Bioenergy Technology (BE). 1; 64-71.
- Khalid, A. and Naz, S. (2013). Isolation and Characterization of Microbial Community in Biogas Production from Different Commercially Active Fermentors in Different Regions in Gujranwala. International Journal of Water Resources and Environmental Sciences. 2(2); 28-33.
- Khalid, A., Arshad, M., Anjum, M., Mahmood, T. and Dawson, L. (2011). The Anaerobic Digestion of Solid Organic Waste. Waste Management. 31(8); 1737-1744.
- Kigozi, R., Aboyade, A. and Muzenda, E. (2014). Biogas Production using the Organic Fraction of Municipal Solid Waste as Feedstock. International Journal of Research in Chemical, Metallurgical and Civil Engineering. 1(1); 1-8.
- Kumar, S. and Mudhoo, A. (2013). Effects of Heavy Metals as Stress Factors on Anaerobic Digestion Process and Biogas Production from Biomass. International Journal of Environmental Science and Technology. 10; 1383-1398.
- Lama, L. Lohani, S. P., Lama, R. and Adhikari, J. R. (2012). Production of Biogas from Kitchen Waste. Rentech Symposium Compendium. 2(12); 14-18.
- Lauwers, J., Appels, L., Degreve, J., Willems, K. and Dewil, R. (2011). Anaerobic Digestion in Global Bio-Energy Production: Potential and Research Challenges. Renewable and Sustainable Energy Reviews. 12; 34-48.
- Lazor, M. Huntan M., Sedleec, S., Kolesarova N, and Spalkova V. (2010). Anaerobic Co-digestion of Poultry Manure and Waste Kitchen Oil. 37th International Conference of Slovak Society of Chemical Engineering. 5; 1399-1406.
- Lehtomaki, G. K., Patrick, C., and Hallenbeck, J. (2007). Anaerobic Co-Digestion of Biomass for Methane Production. Research Gate. 3(6); 13-39.
- Long, W. C., Swiney, K. M., Harris, C., Page, H. N., and Foy, R. J. (2013). Effect of Acidification on Anaerobic Digestion and Biogas Production. PLOS, ONE. 8(4); 1371-1384.
- Manyi-loh, ., Mamphweli, S., and Meyer, E. (2013). Microbial Anaerobic Digestion (Biodigesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and Generation of Renewable Energy. International Journal for Environmental Research and Public Health. 10(9); 4390-4417.
- Odejimi, R. A. O. and Udotong, I. R. (2005). The Prospects for the Adoption of Biogas for Solid Waste Management in Urban Markets in Nigeria. Journal of Environmental Sciences. 9(2); 62-67.
- Ogunrinola, I. O. and Adepegba, E. O. (2012). Health and Economic Implications of Waste Dumpsites in Cities: The Case of Lagos, Nigeria. International Journal of Economics and Finance. 4(4); 239-251.
- Okoroigwe, E. C., Ibeto, C. N. and Ezema, C. G. (2014). Study of Anaerobic Digestion of Dog Waste. Scientific Research and Essays. 9(6); 121-127.
- Olufunmi, A. O. (2014). Microbiological Potentials of Co-digestion of Chicken Droppings and Banana Peels as Substrates for Biogas Production. Journal of Chemical and Pharmaceutical Research. 6(4); 1088-1092.
- Onojo, O. J., Chukwudebe, G. A., Okafor, E. N. C., and Ononiwu, G. C. (2013). Anaerobic Co-Digestion of Biodegradable Municipal Solid Waste. American Journal of Engineering Research. 23(7); 198-209.
- Opeh, R. and Okezie, U. (2011). The Signifance of Biogas Plants in Nigeria’s Energy Strategy. Journal of Physical Sciences and Innovation. 3(3); 11-17.
- Owamah, H. I., Dahunsi, S. O., Oranusi, U. S. and Alfa, M. I. (2014). Fertilizer and Sanitary Quality of Digestate Biofertilizer from the Co-digestion of Food Waste and Human Excreta. Waste Management. 34(4); 747-752.
- Oyewole, O. A. (2010). Biogas Production from Chicken Droppings. Science World Journal. 5(4); 11-14.
- Ozturk, Bahitiyar. (2013). Evaluation of Biogas Production Yields on Different Waste Materials. Journal of Sciences. 2(1); 165-174.
- Park, S. and Li, Y. (2012). Evaluation of Methane Production and Macronutrient Degradation in the Anaerobic Co-digestion of Algae Biomass Residue and Lipid Waste. Bioresourse Technology. 111; 42-48.
- Pound, B., Done, F,. and Preston, T. (2011). Biogas Production from Mixtures of Cattle Slurry and Pressed Sugarcane Stalk With and Without Urea. Tropical Animal Production, 6(1); 1-11.
- Rabah, A. b., Baki, A. S., Hassan, L. G., Musa, M. and Ibrahim, A.D. (2010). Production of Biogas Using Abattoir Waste at Different Retention Time. Science World Journal. 5(4); 23-26.
- Rasi, S., Rossi, D., Trifonov, V., Grunn, A., and Fangazio, M. (2011). Bioenergy Production by Anaerobic Digestion: Using Agricultural Biomass and Organic Wastes. Bioresource Technology. 76(5); 326-331.
- Rintala, J. and Salminen, E. (2012). Anaerobic Digestion of Organic Solid Poultry Slaughterhouse Waste – A Review. Bioresoure Technology. 83; 13-26.
- Sousa, A. R., Penalva, L. O., Marcotte, E. M., and Vogel, C. (2009). Isolation and Characterization of Fungi Involved in Biogas Production. Science World Journal. 4(7); 61-72.
- Themilis, N. J., Moreno, P. C., and Yue, D. (2010). Waste Characterization and Recoverable Energy Potential: Waste-to-Energy Technology in Mexico. Journal of Ecology and Earth Sciences. 1; 1-5.
- Ugwu, G. Z., Nnabuchi, N. M., Akubuko, F. O. and Augustine, C. (2012). Assessment of the Effect of Co-digestion of Chicken Dropping and Cow Dung on Biogas Generation. Global Journal of Science Frontier Research Physics and Space Sciences. 12(7); 2-6.
- UNEP (2010). Environmental Pollution and Impacts on Public Health: Implications of the Dandora Municipal Dumping Site in Nairobi Kenya. United Nations Environmental Programme, Nairobi Kenya. 1; 1-27.
- Usack, M. R., Arsova, L., Bme, T., and Cheme, D. (2014). Design of Scalable Biogas Digesters for the Developing World. Journal of Sciences. 17(11); 23-49.
- Vinneras, B., Nordin, A. and Schonning, C. (2007). Microbial Community in Biogas Systems and Evaluation of Microbial Risks. Energie | Wasser – Praxis. 4; 50-53.
- Wang, J., Zhang, Z., Zheng, P. and Li, C. (2007). The Influence of Aerobic Sludge Retention Time on Anaerobic Co-digestion of Dyeing and Printing Wastewater and Sewage Sludge. African Journal of Biotechnology, 6(7); 902-907.
- Ward, A. J., Hobbs, P. J., Holliman, P. J. and Jones, D. L. (2008). Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresource Technology. 99(17); 7928-7940.
- WBA (2013). World Bioenergy Association. (2013). Biogas – An Important Renewable Energy Source. World Bioenergy Fact Sheet / Pp 1-7.
- Weiland Peter. (2015). Biogas Production: Current State and Perspective. Applied Microbiology and Biotechnology. 85(9); 849-860.
- Willey, J. M., Sherwood, L.M. and Woolverton, C. J. (2008). Prescott, Harley and Klein’s Microbiology. 7.ed. New York. Mcgraw Hill / Pp 493-531.
- Wolfe, R. S. (2011). Techniques for Cultivating Methanogens. Methods in Enzymology. 494; 1-22.
- Wu Wei. (2007). Anaerobic Co-digestion of Biomass for Methane Production: Recent Research Achievements. Energie. 4; 1-10.
- Yin, J., Overpeck, J. T., Griffles, S. M., Hu, A., Russell, J. L., and Stouffer, R. J. (2011). Different Magnitudes of Anaerobic Digestion around Greenland and Antarctica. Nature Geoscience. 4(3); 524-528.

This work is licensed under a Creative Commons Attribution 4.0 International License.






