How to Cite | Publication History | PlumX Article Matrix
Low Cost Microfluidic Device for Assaying Blood Glucose
Azmi Naqvi*1, Dinesh C. Sharma2 and Pradip Nahar3
1Women Scientist (WOSB, DST), Department of Zoology, Km. Mayawati Government Girls P.G. College, Badalpur, Gautam Buddha Nagar (U.P.) – 203207, India.
2Department of Zoology, Km. Mayawati Government Girls P.G. College, Badalpur, Gautam Buddha Nagar (U.P.) – 203207, India.
3New Idea Lab, C-202, Ramkrishnan Cooperative Group Housing Society, Plot-12, Sector 23, Dwarka, New Delhi 110077, India.
Corresponding Author E-mail: azminqv@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2744
ABSTRACT: Herein, gravitational force based low cost colorimetric microfluidic device is developed for diagnostic purpose. Microfluidic system is developed by using discarded pen refills. Refill is filled with three layers of polymer. Bottom of the refill is filled with the polymer polyvinyl chloride (PVC). Second layer from the bottom i.e the layer above PVC layer is filled with silica gel immobilized with horse radish peroxidase (HRP), glucose oxidase (GOD) and o- dianisidine (dye). Whereas, third and the top most layer is filled with untreated silica gel. One drop of blood is poured at the inlet of microfluidic device. Without applying any external power, blood moves through the silica packed region by gravitational pull and capillary action of silica gel. Serum separation started within 30 seconds and subsequently within 2 min., serum successfully separates from blood by pure silica gel. The separated serum then comes in contact with the silica gel immobilized with enzymes and dye. The colour of the silica beads immobilized with enzymes and dye changes from white to orange when comes in contact with glucose in serum. Determination of the glucose in the blood is carried out on a desktop scanner. The developed microfluidic device do not require (i) pump or device to propel the fluid (ii) any type of special mesh or sieve to separate the serum from the blood. Microfluidic device developed is cheap and suitable for low cost setting areas.
KEYWORDS: Capillary Driven Microfluidic Device; Point-of-Care Diagnostics; Silica Gel
Download this article as:| Copy the following to cite this article: Naqvi A, Sharma D. C, Nahar P. Low Cost Microfluidic Device for Assaying Blood Glucose. Biosci Biotech Res Asia 2019;16(2). |
| Copy the following to cite this URL: Naqvi A, Sharma D. C, Nahar P. Low Cost Microfluidic Device for Assaying Blood Glucose. Biosci Biotech Res Asia 2019;16(2). Available from: https://bit.ly/2G27m1Z |
Introduction
The field of microfluidics is characterized by the study and manipulation of fluids at the submillimetre length scale.1 Most of the microfluidic devices often require one or more pumps to propel fluid through microchannels or additional power supply as driving force2 which makes the system quiet expensive and complicated.
Gravitational pull based microfluidic devices based on gravitational force are cheap and simple to operate.3 However, these devices are hard to develop since they are based on only gravitational force. Rhee et. al. has describes the hydrodynamic and gravitational force-based positioning of cells within microfluidic devices that can be implemented without special equipment or fabrication steps.4 Yao et. al. has developed a gravity and electric force driven microfluidic device for cell sorting.5 Most of the work related to gravitational pull based microfluidic device reported till now is mainly related to the area of cell sorting and particle separation. There are hardly any reports on the gravitational pull based microfluidic device, which are used for detecting glucose in blood.
This paper reports a silica gel based low cost glucose detection system in a discarded pen refills. Silica serves as a well-established dispersing material for proteins because it is non-toxic, chemically and biologically inert, subject to negligible hydration swelling, hydrophilic, and inexpensive to synthesize.6,7
Diabetes is one of the principal causes of death and disability in the developing world, and is highly responsible for heart disease, kidney failure, and blindness. It is a major cause of mortality in the age group of 20–79 years. Based on its rapidly increasing incidence, diabetes has been declared a global epidemic by the World Health Organization (WHO).8 Frequent testing of physiological blood glucose levels to avoid diabetic emergencies, is crucial for the confirmation of effective treatment. There have been continuously increasing research efforts in the field of glucose monitoring during the last few decades. The frequent monitoring of blood glucose is critical for diabetic management, as the maintenance of physiological glucose level, i.e., 4–8 mM (72–144 mg/dL), is the only way that a diabetic can lead a healthy lifestyle by avoiding life-threatening diabetic complications, such as diabetic retinopathy, kidney damage, heart diseases, stroke, neuropathy and birth
The most widely used glucose monitoring devices are blood glucose meters which is based on minimally-invasive fingerstick tests that have a substantial market. Several continuous glucose monitoring systems (CGMS), which are presently more than five years old, can also detect glucose at low levels9 Most of the CGMS employ minimally-invasive approaches the use of subcutaneous sensors to determine the glucose concentration in interstitial fluid. Therefore, they cause discomfort to patients, require more frequent calibration by fingerstick tests and cannot be used for more than few days, as the sensor is prone to biofouling. However,the main limitation is their extremely high cost, which is beyond the reach of most diabetics. Besides this, it also requires calibration with blood glucose testing and change of the sensor after a few days. This strictly limits their use to only selected clinical scenarios, where they are critically required.
Glucose oxidase (GOD)-based glucose biosensors have prevalently held the glucose sensor research and development over the last four decades and the market place as well. This is the due to the good stability of GOD that make the glucose/glucose oxidase system a very convenient model for glucose detection. There is a high demand for sensitive and reliable blood glucose monitoring in biological and clinical aspects. However, high cost of glucose sensors available in the market is one of the main disadvantages of enzyme-based glucose determination. Therefore, the development of low-cost detection system that can reduce the operational cost, calibration and warm up periodshas been the subject of concern.
In the present work, low cost colorimetric microfluidic device for detection of glucose is developed. Capillary force driven microfluidic system is developed by using discarded pen refills of 2.66 mm width and 4 cm height.Pen refills are generally made up polypropylene polymer. Polypropylene is inert and is insoluble in most of the organic solvents, hence is suitable for the present work. Refill is packed with three layers of polymer. Bottom of the refill is filled with polyvinyl chloride (PVC) 20mgto prevent the leakage of fluid from the refill. The strong and linear nature of the polymer prevents the dispersion of the liquid from the loaded refill. Second layer i.e the layer above PVC layer is filled with 5mgof treated silica gel i.e silica gel immobilized with Horse radish peroxidase (HRP) Glucose Oxidase (GOD) and o- dianisidine (dye), henceforth named as treated silica. Whereas, third and the top most layer is filled with pure silica gel.In the present study, pure silica gel is used to separate the serum from blood within a short period of time. Whereas treated silica is used for detecting glucose in the blood. Glucose oxidase oxidizes glucose into D- glucono -1,5 lactone with formation of hydrogen peroxide. Horseradish peroxidase enzyme further breaks hydrogen peroxide to water and oxygen. Oxygen then react with an oxygen acceptor dye (OPD) which itself converted to a coloured compound, the amount of which can be measured colorimetrically.
To check the workability of the device, glucose is detected in the blood samples. Four microliter of freshly drawn human blood was carefully dropped at the inlet of microfluidic device. Without applying any external power, blood moves through the silica packed region by capillary force. The movement of blood cells was impeded by small pores between the packed-beads. The hydrophilic surface of the packed beads induced the capillary flow of serum. After dropping 4μl of whole human blood, the serum separation started within 30 seconds and serum has been successfully separated within 2 minutes by the silica gel. The separated serum then comes in contact with the treated silica beads. The colour of the treated silica beads change its colour from white to orange when comes in contact with glucose in serum.
Determination of the glucose in the blood is carried out on a desktop scanner (HP photo smart C6388) by placing the tube in the scanner to get the image. From Adobe Photoshop the mean value of each R, G and B of the scanned image was obtained which was then converted to HSB (Hue, Saturation, and Brightness) using freely available “Macbeth color calculator” software. Image was then quantified as saturation percentage. We have termed “saturation” as “color-saturation” to make it clear as well as to differentiate it from conventional chemical understanding of saturation.10
Experimental
Material and Methods
Horse radish peroxidase (HRP) was purchased from Sigma (USA). Glucose Oxidase (GOD) was purchased from Boehringerb Mannheimn GmbH ,Silica (Sisco Research Laboratories Pvt. Ltd.) D- Glucose, o-dianisidine (dye) and polyvinyl chloride (PVC) were of analytical grade and purchased either from SRL, Glaxo or Merck (India). All buffer solutions were freshly prepared in triple distilled water before use.
Phosphate Buffered Saline (PBS) was prepared by mixing 0.85 % NaCl to 0.01 M phosphate buffer (pH 7.2). A 1.5mg/ ml stock solution of enzyme HRP and GOD was prepared in PBS and stored in refrigerator for further use. Citrate-buffer (pH 5) was prepared by mixing 0.025 M citric acid and 0.05 M di-sodium-hydrogen phosphate in 100 ml distilled water. o-dianisidine (dye) was prepared in Citrate buffer. Glucose solution is prepared in distilled water.
Human blood was obtained from healthy volunteer as well as from diabetic one. Blood sugar is checked with Blood Glucose meter (ACCU-CHEK)
Microfluidic Device Fabrication
Microfluidic device was made from discarded pen refill. Gravity driven microfluidic system is developed by using discarded pen refills having 2.66 mm width and 4 cm height. Refill is washed by acetone to remove the ink remaining in the refill and dried. Bottom of the refill is sealed with parafilm at the end to prevent the leakage of the liquid. 20 mg of PVC is filled at the bottom of the refill to prevent the diffusion of colour. Above the PVC layer,10 mg dried treated silica is loaded and subsequently, 40 mg of pure silica is loaded onto the treated silica. Refills are now ready for glucose estimation.
Optimization of Amount of Glucose Oxidase (GOD) for Immobilization Onto the Pure Silica Gel to Get Treated Silica Gel
Petri plate containing 50 mg pure silica gel is taken and mixed with HRP (20 µg/ 50 µl PBS)and dye (400 µg/50 µl buffer). This mixture is divided into 4 parts each having 10 mg of the mixture. Four parts of the prepared mixture are taken and varying amounts of GOD corresponding to 6, 7, 8 and 9 µg/10 µl PBS is added respectively to get treated silica. Treated silica gels are then kept for drying in dark.
Four clean refills are taken and one end of each refill is closed with parafilm. PVC (20 mg/refill) is then filled in the refill as a bottom layer of the device. Then, 10 mg oftreated silica containing varying amount of GOD is loaded into the refill separately. Subsequently, 40 mg of pure silica is loaded onto the treated silica to get four separate refills containing varying amount of HRP loaded refill.
To get optimum amount of GOD, 4 µl of glucose solution (200 mg/dl) is poured to each refill. Subsequently PBS (76 µl) is poured into each refill. After 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) to get an image. From Adobe Photoshop the mean value of R, G and B of the scanned image was obtained which was then converted to HSB (Hue, Saturation, and Brightness) using freely available “Macbeth colour calculator” software. Image was then quantified as saturation percentage.
Optimization of Amount of HRP for Immobilization Onto Pure Silica Gel to Treated Silica Gel
Petri plate containing 50 mg pure silica gel is taken and mixed with GOD (35 µg/50 µl) and dye (400 µg/50 µl). This mixture is divided into 4 parts each having 10 mg of the prepared mixture. Four parts are taken and varying amounts of HRP corresponding to 2, 3, 4 and 5 µg/ 10 µl PBS buffer is added separately and respectively. These mixtures are then kept for drying in dark.
Four clean refills are taken and one end of each refill is closed with parafilm. PVC (20 mg/refill) is then filled in the bottom layer of the refill. 10 mg of treated silica with varying amount of HRP is loaded into the refill separately. Subsequently, 40 mg of pure silica is loaded onto the silica mixture to get four separate fully loaded refill of varying HRP concentration.
To get the optimum amount of HRP, 4 µl of glucose solution (200 mg/dl) is poured to each refill. Subsequently PBS (76 µl) is poured into each refill. After 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) to get the image and saturation percentage was calculated as described above.
Optimization of Amount of o-Phenyledene Diamine (Dye) for Immobilization Onto Silica Gel
Petri plate containing 50 mg pure silica gel is taken and mixed with GOD (35 µg/50µl) and HRP (20 µg/50 µl). This mixture is divided into 4 parts each having 10 mg of the prepared mixture. Four parts are taken and varying amounts of dye corresponding to 40, 60, 80 and 100 µg/10 µl citrate buffer is added. Four petriplates containing mixtures are then kept for drying in dark.
Four clean refills are taken and one end of each refill is closed with parafilm. PVC (20 mg/refill) is then filled in the bottom layer of the refill (device). 10 mg of dried treated silica with varying amount of dye is loaded into the each refill respectively. Subsequently, 40 mg of pure silica is loaded onto the silica mixture to get fully loaded refill.
For detection, 4 µl of glucose solution (200 mg/dl) is poured to each refill. Subsequently PBS (76 µl) is poured into each refill. After 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) to get the image and saturation percentage was calculated as described above.
Determination of Glucose in Synthetic Sample
Treated silica is prepared by adding HRP (40 µg/100 µl), GOD (70 µg/100 µl) and dye (800 µg/100 µl) with 100 mg of pure silica gel. Treated silica is filled in the different tube as described in earlier sections.
Four µl of varying concentration of D-glucose (50, 80, 100,150, 200, 250, 300 and 400 mg/dl) is added to each refill. Subsequently, PBS (76 µl) is poured and after 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) to get the image and saturation percentage was calculated as described above.
Determination of Glucose in Blood Samples with Added Synthetic Glucose
Treated silica is prepared by adding HRP (40 µg/100 µl), GOD (70 µg/100 µl) and dye (800 µg/100 µl) with 100 mg of pure silica gel. Treated silica is filled in the different tube as described in earlier sections.
Fresh blood sample from fasting individual is taken and glucose level is measured in blood sample with digital glucose meter which was found to be 80 mg/dl. Varying concentration of synthetic glucose (0, 20, 70, 120, 170, 220, and 320 mg/dl) is added in 4 µl of blood sample to get seven sample of varying concentration containing 80, 100, 150, 200, 250, 300, 400 mg/dl glucose respectively. For detection, to each refill 4 µl of prepared samples were poured. Subsequently, PBS (76 µl) is poured onto each refill and after 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) to get the image and saturation percentage was calculated as described above.
Determination of Glucose in Blood Samples Without Adding Synthetic Glucose
Treated silica is prepared by adding HRP (40 µg/100 µl), GOD (70 µg/100 µl) and dye (800 µg/100 µl) with 100 mg of pure silica gel. Treated silica is filled in the different tube as described in earlier sections.
Four µl of blood sample from three individuals, (i) from fasting individual (ii) from non- fasting individual (iii) from diabetic individual, is poured into each refill respectively for detection. PBS (76 µl) is subsequently poured into the refills and after 8 minutes, the refills were scanned on a desktop scanner (HP photo smart C6388) and to get the image saturation percentage was calculated as described above.
Determination of R.G.B. Values
The colour formed after reaction was scanned on a desktop scanner. The scanned image was opened in Adobe Photoshop Version 7.0. The area of colour was selected using a rectangular marquee. After the region is selected, the median of R.G.B. values for each refill were noted.
Determination of Saturation
Saturation of each reading was calculated by Macbeth calculator. This is used for calculating hue, saturation and brightness. Saturation is calculated by providing R.G.B. values calculated from Adobe Photoshop. Saturation was noted down for each refill.
Preparation of Graphs Based on Concentration and Saturation Values
Graphs were prepared by plotting saturation percentage depending on the colour obtained against their concentrations.
Result and Discussion
The most common way to check glucose levels involves pricking a fingertip with an automatic lancing device to obtain a blood sample and then using glucose strips of glucose meter to measure the blood sample’s glucose level. Glucose test strips of glucose meter are expensive due to expensive enzymes, precious metals, chemicals, and other materials that make up test strips.
We are proposing gravitational force driven and capillary action based low cost microfluidic device for assaying blood glucose. The proposed microfluidic device is made out of discarded pen refillsof 2.66 mm width and 4 cm height. Pen refills are generally made up polypropylene polymer which is inert in nature and is insoluble in most of the organic solvents. Pen refills filled with the polymer viz. silica, is utilized as a multipurpose polymer i.e. it is used for separating sera from blood as well as used for detecting glucose when GOD is immobilized onto it.
Silica serves as a well-established dispersing material for proteins because it is non-toxic, chemically and biologically inert, subject to negligible hydration swelling, hydrophilic, and inexpensive. In the present study, pure silica gel is used to separate the serum from blood within a short period of time.14 Whereas treated silica i.e silica gel immobilized with GOD, HRP and dye is used for detecting glucose in the blood. Glucose oxidase oxidizes glucose into D- glucono -1,5 lactone with formation of hydrogen peroxide. Horseradish peroxidase enzyme further breaks hydrogen peroxide to water and oxygen. Oxygen then react with an oxygen acceptor dye (OPD) which itself converted to a coloured compound, the amount of which can be measured colorimetrically.
Figure 1 shows the Schematic illustrations of packed silica beads and blood/ serum separation. Without applying any external power, blood moved through the silica packed region by capillary force and serum separation started within 30 seconds and successful serum separation take place within 2 minutes of loading.
 |
Figure 1: Schematic illustrations of packed silica beads and blood/ serum separation.
|
Enzymatic determination of Glucose onto the treated silica gel is carried out and is noted that the treated silica gel (silica immobilized with GOD, HRP and dye) can detect the glucose concentration successfully.In order to obtain optimum amount of HRP, GOD and dye and to get best treated silica gel, three separate experiments were carried out. As shown in figure 2, optimum amount of GOD required to prepare treated silica gel is 0.7 µg per 50 mg silica gel, whereas optimum amount of HRP required is 0.4 µg per 50 mg silica (figure 3). o-dianisidine (dye) required to act appropriately with optimum amount of HRP and GOD is found to be 80 µg per 50 mg silica (figure 4).
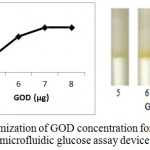 |
Figure 2: Optimization of GOD concentration for the preparation of microfluidic glucose assay device.
|
Varying amounts of GOD (5 µg, 6 µg, 7 µg and 8 µg per 10 µl PBS ) are taken in the glucose assay device. Optimization of GOD is subsequently carried out by pouring glucose 200 mg/dl into glucose assay device. Images were scanned in HP scanner. Colour saturation was obtained from the scanned images.
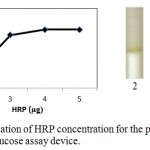 |
Figure 3: Optimization of HRP concentration for the preparation of microfluidic glucose assay device.
|
Varying amounts of HRP (2 µg, 3 µg, 4 µg and 5 µg per 10 µl PBS buffer) are taken in the glucose assay device. Optimization of HRP is subsequently carried out by pouring glucose 200 mg/dl into glucose assay device. Images were scanned in HP scanner. Colour saturation was obtained from the scanned images.
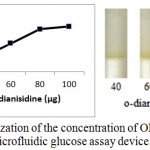 |
Figure 4: Optimization of the concentration of OPD for the preparation of microfluidic glucose assay device.
|
Varying amounts of OPD (40 µg, 60 µg, 80 µg and 100 µg per 10 µl citrate buffer) are taken in the reaction mixture prepared for glucose assay device. Optimization of OPD is subsequently carried out by pouring glucose 200mg/dl into glucose assay device. Images were scanned in HP scanner. Color saturation was obtained from the scanned images.
Figure 5 shows enzymatic determination of glucose onto the treated silica gel (silica immobilized with GOD, HRP and dye). Standard plot is made using varying glucose concentration. It is noted from figure 5 that the % saturation increases with increase in glucose concentration.
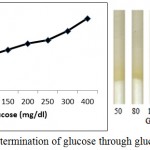 |
Figure 5: Enzymatic Determination of glucose through glucose assay device.
|
Varying concentration of D-Glucose (50 mg/dl, 80 mg/dl, 100 mg/dl, 150 mg/dl, 200 mg/dl, 250 mg/dl, 300 mg/dl and 400 mg/dl) is poured onto the microfluidic glucose assay device. Images were scanned in HP scanner. Colour saturation was obtained from the scanned images.
Determination of Glucose in blood sample with added synthetic D-glucose is further carried out. In this experiment, blood sample is taken from fasting individual and glucose level is checked with digital glucose meter. The blood glucose level in the fasting individual is found to be 55 mg/dl. Four microliter of blood sample is taken from the same fasting individual (with glucose level 55 mg/dl) in seven different eppendorf and varying glucose concentration of 25, 45, 95, 145, 195, 245 and 345 mg/dl is added in the blood sample to get seven sample of varying concentration of 80, 100, 150, 200, 250, 300, 400 mg/dl respectively. These prepared samples were then poured in the loaded pen refills and % saturation is determined (fig. 6)
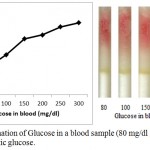 |
Figure 6: Determination of Glucose in a blood sample (80 mg/dl glucose) with added synthetic glucose.
|
Blood samples from fasting individual is taken and varying concentration of synthetic glucose (0, 20, 70, 120, 170, and 220 mg/dl) is added in the blood sample to get eight samples of varying concentration of glucose (80, 100, 150, 200, 250, and 300 mg/dl). These samples are poured into the microfluidic glucose assay device. Images were scanned in HP scanner. Color saturation was obtained from the scanned images.
On comparing the % saturation of figure 5 (wherein the % saturation of only synthetic glucose is demonstrated and standard glucose plot is made) with that of figure 6, it is very clear that the amount of sugar can be calculated using figure 4, since the results are comparable.
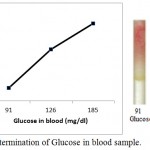 |
Figure 7: Determination of Glucose in blood sample.
|
Blood samples from three individuals (i) from fasting individual (ii) from non- fasting individual (iii) from diabetic individual are poured into the microfluidic glucose assay device. Images were scanned in HP scanner. Color saturation was obtained from the scanned images.
Further, we also detected the blood glucose level in three individual, where we have not added any synthetic sugar in human blood (fig. 7). In this case we took blood sample from (i) from fasting individual (ii) from non- fasting individual (iii) from diabetic individual. It is very clear from figure 7 that the detection of the sugar can be carried out with the help of glucose standard plot (figure 5), since the results are comparable
Conclusion
A simple, rapid and economical microfluidic device for assaying blood glucose level is developed. Developed glucose detection system is far simpler and cheaper than the systems/ machine available in the market. With minor modifications in the treated silica region, the device can be used in other areas of diagnostics also.
Acknowledgements
A.N thanks the Depoartment of Science and Technology, Government of India for the award of a “Women Scientist (WOS B)” Fellowship.
References
- Sackmann, K., Fulton, A. L. and Bebee, D.J.: The present and future role of microfluidics in biomedical research, Nature, 507,181–189 (2104).
CrossRef - Araci, I.E. and Quake, S. R.: Microfluidic very large scale integration (mVLSI) with integrated micromechanical valve. Lab Chip, 2803-2806 (2012)
CrossRef - Huh,D., Bahng, H., Ling, Y., Wei, H., Kripfgans, O. D., Fowlkes, J. B., Grotberg, J. B., and Takayama, S.: A Gravity-Driven Microfluidic Particle Sorting Device with Hydrodynamic Separation Amplification. Anal Chem., 79(4), 1369–1376 (2007).
CrossRef - Rhee, W., Taylor, A. M., Cribbs, D. H., Cotman, C.W. and Jeon, N. L.:External force-assisted cell positioning inside microfluidic devices. Biomed Microdevices, 9(1):15-23 (2007).
CrossRef - Yao, B., Yao, Lao, G.A.,Feng , X., Wang W., Chen, L.X., Wang, Y.M. and Yao, Y. M.: A microfluidic device based on gravity and electric force driving for flow cytometry and fluorescence activated cell sorting, Lab Chip, 4(6), 603-7 (2004) .
CrossRef - Nassif, N, Coiffier,A, Coradin, T, Roux, C, Livage, J. and Bouvet, O. Viability of Bacteria in Hybrid Aqueous Silica Gels. Journal of Sol-Gel Science and Technology,26:1141–1144 (2003).
CrossRef - Chiriac, PN, Nita, I, Nistor, L, Sol, M. Gel Method Performed for Biomedical Products Implementation. Mini Reviews in Medicinal Chemistry. 10:990–1013 (2010).
CrossRef - Derek, Y., David, S. and Kelly, D. B. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nature Medicine, 12, 62 – 66 (2006).
CrossRef - Bode, B., Silver, M. Weiss, R., Martin, K. Evaluation of a continuous glucose monitoring system for home-use conditions. Manag. Care, 17, 40–45 (2008).
- Parween S. and Nahar, P., Image-based ELISA on an activated polypropylene microtest plate—A spectrophotometer-free low cost assay technique, Biosensors and Bioelectronics, 48,287–292 (2013).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





