Manuscript accepted on : 22-Sep-19
Published online on: 30-09-2019
Plagiarism Check: Yes
Antiapoptic Activity of Cinnamon on Some Organs of 18 Days Rat Fetuses of Diabetic Mother
Mohamed E El-Beeh1, 2 ![]() , Yousra A. Fouda1, 3, Dina A El-badry1 and Hassan IH El-Sayyad1*
, Yousra A. Fouda1, 3, Dina A El-badry1 and Hassan IH El-Sayyad1*
1Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt
2Biology Department, El-Jumum University College, Umm Al-Qura University, KSA
3Biology Department, Faculty of Science and Art, Baha University, KSA
Corresponding Author E-mail: elsayyad@mans.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2779
ABSTRACT: Diabetes is a public health problem affected pregnant rats associated with developmental defects of their growing fetuses and histopathological abnormalities of their body organs. The traditional application of phytotherapy encourages author to develop the more safety plants which exerts antidiabetic activity and improve the histological structure. The present study aimed to evaluate the intensity of lesions induced in liver, kidney, heart and lingual mucosa of 18-day old fetuses of diabetic mother. Also, how can cinnamon-extract supplementation exert antiapoptic activity and improved the histological picture during in utero treatment. Twenty pregnant rats were used in the present work. They were categorized into four groups (n = 5); control, cinnamon extract group, diabetes, diabetes and cinnamon supplementation. Diabetes was developed by single i.p. administration of streptozotocin (60 mg/kg in citrate buffer pH 4.5 plus 100mg/kg nicotinamide). Cinnamon watery extract (300mg/kg body weight) was daily orally administrered from 6th day of gestation until 18th day of gestation. At the end of treatment, the mother was sacrificed, and their fetuses were removed and liver, kidney, heart and tongue were dissected and preserved in 10% phosphate buffered formalin pH 7.4. Also, immunohistochemistry of caspase 3 and P53 were carried out. At 18th day of gestation, maternal blood glucose levels were monitored in the investigated groups. The present findings revealed that diabetes induced damage of hepatocytes, deformation of renal tubules and renal corpuscles, fragility of myocardial muscles and damage of epithelium lining the lingual mucosa and retarded the differentiation of lingual papillae especially fungiform papillae. Increase average of apoptic cells were detected in the examined tissues of diabetic mother. Cinnamon-treatment reduced the incidence of apoptosis and improved the histological picture of liver, kidney, heart and tongue of fetuses maternally diabetic compared to the control. Image analysis revealed overexpression of immunohistochemical reaction of caspase 3 in liver, kidney and heart as well as caspase 3 and p53 in heart of fetuses of diabetic mother compared to those of diabetic mother supplemented cinnamon extract and control. The authors finally concluded that cinnamon extract showed a hypoglycaemic activity, reduced the streptozotocin associated diabetes and ameliorated the fetal liver, kidney, heart and tongue histological and immunohistochemical picture.
KEYWORDS: Cinnamon; diabetes; fetuses; liver; heart; kidney; tongue
Download this article as:| Copy the following to cite this article: El-Beeh M. E, Fouda Y. A, El-badry D. A, El-Sayyad H. I. Antiapoptic Activity of Cinnamon on Some Organs of 18 Days Rat Fetuses of Diabetic Mother. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: El-Beeh M. E, Fouda Y. A, El-badry D. A, El-Sayyad H. I. Antiapoptic Activity of Cinnamon on Some Organs of 18 Days Rat Fetuses of Diabetic Mother. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2kI1kMy |
Introduction
Diabetes mellitus (DM) is public health disease affected almost 184 million diabetic women in 2013, and the incidence may reach to 288 million by 2030 (IDF, 2015).
The fetal liver represents the metabolic brain, via managing and consuming of nutrient materials from the placenta. There are two ways of venous blood supply to the liver; oxygenated blood come from the placenta by the umbilical vein and decreased oxygenated one from the portal vein. The umbilical venous blood is important for cell proliferation of liver, heart and kidney and consequently fetal growth (Tchirikov et al., 2002). Also, diabetes was associated with increased umbilical venous flow to the liver and development of newborn adiposity (Brumbaugh et al., 2013; Ikenoue et al., 2017). Neonates of mothers fed on a hiigh fat diet during pregnancy and lactation altered hepatic structure and function parallel with Non-Alcoholic Steatohepatitis (Oben et al., 2010; Dudley et al., 2011). Offspring of gestation diabetic mother exhibited abnormal accumulation of subcutaneous fat at birth (Schaefer-Graf et al., 2008) and at increased risk of metabolic syndrome at 6 years-old (Boney et al., 2005).Human and animal model of diabetes have been associated that intrauterine hyperglycemia led to impairment of glucose tolerance in offspring and transmitted to the next generation suggesting an epigenetic impact. This was associated with impaired pancreatic B-cell function (Fetita et al., 2006).
Concerning heart development, diabetes was found to decrease cardiac morphogenesis and alter fetal circulation (Homberger, 2006) as well as fetal hypertrophic cardiomyopathy (Fontes‐Pedra et al., 2002). Expose culture of neural crest cells to glucose concentrations of 10, 30, or 50 mmol/l for 48 and 72hours led to reduced migratory area expansion which are critical to the development of heart (Suzuki et al., 1996). Diabetes was found to increase the risk of congenital anomalies in infants of diabetic mothers (IDM), to about of 2.5 to 12% (Ramos-Arroyo et al., 1992) and impaired fetal circulation through disrupted diastolic function and malformed the right ventricle (Dervisoglu et al., 2018). Maternal diabetes represent the main etiological factor for the development of congenital heart disease (Basu and Grag, 2018).Neonates of diabetic pregnancy exhibited altered heart rate viability due to maternal hyperglycaemia, fetal acidaemia and fetal glycaemia(Russell et al., 2016) as well as increased fetal epicardial fat thickness (Jackson et al., 2016).
Maternal diabetes was found to develop palate defects and orofacial clefts (Hrubec et al.,2009) such as oral mucosal lesions (Al-Maweri et al., 2013) and periodontitis, salivary, taste dysfunction and hyperceratosis associated damage of the lining epithelial cells (Ortug et al., 2018). Also, diabetes was found to induce neurogenic inflammation leading to vasoconstriction and lesions of the oral mucosa and taste impairment (Hevér et al., 2013) and increase the frequency of micronuclei in lingual epithelium (Quintero Ojeda et al., 2018). Reported that diabetes Increased the number of mast cell nerve contacts in neurogenic inflammation which enhanced vasoconstriction and lesions of the oral mucosa (Batbayar et al., 2003). In experimental animal model, diabetes induced micrognathia with tongue protrusion of rat fetuses (Deuchar, 1977).
Development of chronic kidney diseases in fetuses and neonate were generated by socio-economic factors, along with poor maternal-fetal health and genetic and environmental which predicted early life events of growth defects (Brophy , 2017).
Fu et al. (2016) reported that women with fetal multicystic dysplastic kidneys exhibited increased frequency of chromosomal abnormalities following karyotype investigation, however chromosome microarray analysis revealed increase of the rate of pathogenic detection to 16.7%.
Cinnamon (Cinnamomum zeylanicum), and Cinnamon cassia, are tropical tree belongs to the Lauraceae family and their bark is of great medicinal importance (Shen et al., 2002; Paranagama etal. 20010). Its major bio components are cinnamaldehyde and trans-cinnamaldehyde (Cin) (Chericoni et al., 2005) which are the main components of eugenol, trans-cinnamaldehyde and linalool and constitutes 82.5% of the total composition (Chericoni etal. 2005).
Cinnamon intake to pregnants was found to decrease visceral white adipose tissue mass, increase serum progesterone level and normalize adiponectin, serum insulin, leptin or estradiol levels (Bento-Bernardes et al., 2017). It exerted antidiabetic activity in experimental animals and human patients (Boyle et al., 2010) and improved lipid profile, blood sugar level, leptin and increased insulin serum levels in obese diabetic rats (Shalaby and Saifan, 2014). Diabetic patients given either 1 g (Sahib et al., 2016) or 3gm of cinnamon(Talaei et al., 2017) for 8 and 12 weeks decreased blood sugar level and glycosylated Hb and improved the oxidative stress markers.
The present study aimed to evaluate the intensity of lesions induced in liver, kidney, heart and lingual mucosa of 18-day old fetuses of diabetic mother. Also, how can cinnamon-extract supplementation exerts antiapoptic activity and improved the histological picture during in utero treatment.
Materials and Methods
Cinnamon watery extract-treatment
Dried cinnamon was obtained from the market, weighed and immersed on a known volume of hot water, filtered and cooling at room temperature. The dose freshly prepared per each treatment. Each pregnant rat was orally administered 0.5 ml3 containing 400mg of the extract / rat by stomach tube from 6th day of conception till 18th day of gestation.
Induction of diabetes
Experimental type 2 diabetes mellitus was induced by a single interperitoneal injection of streptozotocin (80 mg/kg) in citrate buffer (0.05 M) (pH 4.5) and 100mg/kg nicotinamide (Ghasemi et al., 2014). Hyperglycemia was determined by measuring the blood glucose levels by glucometer and adjustment the group within the level of 240- 280 mg/dL.
Experimental work
Twenty fertile male and virgin female albino rats (Rattus norvegicus) (1 male : 3 females) weighing about 200g body weight, obtained from, Farm of Ministry of Health, Egypt and used for studying. They were kept in aerated room with almost 12-hour light and dark cycle. Virgin females were mated and zero dates of gestation were determined by monitoring the presence of sperm in vaginal smear. The pregnant were arranged into four groups (n = 4 per each); control, cinnamon watery extract, diabetes, diabetes and cinnamon extract. Free access od diet and water were allowed ad Libitum. Diabetes was induced at 6th day of gestation and experimental work was carried out. Cinnamon extract was dosed orally every other day from 6th day of gestation till 18th day of it. At the end of experiment , the pregnant were sacrificed by light diethyl ether anesthesia and dissected. Blood was collected and sera were separated. For biochemical investigations, the sera levels of total cholesterol (TC) (Deeg and Ziegenhorn, 1982) and high-density lipoproteins (HDL) were determined according to Grove (1979). Low density lipoproteins (LDL), it was calculated from the total concentrations of triglycerides, cholesterol (TC) and HDL-cholesterol (Friedewald et al , (1972). The glucose was assayed by blood glucometers one touch ultra (Life Scan Milipitas, CA, USA).
The fetuses were separated, dissected and their liver, heart, kidney and tongue were removed and fixed in 10% phosphate buffered formalin. The specimens were dehydrated in ascending ethyl alcohol, cleared in toluene and mounted in molten paraplast 58-62C0. Five µm histological sections were carried out and stained with hematoxylin and eosin. For immunohistochemical reaction, the dewaxed tissue sections were digested with 0.05 % trypsin (pH 7.8) and incubated with the antibodies against caspase 3 (dilution 1:100 Thermo Fisher Scientific, Fremont, CA, USA; Cat. No. A1–70007) for overnight at 4°C, followed by treatment with a horseradish peroxidase streptavidin detection system (Dako), and DAB for developing the immunostaining and counterstained with hematoxylin. Negative control was carried out by using 1% non-immune serum phosphate buffer solution (PBS) solution. Extra immunohistochemical staining of P53 of myocardial tissue section as previously mentioned. The specimens were visualized under a Leica BM5000 microscope (Leica Microsystems, Wetzlar, Germany) and photographed. Image analysis was carried out of the slides after photographed using Olympus® digital camera installed on Olympus® microscope with 1/2 X photo adaptor, using 40 X objective. The result images were analyzed by using Intel® Core I5® based computer using Video Test morphology® software (Russia) and % area measurement was carried out.
Results
Biochemical observations:
Diabetic mother supplemented cinnamon-extract exhibited improved blood levels of glucose, and serum HDL, LDL and total cholesterol levels in comparison with diabetic and control groups (Fig.1).
 |
Figure 1: Chart illustrating the blood glucose level and sera levels of LDL-C, HDL and total cholesterol levels of diabetic mother supplemented watery cinnamon extract. |
Kidney
Histological and immunohistochemistry:
Liver
In 18day-old fetuses of control mother, the hepatic tissues are composed of irregular hepatic plates one cell thick. The hepatocytes appeared polygonal with centrally located nuclei. It is dispersed in between the islets of haemopoietic cells containing erythrocytes (Fig. 2A ). Fetuses maternally treated with ginger extract exhibited similar architecture structure of the hepatic tissues like the control (Fig.2A1).
Fetuses of diabetic mother showed massive dissolution of hepatic plates. The hepatocytes were infiltrated by round inflammatory cells and their blood sinusoids were dilated. Many of the hepatocytes exhibited either pyknotic or karyolysed nuclei (Fig. 2A2).
In fetuses of diabetic mother and supplemented ginger extract, amelioration of the hepatic pictures was detected. The hepatocytes restored almost normal pattern structure of polygonal hepatocytes with centrally located nuclei. The blood sinusoids become finely observed with erythrocyte and lymphocytes (Fig.2 A3).
Following immunohistochemical of caspase 3, the fetuses of diabetic mother exhibited increase dark brown reaction in hepatocytes (Fig. 1 B2) in comparison with the negative reaction in control and cinnamon supplementation (Fig.1B&B1). Fetuses of maternally diabetic and supplemented cinnamon extract revealed a marked reduction of the immunohistochemical reaction (Fig.13). Image analysis revealed increased overexpression of immunohistochemical reaction of caspase 3 in hepatocytes of fetuses of diabetic mother compared to decreased immunostaining in those of diabetic mother supplemented cinnamon extract and negative immune reaction in control and those maternally supplemented cinnamon extract (Fig. 2 C).
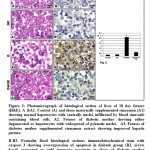 |
Figure 2: Photomicrograph of histological section of liver of 18 day fetuses (H&E). |
Kidney
In the kidney of control and cinnamon extract supplemented fetuses, the structural elements of renal tubules and glomeruli were observed. Each renal tubule is composed of cuboidal epithelial lining cells with prominent of nuclei and cell border. Their tubular lumina were clearly distinct. The glomeruli become unsheathed with mesangial cells and their cellular content fills almost the major part of the Bowman’s capsule (Fig. 3A, A1).
In fetuses of diabetic mother, many of the tubules were swollen associated with degeneration of their epithelial lining cells. The mesangial cell layer lining Bowman’s capsule appeared loose and partially degenerated and the glomeruli become swollen (Fig. 3A2).
In fetuses maternally diabetic and supplemented ginger extract, there were a marked improvement of the histological structure but was still not matched with the control. The renal tissue restored their major histological pattern and their regular arrangement of their elementary structures (Fig. A3).
Applying immunohistochemistry of caspase 3 to renal tissues of the fetuses of diabetic mother revealed increase dark brown reaction in epithelium of renal tubules and nephron (Fig. 3 B2) in comparison with the negative reaction in control and cinnamon supplementation (Fig.3B&B1). Fetuses of diabetic mother and supplemented cinnamon extract revealed a marked reduction of the immunohistochemical reaction (Fig.3 B3). Image analysis revealed increased dark-brown of immunohistochemical reaction of caspase 3 in epithelium lining renal tubules and nephron of fetuses of diabetic mother compared to decreased immunostaining in those of diabetic mother supplemented cinnamon extract and negative immune reaction in control and those maternally supplemented cinnamon extract (Fig. 3 C).
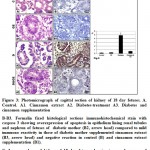 |
Figure 3: Photomicrograph of sagittal section of kidney of 18 day fetuses. A. Control. A1. Cinnamon extract A2. Diabetes-treatment A3. Diabetes and cinnamon supplementation |
Kidney
Heart
In control fetuses and those of mother supplemented cinnamon extract, the myocardial muscle is composed of regularly arranged of muscle fibers with centrally located nuclei (Fig.4 A & A1).
Fetuses of diabetic mothers possessed marked fragility and loosely separated of the myocardial muscle fibers. Many of the muscle fibers were degenerated (Fig. 4 A2).
Fetuses maternally diabetic and supplemented ginger extract showed better improvement of the histological picture of myocardial muscle. The myocardial fibers restored their regular their arrangement and presence of many of their nuclei (Fig. 4A3).
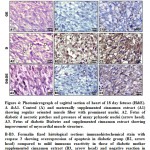 |
Figure 4: Photomicrograph of sagittal section of heart of 18 day fetuses (H&E). A &A1. |
Kidney
Following immunohistochemistry of both caspase 3 and p53, the myocardium of fetuses of diabetic mother possessed overexpression of caspase 3 and p53 (Fig.3 B2) in comparison with the negative reaction in control and cinnamon supplementation (Fig.3B&B1). Fetuses of diabetic mother and supplemented cinnamon extract revealed a marked reduction of the mention immunohistochemical reaction (Fig.3 B3). Image analysis revealed overexpression of immunohistochemical reaction of caspase 3 and p53 in myocardium of fetuses of diabetic mother compared to decreased immunostaining in those of diabetic mother supplemented cinnamon extract and negative immune reaction in control and those maternally supplemented cinnamon extract (Fig. 5).
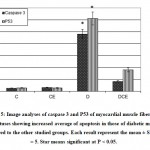 |
Figure 5: Image analyses of caspase 3 and P53 of myocardial muscle fiber of 18 day fetuses showing increased average of apoptosis in those of diabetic mother compared to the other studied groups. Each result represent the mean ± SD of n = 5. Star means significant at P < 0.05. |
Kidney
Tongue
In control fetus and those maternally supplemented ginger extract, the lingual epithelium is composed of stratified squamous epithelium. The fungiform papilla placode is detected in the form of grouping several layers of cuboidal cells. The lamina propria is composed of connective tissue core underneath the lingual mucosa (Figs.6 A &A1).
Fetus of diabetic mother possessed comparatively reduced lingual mucosa associated with vacuolar degeneration of the peripheral epithelial lining layer which become eosinophilic. The underlying connective tissue of the lamina propria become fragile (Fig.6 A2).
Fetuses of diabetic mother supplemented cinnamon showed apparent improvement. The lingual mucosa restored its ordinary structure of and infiltrated by bud like structure of the fungiform papillae. The collagenous lamina propria restored more dense cellular components (Fig.6A3).
Applying immunohistochemistry of caspase 3 to tongue of the fetuses of diabetic mother revealed increase dark brown reaction in lingual mucosa of tongue (Fig. 5 B2) in comparison with the negative reaction in control and cinnamon supplementation (Fig.6B&B1). Fetuses of diabetic mother and supplemented cinnamon extract revealed a marked reduction of the immunohistochemical reaction (Fig.6 B3). Image analysis revealed increased dark-brown of immunohistochemical reaction of caspase 3 lingual mucosa of fetuses of diabetic mother compared to decreased immunostaining in those of diabetic mother supplemented cinnamon extract and negative immune reaction in control and those maternally supplemented cinnamon extract (Fig. 6 C).
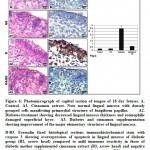 |
Figure 6: Photomicrograph of sagittal section of tongue of 18 day fetuses. A. Control. A1. Cinnamon extract. |
Kidney
Discussion
Studying of the developmental origin of disease during fetal life is of great importance to outline the impact of health in the adult life.
The observed damage of hepatocytes and myocardial cells of fetuses of diabetic mother was supported by previous work carried out by Elsayyad et al. ( ) and ( ). Overexpression of caspase 3 manifesting apoptic cell death was attributed to the marked increase of both hepatic and myocardial alkaline and lactic dehydrogenase Elsayyad et al. (20124) and (2014).
Diabetes was found to be associated with fetal hypertrophic cardiomyopathy (Fontes‐Pedra et al., 2002), impaired fetal circulation and malformed the right ventricle (Dervisoglu et al., 2018) and increased fetal epicardial fat thickness (Jackson et al., 2016). Diabetes was found to increase hyperglycemia which transmitted to the next generation via impairing of pancreatic B-cell function (Fetita et al., 2006; El-Sayyad et al.,2013).
On the other hand, diabetes led to the development of histological abnormalities of renal tissues of fetuses explained by damaging of the epithelium lining the renal tubules and deformation of renal corpuscles. The detected increase of damaging renal cells illustrated by the overexpression caspase 3.
Similar findings damaging nephron was reported in newborn of streptozotocin-treated mother and hyperglycemia at 13th day of gestation (Amri et al., 1999 and Gomes and Gil, 2011 ). Diabetic mother mice was found to upregulation of NF-κB isoforms, p50 and p65, and activation of the NF-κB pathway (Tran et al., 2009).The observed renal damage may be attributed to preeclampsia of diabetic women (Powe and Thadhani, 2011) due to a decrease in glomerular filtration rate (Martins Jde et al., 2014).
It is known that the tongue primordial structure was developed from the occipital myotome, and then migrates into the oral cavity (Uchiyama et al., 2000) and contributed to its development (Achiron et al., 1997).
The present findings illustrated damaging of the epithelium lining the lingual mucosa and deformation of the gustatory lingual papillae. Similar findings were reported by many authors. Diabetes was found to induce neurogenic inflammation leading to vasoconstriction and lesions of the oral mucosa and taste impairment (Hevér et al., 2013) and increase the frequency of micronuclei in lingual epithelium (Quintero Ojeda et al., 2018).
It is known that the development of fetus during in utero life is affected by the interaction between the mother and fetus. Maternal transmission of micronutrients, oxygen and endocrine signals are of great importance for growth of fetus and differentiation of their body organs (Piotrowska et al., 2014). Maternal diabetes was found to induce growth defects during the early stage of life and programming of the metabolic disturbances parallel with the disruption in the intrauterine environment and developed the detected increased apoptic cell death and histological abnormalities of the investigated tissues.
Similar hypoglycemic effects were reported after application of the extract of Allium sativum, Eugenia jambolana, Momordica charantia Ocimum sanctum, Phyllanthus amarus, Pterocarpus marsupium, Tinospora cordifolia, Trigonella foenum graecum and Withania somnifera (Modak et al., 2007).
Also, Kooti et al. (2016) reported the hypoglycemic effect of plant extract such as Acacia Arabica, Achyranthes aspera, Acosmium panamense, Aegle marmelose, Aloe barbadensis Miller, Andrographis paniculata, Annona squamosal, Argyreia nervosa, Artemisia herba, Averrhova bilimbi, Azadirachta indica, Barleria prionitis, Biophytum sensitivum, Brassica nigra, Bryonia alba, Caesalpinia bonducella, Cajanus cajan, Carum carvi, Casearia esculenta, Chamaemelum nobile, Citrulus colocynthis, Dorema aucheri and Eclipta alba. Its hypoglycemic activity was attributed to their contents of carotenoids, flavonoids, terpenoids and alkaloids which improve the pancreatic B cells by increasing insulin secretions or reducing the intestinal absorption of glucose (Afrisham et al., 2015).
The present findings revealed antidiabetic activity of cinnamon through decrease of blood sugar level, reduction of total cholesterol, LDL-C and an increase in HDL-C levels. Similar finding were reported by Allen et al. (2013) following metanalysis of .the phytomedicinal application of cinnamon extract.
The cinnnamaldehyde metabolite of cinnamon was found to manage insulin release, enhancing insulin sensitivity and regulate the protein-tyrosine phosphatase 1B and insulin receptor kinase (Sheng et al., 2008; Liu et al., 2015).
Cinnamon extract decreased triglyceride and increased liver glycogen content and improved insulin action in liver tissues (Sartorius et al., 2014).
The capacity of amelioration of fetal tissues comes from the improvement of the hyperglycaemic condition of diabetes and increased antioxidant activity of the cinnamon extract (Qusti et al., 2016; Hosni et al., 2017) which facilitate growth and differentiation of the investigated tissues. Sunami et al. (2010) explained another route of improvement base on the migration of foetal tissues from fetus to the mother through placental and umbilical circulation in bone marrow, liver, kidney and pancreas which can be flourished post cinnamon-treatment and support the fetal organs for restoring the capacity of differentiation. Improvement may comes from the migration of the early foetal cells with their multilineage potential in maternal body tissues and sustain their population over the long term after delivery (Sunami et al., 2010).
The authors finally concluded that cinnamon–treatment increased the capacity of the antioxidants and decreased the oxidative stress resulted from the diabetes leading to damage of the fetal tissues.
References
- Abi Khalil, C., Travert, F., Fetita, S., Rouzet, F., Porcher, R., Riveline, J.P., Hadjadj, S., Larger, E., Roussel, R., Vexiau, P., Le Guludec, D., Gautier, J.F., Marre, M. exposure to maternal type 1 diabetes is associated with renal dysfunction at adult age. 2010; 59(10), 2631–2636.
CrossRef - Achiron, R., Ben, Arie. A., Gabbay, U., Mashiach, S., Rotstein, Z., Lipitz, S. Development of the tongue between 14 and 26 weeks of gestation: in uteroultrasonographic measurements. Ultrasound Obstet Gynecol. 1997; 9: 39–41.
CrossRef - Afrisham, R., Aberomand, M., Ghaffari, M.A., Siahpoosh, A., Jamalan, M. Inhibitory Effect of Heracleum persicum and Ziziphus jujuba on Activity of Alpha-Amylase. Journal of Botany. 2015; 2015:1–8.
CrossRef - Allen, R.W., Schwartzman, E., Baker, W.L., Coleman, C.I., Phung, O.J. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013 ;11(5):452-9.
CrossRef - Al-Maweri, S.A., Ismail, N.M., Ismail, A.R., Al-Ghashm, A. Prevalence of oral mucosal lesions in patients with type 2 diabetes attending hospital universiti sains malaysia. Malays J Med Sci.2013;20(4):39-46.
- Amri, K., Freund, N., Vilar, J., Merlet-Bénichou, C., Lelièvre-Pégorier, M. Adverse effects of hyperglycemia on kidneydevelopment in rats: in vivo and in vitro studies. Diabetes. 1999;48(11):2240-5.
CrossRef - Basu, M., Garg, V. Maternal hyperglycemia and cardiac Maternal hyperglycemia and cardiac development: Clinical impact and underlying mechanisms. Birth Defects Res.2018;110(20):1504-1516.
CrossRef - Batbayar, B., Somogyi, J., Zelles, T., Fehér, E. Immunohistochemical analysis of substance P containing nerve fibres and their contacts with mast cells in the diabetic rat’s tongue. Acta Biol Hung.2003;54(3-4):275-83.
CrossRef - Bento-Bernardes, T., Toste, F.P., Pazos-Moura, C.C., Oliveira, K.J. Maternal cinnamon extract intake during lactation leads to sex-specific endocrine modifications in rat offspring. J Sci Food Agric.2017;97(11):3855-3863.
CrossRef - Boney, C.M., Verma, A., Tucker, R., Vohr, B.R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. 2005;115:e290–6.
CrossRef - Bosch, J.P., Lew, S., Glabman, S., Lauer, A. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am J Med. 1986; 81:809–815
CrossRef - Boyle, S.M., Simon, B., Kobrin, S.M. Antidiabetic Therapy in End-Stage Renal Disease. Semin Dial.2015;28(4):337-44.
CrossRef - Brophy, P. Maternal determinants of renal mass and function in the fetusand neonate. Semin Neonatal Med. 2017;22(2):67-70.
CrossRef - Brumbaugh, D.E., Tearse, P., Cree-Green, M., Fenton, L.Z., Brown, M., Scherzinger, A., Reynolds, R., Alston, M., Hoffman, C., Pan, Z., Friedman, J.E., Barbour, L.A. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162(5):930-6.e1.
CrossRef - Chericoni, S., Prieto, J.M., Iacopini, P., Cioni, P., Morelli, I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J Agric Food Chem.2005; 15;53(12):4762-5.
CrossRef - Correa-Costa, M., Landgraf, M.A., Cavanal, M.F., Semedo, P., Vieira, D.A., De Marco, D.T., Hirata, A.E., Câmara, N.O., Gil, F.Z. Inflammatory milieu as an early marker of kidneyinjury in offspring rats from diabetic Eur J Pharmacol. 2012;689(1-3):233-40.
CrossRef - Deeg, R., Ziegenhorn, J. Kinetic enzymic method for automated determination of total cholesterol in serum. ClinChem 1983; 29: 1798–1802.
- Dervisoglu, P., Kosecik, M., Kumbasar, S. Effects of gestational and pregestational diabetes mellituson the foetal heart: a cross-sectional study. J Obstet Gynaecol. 2018;38(3):408-412.
CrossRef - Deuchar, E.M. Embryonic malformations in rats, resulting from maternal diabetes: preliminary observations. J Embryol Exp Morphol.1977;41:93-9.
- Dudley, K.J., Sloboda, D.M., Connor, K.L., Beltrand, J., Vickers, M.H. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011;6:e21662.
CrossRef - El-Sayyad, H.I., Al-Haggar, M.S., El-Ghawet, H.A. , Bakr, H. Cardiomyopathy and angiogenesis defects of Wistar rat fetuses of diabetic and hypercholesterolemic mothers. Nutrition. 2012; 28(7-8): e33-43.
CrossRef - El-Sayyad, H.I.H, Al-Haggar, M.M.S., El-Ghawet, H.A., Bakr, I.H.M. Effect of maternal diabetes and hypercholesterolemia on liver of albino Wistar rats. Nutrition 30. 2014; 326–336.
CrossRef - El-Sayyad, H.I.H., El-Shershaby, E.M., Saleh, D.F. Effects of experimental diabetes and gliclazide on pregnant albino rats and their pups. JIMR 2013;1(1):22-29.
CrossRef - Fetita, L.S., Sobngwi, E., Serradas, P., Calvo, F., Gautier, J.F. Consequences of Exposure to Maternal Diabetes in Offspring, J Clin Endocrinol Metab, 2006;91(10):3718 –3724
CrossRef - Fontes‐Pedra, S. R. F., Smallhorn, J., Ryan, G. cardiomyopathies: etiologies, hemodynamic findings and clinical outcome. Circulation2002;106585–591.
- Friedewald, W.T., Levy, R.I., Fredrickson, D.S. () Estimation of low-density lipoprotein cholesterol in plasma without use preparative ultracentrifuge. 1972; 18: 499–502.
- Fu, F., Chen, F., Li, R., Zhang, Y., Pan, M., Li, D., Liao, C. Prenatal diagnosis of multicystic dysplastic kidney via high-resolution whole-genome array. Nephrol Dial Transplant. 2016;31(10):1693-8.
CrossRef - Ghasemi, A., Khalifi, S., Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. ActaPhysiol Hung 2014;10: 408–20.
CrossRef - Gomes, G.N., Gil, F.Z. Prenatally programmed hypertension: role of maternal diabetes. Braz J Med Biol Res.2011;44(9):899-904.
CrossRef - Grove, T.H. Effect of reagent PH on determination of the high-density lipoprotein cholesterol by precipitation with sodium phototungestate-magnesium. ClinChem 1979; 25: 560–564.
- Hevér, H., Altdorfer, K., Zelles, T., Batbayar, B., Fehér, E. Changes in the innervation of the taste buds in diabetic rats. Orv Hetil.2013;154(12):443-8.
CrossRef - Hornberger, L. K. Maternal diabetes and the Heart. 2006; 92(8):1019-21.
CrossRef - Hosni, A.A., Abdel-Moneim, A.A., Abdel-Reheim, E.S.1., Mohamed, S.M., Helmy, H. Cinnamaldehyde potentially attenuates gestational hyperglycemia in rats through modulation of PPARγ, proinflammatory cytokines and oxidative stress. Biomed Pharmacother. 2017;88:52-60.
CrossRef - Hrubec, T.C., Toops, K.A., Holladay, S.D. Modulation of diabetes-induced palate defects by maternal immune stimulation. Anat Rec (Hoboken). 2009;292(2):271-6.
CrossRef - Ikenoue, S., Waffarn, F., Ohashi, M., Sumiyoshi, K., Ikenoue, C., Buss, C., Gillen, D.L., Simhan, H.N., Entringer, S., Wadhwa, P.D. Prospective association of liver blood flow at 30 weeks gestation with newborn adiposity. Am J Obstet Gynecol. 2017;217(2):204.e1-204.e8.
CrossRef - International Diabetes Federation (IDF). Diabetes Atlas. IDF: Brussels, Belgium, 7th ed. 2015 1–144.
- Jackson, D., Deschamps, D., Myers, D., Fields, D., Knudtson, E., Gunatilake, R. epicardial fat thickness in diabetic and non-diabetic pregnancies: A retrospective cross-sectional study. Obesity (Silver Spring). 2016; 24(1):167-71.
CrossRef - Kooti, W., Farokhipour, M., Asadzadeh, Z., Ashtary-Larky, D., Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832-42.
CrossRef - Liu, Y., Cotillard, A., Vatier, C., Bastard, J.P., Fellahi, S. , Stévant, M. A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-DiabeticSubjects: A Randomized, Placebo-Controlled Trial. PLoS One. 2015;10(9):e0138646.
CrossRef - Martins, Jde. O., Panício, M.I., Dantas, M.P., Gomes, G.N. Effect of maternal diabetes on female offspring. Einstein (Sao Paulo).2014 ;12(4):413-9.
CrossRef - Modak, M., Dixit, P., Londhe, J., Ghaskadbi, S., Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40(3):163-73.
CrossRef - Oben, J.A., Mouralidarane, A., Samuelsson, A.M., Matthews, P.J., Morgan, M.L., McKee, C. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–20.
CrossRef - Ortug, G., Ignak, S., Ortug, A. Characteristics of lingual papillae in diabetic rats. 2018;102(339):250-254.
CrossRef - Paranagama, Priyani Paranagama,S. WimalasenaGamini, S. JayatilakeGamini, S. Jayatilake Mubarak, Mariam. A comparison of essential oil constituents of bark, leaf, root and fruit of cinnamon (Cinnamomum zeylanicum Blum) grown in Sri Lanka. Journal of the National Science Foundation of Sri Lanka. 2001; 29(3-4):147-153
CrossRef - Piotrowska, I., Zgódka, P., Milewska, M., Błaszczyk, M., Grzelkowska-Kowalczyk, K. Developmental programming of metabolic diseases–a review of studies on experimental animal models. Postepy Hig Med Dosw.2014;68:899-911.
CrossRef - Powe, C.E., Thadhani, R. Diabetes and the kidney in pregnancy. Semin Nephrol.2011;31(1):59-69.
CrossRef - Pucci, K.R., Pereira, Júnior. C.D., Idaló, P.B., Moreira, A.C., Rocha, L..P, Rodrigues, A.R., Reis, L.C., Gomes, R.A., Rocha, L.B., Guimarães, C.S., Reis, M.A., Câmara, N.O., Corrêa, R.R. Morphological and functional aspects of acute kidneyinjury after programing in the offspring of diabetic rats. J Matern Neonatal Med. 2015;28(4):403-8.
CrossRef - Quintero, Ojeda. J.E., Aguilar-Medina, M., Olimón-Andalón, V., García, Jau. R.A., Ayala, Ham. A., Romero, Quintana, J.G., Silva-Benítez, E.L., Sanchez-Schmitz, G., Ramos-Payán, R. Increased Micronuclei Frequency in Oral and Lingual Epithelium of Treated Diabetes Mellitus Patients. Biomed Res Int.2018; 2018:4898153.
CrossRef - Qusti, Safaa Y., Abo-khatwa, Ahmed N., Mona, A. Bin, Lahwa. SCREENING OF ANTIOXIDANT ACTIVITY AND PHENOLIC CONTENT OF SELECTED FOOD ITEMS CITED IN THE HOLLY QURAN. 2010, 2 (1)
- Ramos-Arroyo, M.A., Rodriguez-Pinilla, E., Cordero, J.F. Maternal diabetes: the risk for specific birth defects. Eur J Epidemiol. 1992; 8:503–508.
CrossRef - Russell, N.E., Higgins, M.F., Kinsley, B.F., Foley, M.E., McAuliffe, F.M. Heartrate variability in neonates of type 1 diabetic pregnancy. Early Hum Dev. 2016; 92:51-5.
CrossRef - Sahib, Ahmed Salih . Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016; Mar-Apr; 5(2): 108–113.
CrossRef - Sartorius, T., Peter, A., Schulz, N., Drescher, A., Bergheim, I., Machann, J. Cinnamonextract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9(3):e92358.
CrossRef - Schaefer-Graf ,U.M., Graf, K., Kulbacka, I., Kjos, S.L., Dudenhausen, J., Vetter, K. Maternal lipids as strong determinants of environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008; 31:1858–63.
CrossRef - Shalaby, M.A., Saifan, H.Y. Some pharmacological effects of cinnamon and ginger herbs in obese diabetic rats. J Intercult Ethnopharmacol. 2014 ;3(4):144-9..
CrossRef - Sheng, X., Zhang, Y., Gong, Z., Huang, C., Zang, Y.Q. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008; 581348.
CrossRef - Sunami, R., Komuro, M., Yuminamochi, T., Hoshi, K., Hirata, S. cell microchimerism develops through the migration of fetus-derived cells to the maternal organs early after implantation. J Reprod Immunol. 2010;84(2):117-23.
CrossRef - Suzuki, N., Svensson, K., Eriksson, U. J. High glucose concentration inhibits migration of rat neural crest cells in vitro. Diabetologia1996; 39401–411.
- Talaei, B., Amouzegar, A., Sahranavard, S., Hedayati, M., Mirmiran, P., Azizi, F. Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. 2017 ;8;9(9).
CrossRef - Tchirikov, M., Kertschanska, S., Sturenberg, H.J., Schroder, H.J. Liver blood perfusion as a possible instrument for growth regulation. 2002;23 Suppl A:S153–8. pmid:11978076.
CrossRef - Tran, S., Chen, Y.W., Chenier, I., Chan J.S., Quaggin, S., Hébert, M.J., Ingelfinger, J.R., Zhang, S.L. Maternal diabetes modulates renal morphogenesis in offspring. J Am Soc Nephrol. 2008;19(5):943-52.
CrossRef - Uchiyama, K., Ishikawa, A., Hanaoka, K. Expression of lbx1 involved in the hypaxial musculature formation of the mouse embryo. J Exp Zool 2000; 286: 270–9.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





