How to Cite | Publication History | PlumX Article Matrix
Isolation and Identification of Actinomycetes with Anticandida Activity from Mangrove Soil
Mohammad Sharifi and Nirichan Kunchirman Bipinraj*
and Nirichan Kunchirman Bipinraj*
Rajiv Gandhi Institute of I.T. and Biotechnology, Bharati Vidyapeeth Deemed to be University, Pune, Maharashtra.
Corresponding Author E-mail: bipinrajnk@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2776
ABSTRACT: Candida albicans, a common human commensal, is the leading cause of nosocomial infections due to the emergence of drug resistance. The present study reports the isolation and identification of actinomycetes from mangrove soil and characterization of its antagonistic activity against drug resistant Candida species. Mangrove soils from Khargar, Navi Mumbai were screened for actinomycetes with anti-candida activity. In total, 20 actinomycetes culture were isolated from mangrove soil sample, amongst the culture designated as MB was found to inhibit all tested pathogenic Candida cultures. The isolate MB was identified using biochemical tests and 16S rRNA sequencing as Streptomyces viridocromogenes. MB culture showed maximum activity after incubation period of 48 to 72 h, pH of 6.2 and temperature of 30℃. Partially purified active molecule was found to be inactivated by heat treatment but resisted proteinase K, indicating the compound can be an antibiotic in nature. The study highlights the isolation of Streptomyces viridocromogenes with antagonistic activity against multidrug resistant Candida from mangrove soil. This culture is an ideal candidate for further characterization studies for anti-candida molecules.
KEYWORDS: Actinomycetes; Anti-candida; Mangrove soil; Streptomyces viridocromogenes
Download this article as:| Copy the following to cite this article: Sharifi M, Bipinraj N. K. Isolation and Identification of Actinomycetes with Anticandida Activity from Mangrove Soil. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Sharifi M, Bipinraj N. K. Isolation and Identification of Actinomycetes with Anticandida Activity from Mangrove Soil. Biosci Biotech Res Asia 2019;16(3). Available from: https://www.biotech-asia.org/?p=34156 |
Introduction
The rate of nosocomial infections due to Candida is in a steady state of increase since the past three decades (1). Anti-fungal drugs such as clotrimazole and fluconazole are widely available to treat candida infections, however, owing to the drug resistance against these drugs and various side effects of such drugs there is a need of safe anti-candida molecule (2). Mangrove soils are a brimful resource of microorganisms, and due to the dynamic physicochemical environment, microorganisms surviving in this area are equipped with various biomolecules, which has many potential applications in pharmaceutical industry. Moreover, microorganisms isolated from mangroves are reported for anti-fungal activity(3,4).
Actinomycetes are filamentous, aerobic and Gram-positive bacteria which belongs to class actinobacteria, reported to produce different important biomolecules such as anti-parasitic, antibiotics, fungicides, and several industrial enzymes(5). Anti-microbial compounds such as surfactin, iturinic, fengymycin, bacilysocin, and fengycin etc. are produced by actinomycetes(3).
The present study aimed to isolate actinomycete from mangrove soil and to characterize its anti-candida activity in order to find out newer anti-candida cultures/ molecules.
Material and Methods
Sample collection and isolation of actinomycetes
Rhizospheric soil (5-10 deep) of the mangrove plants were collected from Indian coast at Kharghar, and Navi Mumbai. The samples (100-200g) were transferred to laboratory in sterile polyethylene bags (6). The soil was then heated at 70℃ for 30 min before using it for culture isolation (3,6).
Treated soil sample (1g) from different locations were mixed in 100 ml of 50% sterile sea water separately and kept in shaker for 30 min at room temperature. The mixture was then serially diluted using 50% sterile sea water and 100µl was spread plated on starch casein agar, (SCA) containing soluble starch- 10 g/L, casein- 0.3 g/L, potassium nitrate- 2 g/L, sodium chloride- 2g/L, dipotassium hydrogen phosphate- 2g/L, magnesium sulphate- 0.05 g/L, ferrous sulphate- 0.01g/L, calcium carbonate- 0.02g/L and agar- 15g/L, with 30µg/ml tetracycline and 30µg/ml fluconazole. The plates were incubated at 28℃ for 7-10 days under aerobic condition. Morphologically distinct colonies were isolated, purified and inoculated in yeast extract, malt extract agar (YMA) containing yeast extract- 3g/L, malt extract- 3g/L, peptone- 5g/L, dextrose- 10g/L, agar- 20g/L for preservation. Cultures were grown in YMB medium incubated for 72 h. and used for all experiments.
Screening of Anti-candida activity
Anti-candida activity of the isolates were performed by agar well diffusion method on YMA plates against Candida albicans (NCIM 3557), Candida glabrata NCYC 388, Candida tropicalis NCIM 3118 and Candida albicans clinical isolates CA-14, ROC 1 and ROC 2. All Candida cultures (100µl 1 OD ) were spread plated on Sabouraud Dextrose Agar (SDA) plates and incubated at 37℃ for 30 min. Wells were punctured in agar using well borer (5mm dia.), and 10 µl of culture supernatant were added and incubated at 37℃ for 48h. Anti-candida activity of the isolate was scored by measuring the zone of inhibition around the wells (7).
Biochemical and phenotype characterization
Isolates were characterized for Gram’s nature, starch hydrolysis, citrate utilization, catalase, urease, casein hydrolase, H2S production and MR-VP tests using standard procedures(8,13).
Hemolysis
Isolated cultures were spot inoculated on sheep blood agar medium and incubated at 37℃ for 24h after which it was observed for hemolytic pattern on agar plate (9).
Molecular identification
Total genomic DNA of the culture was purified as per the manufacturer’s instructions using genomic DNA isolation kit (Sigma, USA). It was then used as template for PCR reaction mixture containing approximately 10ng of DNA; 2.5 mM MgCl2; 1x PCR buffer (Bangalore Genei, Bangalore, India) 200µM each dCTP, dGTP, dATP, and dTTP; 2pmol of each, forward and reverse primers. PCR product was sequenced using ABI Prism Big Dye Terminator Cycle Sequencing reaction kit. Universal primers 27f–1525r (universal primers for 1.5 kb fragment amplification for actinobacteria) was used to sequence the nearly completed gene. The sequencing reaction and template were purified as per the manufacturer’s instructions (Applied Biosystems). Samples were run on ABI prism 3100 Genetic Analyzer and sequencing output was analyzed using DNA sequence analyzer computer software. The sequence was compared with National Centre for Biotechnology Information GenBank entries by using the BLAST algorithm (10).
Optimization of growth parameters
Selected isolates were inoculated in 100 ml YMB broth with varying pH (4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5 and 8) and incubated at 25ºC on shaker incubator (120 rpm). To check the optimum temperature, isolates were inoculated in YMB medium with optimized pH and incubated at various temperature (20, 25, 30, 35, 40, 45, 50, 55ºC). After the incubation, activity of the cultures was checked with well diffusion method against Candida strains (2,11).
To optimize the incubation time, cultures were inoculated in YMB medium and incubated for 120 h at optimized pH and temperature. Growth and activity of the cultures were checked at regular intervals by well diffusion assay (2,11).
Partial purification of active compound
Isolates were inoculated (1 OD, 1%) in YMB medium and incubated at 300C on shaker at 120 rpm for 48 h. Culture supernatant was collected by centrifugation at 10000 rpm for 20 min at 4 ºC. Supernatant was mixed with different solvents such as methanol, acetone, as well as combination of chloroform and methanol (1:1 V/V) and chloroform and ethyl acetate (1:1 V/V). After vigorous mixing the solution was kept at 20℃ for 2 h. Solvent was collected and vacuum evaporated at 50ºC to collect the precipitate which was then suspended in 1 ml of 1M phosphate buffer (pH 6.5) before checking anti-candida activity.
Characterization of the activity
Sensitivity to temperature and hydrolyzing enzymes
Temperature sensitivity was checked by exposing the precipitate obtained by solvent extraction to various temperature (60°C for 90 min, 80°C for 20 min, 100°C for 20 and 30 min or autoclaved). Similarly the suspension was exposed to equal volume of proteinase K at a final concentration of 1.0 mg mL-1 for 30 min at room temperature. The residual activity of the suspension was determined by well diffusion assay (12).
Results and Discussions
Culture isolation, screening and characterization
Total 20 morphologically distinct colonies with characters similar to actinomycetes were selected from SCA agar. Out of which only two cultures designated as MB and MB2 that showed inhibitory activity against all pathogenic Candida cultures tested (Fig. 1) by agar well diffusion assay. Among the two cultures MB was found to be non-hemolytic (Fig.2) and selected for further characterization. Isolate MB was found to be Gram positive rod, catalase, casein and starch hydrolysis positive and negative for indole, MR-VP and citrate utilization (Table 1). Isolate MB showed maximum anti-candida activity when grown at pH 6, temperature 30℃ and after 48 h to 72 h of incubation. (Table 2).
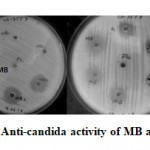 |
Figure 1: Anti-candida activity of MB and MB2 |
 |
Figure 2: Result of hemolytic study. |
The culture MB was further identified by 16S rRNA sequencing as Streptomyces viridocromogenes with 99% strain similarity with NBRC 3113 with accession no. AB182748.
Purification and characterization of active compound
When extracted with different solvents precipitates from methanol treatment showed better activity (Figure 3). The precipitate however, lost its activity after heating at 80℃ but retained its activity even after proteinase K treatment.
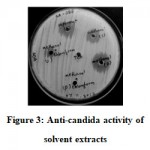 |
Figure 3: Anti-candida activity of solvent extracts |
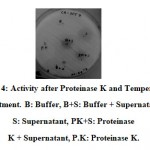 |
Figure 4: Activity after Proteinase K and Temperature treatment. B: Buffer, B+S: Buffer + Supernatant, S: Supernatant, PK+S: Proteinase K + Supernatant, P.K: Proteinase K. |
Actinomycetes are known to produce industrially important secondary metabolites including many anti-microbial compounds(4). Since mangrove soil is a diverse source of actinomycetes(6), we have selected mangrove soil for isolation of actinomycetes. Soil was collected from mangrove region at Khargar, Navi Mumbai, a polluted area owing to various industrial effluents and other man made activities(5,8). The samples yielded 20 distinct actinomycetes like colonies. Out of twenty isolates, only two cultures showed anti-candida activity and among the two only MB was found to be non-hemolytic. Hemolysis of sheep blood agar is used as a screening method to find out toxic nature of microorganism(9). Here the culture, designated as MB was found to be non-toxic and possessed excellent anti-candida activity. Studies showed that anti-candida compound produced by MB is extracellular and it showed maximum growth and activity when grown at 30ºC and pH 6 after 72 h in YMB medium. Moreover, it was found out that the activity of the culture was stable up to pH 8.5 and temperature of 55ºC. Ability to tolerate high pH and temperature is an important property with respect to industrial application(2,4,11). The culture was then identified as Streptomyces viridocromogenes by 16S rRNA analysis. Streptomyces viridocromogenes which has been reported previously for production of oligopeptide antibiotic compound(3). The present isolate MB showed significant activity against different pathogenic Candida species. The activity of the culture was inhibited by high temperature treatment (80ºC) and was not suppressed by proteinase K treatment indicating the compound of interest may be an antibiotic (6).
Conclusion
Mangrove soil has been always recognized as a diverse source for microorganism with pharmaceutically important molecule. The present study showed isolation of Streptomyces viridocromogenes from mangrove soil from Khargar, Navi Mumbai a polluted area near Mumbai, India. In the current scenario of emergence of multidrug resistance in pathogenic microorganisms especially Candida, it is important to find out new organisms from various sources that can be explored for industrial application. The isolate MB was found to be non-toxic and showed significant anti-candida activity against standard Candida species. The activity was stable at wide range of pH and temperature. Hence, this culture is an ideal organism for further characterization and exploration for new anti-candida molecule.
Acknowledgments
The authors are indebted to BV- Rajiv Gandhi Institute of IT and Biotechnology, Bharati Vidyapeeth Deemed University (BVDU), Pune for allowing them to undertake this work.
References
- Ahimou F., Jacques P., Deleu M.S. Surfactin and Iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme and Microbial Technology 2000; 27(10):749-54.
CrossRef - Vikrant P., Rajeev M., Rahul M.S., Monalisa M., Meera S.M., Neelam K., Prashant K.D., Prabhkar K.R., and Bipinraj N.K. Inhibition of pathogenic stains of Candida albicans and non albicans by Bacillus species isolated from traditional Indian fermented food preparation. International Journal of Current Microbiology and Applied Sciences 2015; 4(3) 691-699.
- Nurfathiah A.M., Ahmed J.K., Chowdhury Z.Z., and Zaima A.Z.A. Selective isolation of actinomycetes from Mangrove forest of Pahang, Malaysia. International Conference on Agriculture, Biology and Environmental Sciences (ICABES 14) Dec. 8-9 2014.
- Gunasekaran M., Thangavel S. Isolation and screening of actinomycetes from marine sediments for their potential to produce antimicrobial, International Journal of Life Sciences Biotechnology and Pharma Research 2013; 2(3):115-126.
- Aparanji P., Venkata R.L., and Murali K.R. Isolation of potent antibiotic producing actinomycetes from marine sediments of Andaman And Nicobar marine island. Journal of Microbiology and Antimicrobials 2013; 5(1):6-12.
CrossRef - Emilda R., Saramma A.V. Isolation of actinomycetes from mangrove and estuarine sediments of cochin and screening for antimicrobial activity. Journal of Coastal Life Medicine 2016; 4(3):207-210.
- Shinde C.H., Anand P.K., Kunchiraman B.N., Arun B.J. In-vitro study for the anticandida activity of homeopathic medicines against Candida albicans. International Journal of Health Sciences and Research 2018; 8(9):57-61.
- Sweetline C., Usha R. and Palaniswamy M. Antimicrobial activity of actinomycetes from Pichavaram Mangrove, Tamil Nadu. Applied Journal of Hygiene, 2012; 1(2):15-18.
- Chelliah R., Ramakrishnan S.R., Prabhu P.R., and Antony U. Evaluation of antimicrobial activity and probiotic properties of wild-strain Pichia kudriavzevii isolated from frozen idli batter. YEAST 2016; 33:385-401.
CrossRef - Ashish V.P, Venkata V.R, Amaraja J., Larrisa P., Yogesh S.S. Rufibacter immobilisnov., isolated from high-altitude saline lake. International Journal of Systemic and Evolutionary Microbiology 2015; 65:1592-1597.
CrossRef - Chavan N.P., Pandey R., Navani N., Nanda R.K., Tandon G.D., Khetmalas M.B. Biocontrol potential of actinomycetes against Xanthomonas axonopodis pv punicae, A causative agent for oily spot disease for pomegranate. Biocontrol Science and Technology 2015; 26(3):351-372.
CrossRef - Raaesh M.S., and Utpal R. Biochemical characterization of an anti-Candida factor produced by Enterococcus fecalis. BMC Microbiology 2012; 12(1):132.
CrossRef - Raghavarao K.V., Siva K.K., Bhaskar R.D., Raghava R.T. Isolation and characterization of antagonistic Actinobacteria from Mangrove soil. Journal of Biochemical Technology 2012; 3(4):361-365.

This work is licensed under a Creative Commons Attribution 4.0 International License.





