How to Cite | Publication History | PlumX Article Matrix
Occurrence of Organochlorine Pesticide (OCP) Residues in Chicken Skin, Liver and Muscle in Egypt
Tamer M. Abd El-Aziz El-Mergawy1, Wafai Z. A. Mikhail2*  , Islam Noeman Nasr1, and Waiel M. Salah El Dien3
, Islam Noeman Nasr1, and Waiel M. Salah El Dien3
1Central Agricultural Pesticides Laboratory, Agricultural Research Centre, Giza, Egypt.
2Department of Natural Resources, Faculty of African Postgraduate Studies, Cairo University.
3Animal Health Research Institute, Zagazig Provincial Lab., Food Hygiene Department.
Corresponding Author E-mail: wafai47@hotmail.com
DOI : http://dx.doi.org/10.13005/bbra/2781
ABSTRACT: This study was conducted for determination of organochlorine (OCPs) pesticide residues in muscle, skin and liver of chickens (laying hens). The examined samples were collected from three laying hen farms (white Hy- Line breed) in El- Dakahlia, El-Sharkia and El-Giza Governorates, Egypt. Five samples of each tissue were collected at 3 stages; start, peak and end, of egg production from the three farms with the total of 45 samples for each tissue type. The examined samples were extracted and prepared to organochlorine pesticide detection by gas Chromatography system equipped with electron capture detector (ECD). Exactly 14 of organochlorine pesticide residues were analyzed. The obtained results revealed that the Methoxychlor, PP-DDE, Dieldrin, Aldrin, Heptachlor epoxide and α-BHC residues were not detected in all the examined samples. Meanwhile, PP-DDT, PP-DDD, Endrin, Endosulfan, Heptachlor, Δ-BHC, ɤ-BHC (lindane) and ɤ-chlordane were detected in the examined samples in different concentrations. No considerable difference in organochlorine residues could be noticed either between the different Governorates or between the different stages of egg production. The results exhibited relatively higher levels of these residues in skin and liver samples comparing with those in the muscle samples.
KEYWORDS: Chicken Liver; Chicken Muscle; Chicken Skin; Organochlorine Pesticide; Pesticide Residues
Download this article as:| Copy the following to cite this article: El-Mergawy T. M. A. E, Mikhail W. Z. A, Nasr I. N, Dien W. M. S. E. Occurrence of Organochlorine Pesticide (OCP) Residues in Chicken Skin, Liver and Muscle in Egypt. |
| Copy the following to cite this URL: El-Mergawy T. M. A. E, Mikhail W. Z. A, Nasr I. N, Dien W. M. S. E. Occurrence of Organochlorine Pesticide (OCP) Residues in Chicken Skin, Liver and Muscle in Egypt. Available from: https://bit.ly/33QFeIn |
Introduction
Pesticides are toxic chemicals which are used for control the unwanted organisms especially those which have public health significance. Organochlorine pesticides developed in 1940s for use mainly as insecticides in 2nd world war. This pesticide group is fat soluble, chemically stable, and has a slow rate of bio- transformation and degradation1. Owing to their environmental persistence and serious risk on the public health, organochlorine pesticides were prohibited in most countries. In spite of banning of this pesticide group in Egypt since 19802, many recent studies detect organochlorine residues in foods3-4-5, and in human breast milk6.
The probable sources of this pesticide group originated from previous and/or illegal use. They cause serious toxic symptoms including developmental abnormalities, growth suppression, disruption of the endocrine system, impairment of immune function, and cancer promotion7. OCPs are characterized by their bio-accumulation in the environment, especially in the food chain, where they find their way into the human body8.
During the last few decades, poultry meat became the most common and popular supply of animal protein in Egypt. It contained all essential amino acids, many minerals and vitamins, which are required for maintaining life and promoting growth9. A large part of these organic pesticides are accumulated in soil for many years, where the plants absorb some of these chemical pesticides and store them in their stems, leaves, fruits and cereals, and then move to the poultry feeds on, and therefore appears in tissues and organs. Subsequently, these chemicals reach to the human bodies via consuming the contaminated poultry meat and eggs10-11-12.
Therefore, the aim of the present investigation is to monitor the organochlorine pesticide residues in muscle, skin and liver samples from the laying hens during different stages of the laying period.
Material and Methods
Experimental hens
This study was conducted for determination of organochlorine pesticide residues (OCPs) in laying hen tissues (muscle, skin and liver). The examined samples were collected from three laying farms (white Hy-Line breed) from each of El-Dakahlia, El-Sharkia and El-Giza Governorates, Egypt.
Collection of the samples
The examined tissue samples were collected at the beginning of the egg production period (24 week of age), at the peak of the egg production (32 week of age) and near the end of the egg production period (40 week of age). Five laying hens from each farm were selected in isolated section, slaughtered, defeathered and evisceration steps were conducted under sanitary conditions. Approximately, 50 gm from each of skin, muscle (pooling of breast and thigh muscle) and the whole liver samples were packed in colorless polyethylene bags, stored in an ice-box and brought to the laboratory and finally stored in a deep-freezer at -20°C till the time of analysis.
Pesticides
Pesticide reference standards obtained from Dr. Ehrenstorfer (Augsburg, Germany), with purities >95% were used to prepare stock solutions dissolved in Hexane for standard stock solution of GC-ECD.
Stock Solutions
Mixed reference standard solutions were prepared and kept at -20±2°C, the solvent(s) used is appropriate to the analyte solubility, stability and method of analysis.
Working solutions
Diluted mix of all compounds of each of organochlorine were prepared for spiking and GC-MS injection and stored in refrigerator at 4±2°C.
Monitoring of some OCPs pesticides
Chemicals and reagents
All of the individual pesticide reference standards (purity>98.0%) were obtained from Dr. Ehrenstorfer, Augsburg, Germany. Organochlorines were prepared in n-Hexane.
Organochlorine pesticides examined were
Methoxychlor, PP-DDT, PP-DDD, PP-DDE, Endrin, Dieldrin, Endosulfan, ɣ-Chlordane, Aldrin, Heptachlor epoxide, Heptachlor, Δ-BHC, ɤ-BHC (lindane) and α-BHC.
Reagents
Acetone (99.9% HPLC grade), Acetonitrile (99.9% HPLC grade), n-Hexane (97% HPLC grade), Magnesium sulfate anhydrous, Sodium chloride 99%, Disodium hydrogen citrate sesquihydrate, Trisodium citrate dehydrate, Primary Secondary Amine (PSA) and C18 ( Octadecyl silyl modified silica gel).
Apparatus
Acrodisc® syringe filters nylon membrane, diam. 25mm, pore size 0.45μm.
Vortex® V2H-Boeco Digital, Processing Equipment.
Polypropylene centrifuge tubes with screw caps, 15 ml and 50 ml.
Lab Centrifuge, Heraeus Labofuge 400, suitable for the centrifuge tubes employed in the procedure and capable of achieving at least 4000 rpm.
Injection Vials, 1.5ml, suitable for GC and auto sampler, if necessary with micro-inserts.
Screw capped glass vials, 20ml, for the storage of the excessive amounts of the final extract, if necessary. Vibration Device, e.g. Vortex.
Volumetric Flasks, Grade A: Hirschman Labogerate, and Brand: 10, 25, 50, and 100ml.
Automatic Pipettes, Hirschman Labogerate suitable for handling volumes of 10 to 100 μl, and 100 to 1000 μl. -Analytical Balance, Mettler Toledo AG 204: 0.1 mg to 210 gm. range.
Precision Balance, Mettler Toledo GG 4002-S: Delta range, 0.5 to 4100 gm.
GC-ECD determination of organochlorine in sample
Agilent 6890 N series gas Chromatography system equipped with electron capture detector (ECD). The inlet operating conditions were injection volume, 1 μl, flow rate 3 ml/minute; Agilent technologies: PAS-5 UI capillary column (5% biphenyl-95% dimethylsiloxane), ID: 0.25 mm, Film thickness: 0.52 μm, column length: 30 m.
The temperature program
The oven program was set at 160°C, for 2 minute, programmed to 280°C at rate of 6°C/minute, and kept at this temperature for 2 minutes. The nitrogen carrier gas flow was maintained at a constant flow at 3 ml/minute.
Extraction and preparation of samples
QuEChERS method for analysis of pesticide residues13 was used for extraction of pesticide residues in samples. The procedure involves initial single-phase extraction of 10 g sample with acetonitrile in a 50 mL centrifuge tube, 10 mL acetonitrile the tube is closed and shaken vigorously by hand for 1 minute. After that add a mixture of: 4±0.2 g Magnesium sulphate anhydrous, 1±0.05 g Sodium chloride, 1g±0.05 g Trisodium citrate dehydrate and 0.5±0.03 g Disodium hydrogen citrate sesquihydrate.
The tube is closed and immediately shaken vigorously by hand for 1 minute and use vortex for 1 minute for well blending then centrifuge for 5 minutes at 4000 rpm. An aliquot of the extract is transferred into a single use centrifugation tube and stored overnight in a freezer. Add 150 mg magnesium sulphate¸ 25 mg PSA and 25 mg C18 per ml extract (e.g.: for 8 ml extract 1.2 g Magnesium sulphate are needed). The tube is shaken vigorously for 30s and centrifuged for 2 minutes at 4000 rpm. After centrifugation the cleaned extract is transferred into a screw cap vial (EN 15662:2008). Inject 1µl into Gas Chromatography-Electron Capture Detector (GC-ECD).
Residue calculation
Calculation, the analyte concentration in sample Cs (mg/kg) was calculated as follows
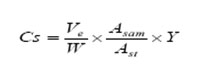
Ve = Extracted volume (ml), Asam= The area of sample peak, Ast = The area of standard in matrix peak, Y = Standard concentration (μg/ml), W = Weight of sample taken for extraction (g).
Method of validation
The selected parameters for in-house validation were mainly taken from guidelines13.
Recovery test
It is defined as the proportion of analyte remaining at the point of the final determination, following its addition (usually to a blank sample) immediately prior to extraction. Untreated samples of muscle, skin and liver were spiked with known amount of the tested compounds prior to extraction and clean-up for recovery tests. Samples were passed through the entire process of extraction, clean up and analysis as previously described. Recovery is usually expressed as a percentage (%) of the orgaochlorine pesticides. The recovery sample was repeated and relative standard deviation (RSD%= CV%) was calculated according to the equation of atomic emission spectroscop14:
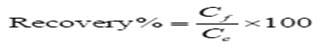
Where : Cf = found concentration, Ce = added concentration; relative standard deviation will be calculated by equation
Standard deviation can be calculated by the equation.
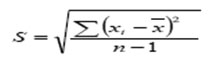
S = standard deviation, xi = found concentration in samples, ͞x = Mean of found concentration in n samples, n = number of samples.
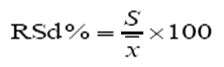
RSD % = Relative standard deviation, S = Standard deviation, x = Mean of the found concentration in n samples. Spiked level of 0.01 mg/kg of the obtained results were corrected according to the recovery rate.
Repeatability
Conditions where independent test results are obtained using the same method on the same sample(s) in a single laboratory over a short period of time, during which differences in the materials and equipment used and/or the analysts involved will not occur. The precision (standard deviation) of measurement of an analyte (usually obtained from recovery or analysis of reference materials). Repeatability experiments were done by fortification of blank samples with the concentration level used and the RSD% was calculated.
Recovery of organochlorine pesticide (OCPs)
Untreated samples of muscle, skin and liver were spiked with known amount of the tested compounds prior to extraction and clean-up for recovery tests. Samples were passed through the entire process of extraction, clean up and analysis as previously described. Percent of recovery was calculated by the following equation:
% Recovery = ((µg) present / (µg) added) × 100.
Spiked level 0.1 mg/kg, the obtained results were corrected according to the recovery rate (Table 1).
Statistical analysis
Statistical analysis of data of organochlorine pesticides residues was conducted as described15.
Table 1: Recovery rate of organochlorine pesticides (OCPs)
|
Compounds |
Muscles | Skin | Liver | |||
| Recovery% ± SD | RSD% | Recovery%± SD | RSD% | Recovery%±SD | RSD% | |
| Methoxychlor | 99.20±5.19 | 5.24 | 94.55±2.49 | 2.63 | 90.34±7.70 | 8.63 |
| PP-DDT | 90.43±11.22 | 12.41 | 89.56±2.46 | 2.74 | 88.12±3.65 | 4.15 |
| PP-DDD | 79.72±3.27 | 4.11 | 84.54±5.47 | 6.47 | 88.43±8.93 | 10.00 |
| PP-DDE | 90.76±8.74 | 9.64 | 89.56±9.02 | 10.07 | 90.65±8.42 | 9.28 |
| Endrin | 77.98±5.43 | 6.97 | 79.43±7.68 | 9.67 | 79.56±5.75 | 7.22 |
| Dieldrin | 90.76±5.36 | 5.92 | 92.66±3.45 | 3.72 | 87.98±5.70 | 6.48 |
| Endosulfan | 87.88±10.19 | 11.60 | 89.98±4.62 | 5.13 | 97.50±5.81 | 5.96 |
| ɤ-chlordane | 88.78±7.67 | 8.65 | 79.98±3.40 | 4.26 | 82.21±5.22 | 6.35 |
| Aldrin | 99.05±4.88 | 4.94 | 79.88±4.87 | 6.00 | 87.99±2.31 | 2.63 |
| Hepta epoxide | 90.05±6.86 | 7.62 | 89.76±1.89 | 2.10 | 90.11±3.33 | 3.69 |
| Heptachlor | 88.54±7.19 | 8.13 | 90.95±3.74 | 4.11 | 89.67±1.29 | 1.44 |
| ∆-BHC | 85.44±4.05 | 4.75 | 88.76±5.56 | 6.31 | 87.77±3.80 | 4.44 |
| ɤ-BHC (lindane) | 88.66±1.68 | 1.90 | 93.45±10.80 | 11.66 | 89.34±1.82 | 2.02 |
| α-BHC | 98.87±1.04 | 1.06 | 9.05±6.24 | 6.20 | 90.98±2.01 | 2.21 |
Results and Discussion
Organochlorine pesticide (OCPs) residues (µg/gm) for chicken muscles in El-
Dakahlia, El-Sharkia and El-Giza Governorates.
The present study were conducted to estimate 14 of organochlorine pesticide residues in the muscle, skin and liver samples of laying hens obtained from different farms located at El-Dakahlia, El-Sharkia and El-Giza Governorates in different stages of the egg production period.
Table (2) shows results of organochlorine pesticide (OCPs) residues in µg/gm, for chicken muscles in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production. Only, each of PP-DDD, Endrin and Δ-BHC were detected in the muscle samples of chickens. PP-DDD was detected in muscle samples taken from each of El-Sharkia and El-Giza Governorates. In El-Sharkia Governorate, PP-DDD residue is 0.02 µg/gm at start, peak and end of egg production. On the other hand, in El-Giza Governorate, it was 0.04 µg/gm at the start and 0.06 µg/gm at each of peak and end of egg production.
Table 2: Organochlorine pesticide (OCPs) residues (µg/gm) for chicken muscles in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production, ND=not detected.
| Organochlorine pesticides | El-Dakahlia Governorate | El-Sharkia Governorate | El-Giza Governorate | ||||||
| start | Peak | end | start | peak | end | start | peak | end | |
| Methoxychlor | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PP-DDT | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PP-DDD | ND | ND | ND | 0.02 | 0.02 | 0.02 | 0.04 | 0.06 | 0.06 |
| PP-DDE | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endrin | ND | ND | 0.02 | ND | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 |
| Dieldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endosulfan | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ɤ-chlordane | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Aldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor epoxide | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Δ-BHC | ND | 0.16±0.06 | ND | ND | 0.02 | ND | 0.06±0.04 | 0.30±0.16 | 0.32±0.15 |
| ɤ-BHC (lindane) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| α-BHC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Each of Endrin and Δ-BHC are detected in muscle sampled from all Governorates. Endrin was detected in El-Sharkia Governorate at the peak and end of egg production (0.04 µg/gm each), while in El-Giza Governorates, detected in all stages of egg production, whereas, in El-Dakahlia Governorate at the end of egg production only (0.02 µg/gm). Δ-BHC, was detected in the three Governorate. It detected at peak of egg production only, in each of El-Dakahlia and El-Sharkia Governorates, with concentration of O.16±0.06 and 0.06 µg/gm, respectively. In El-Giza Governorate it was detected in different periods of egg production, start, peak and end, with 0.06±0.04, 0.30±0.16 and 0.32±0.15 µg/gm, respectively.
Organochlorine pesticide (OCPs) residues (µg/gm) for chickens skin in El- Dakahlia, El-Sharkia and El-Giza Governorates.
Table (3) shows results of organochlorine pesticide (OCPs) residues in µg/gm of chicken skin in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production. Six organochlorine pesticide residues were detected. These are: PP-DDT, PP-DDD, Endrin, Endosulfan, ɤ-chlordane and Δ-BHC. PP-DDT is detected at start and peak of egg, each with 0.02 µg/gm in El-Dakahlia. In El-Giza Governorates, it present only with 0.04 µg/gm at the peak period. PP-DDD is detected in all stages of egg production in each of El-Sharkia and El-Giza Governorates. Endrin was detected in all stage of egg production except the end period in El-Dakahlia Governorate, with a peak of 0.14 µg/gm at the end period in El-Sharkia Governorate. Endosulfan and ɤ-chlordane were detected at the end and start periods in each of El-Sharkia and El-Giza Governorates, respectively. Δ-BHC was detected in the three periods of egg production in the three Governorates, except the end period of El-Dakahlia Governorate. Maximum value was 0.48±0.08 µg/gm detected at start period of egg production in El-Sharkia Governorate.
Table 3: Organochlorine pesticide (OCPs) residues (µg/gm) for chicken skin in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production, ND=not detected.
| Organochlorine pesticides | El-Dakahlia Governorate | El-Sharkia Governorate | El-Giza Governorate | ||||||
| start | peak | end | start | peak | end | start | peak | end | |
| Methoxychlor | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PP-DDT | 0.02 | 0.02 | ND | ND | ND | ND | ND | 0.04 | ND |
| PP-DDD | ND | ND | ND | 0.10 | 0.10 | 0.10 | 0.08 | 0.08 | 0.04 |
| PP-DDE | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endrin | 0.02 | 0.06 | ND | 0.02 | 0.06 | 0.14 | 0.04 | 0.06 | 0.06 |
| Dieldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endosulfan | ND | ND | ND | ND | ND | 0.02 | 0.02 | ND | ND |
| ɤ-chlordane | ND | ND | ND | ND | ND | ND | 0.02 | ND | ND |
| Aldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor epoxide | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Δ-BHC | 0.02 | 0.06±0.04 | ND | 0.48±0.08 | 0.32 | 0.70 | 0.04 | 0.60±0.27 | 0.18±0.09 |
| ɤ-BHC (lindane) | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| α-BHC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Organochlorine pesticide (OCPs) residues (µg/gm.) for chickens liver in El-Dakahlia, El-Sharkia and El-Giza Governorates.
Table (4) shows results of organochlorine pesticide (OCPs) residues in µg/gm of chicken liver in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production. Five organochlorine pesticide residues were detected. These are PP-DDD, Endrin, Heptachlor, Δ-BHC and ɤ-BHC (lindane).
Table 4: Organochlorine pesticide (OCPs) residues (µg/gm) for chickens liver in El-Dakahlia, El-Sharkia and El-Giza Governorates at start, peak and end of egg production, ND=not detected.
| Organochlorine pesticides | El-Dakahlia Governorate | El-Sharkia Governorate | El-Giza Governorate | ||||||
| start | peak | end | start | peak | end | start | peak | end | |
| Methoxychlor | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PP-DDT | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PP-DDD | 0.04 | 0.02 | ND | 0.02 | ND | ND | 0.02 | 0.04 | 0.02 |
| PP-DDE | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endrin | 0.08 | ND | 0.02 | 0.02 | 0.02 | 0.04 | 0.06 | 0.08 | 0.08 |
| Dieldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Endosulfan | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ɤ-chlordane | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Aldrin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor epoxide | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heptachlor | ND | ND | ND | 0.02 | ND | 0.04 | ND | 0.06 | ND |
| Δ-BHC | 0.20 | 0.10 | ND | ND | ND | ND | 0.16 | 0.18±0.08 | ND |
| ɤ-BHC (lindane) | ND | ND | ND | ND | ND | ND | ND | 0.16 | 0.10 |
| α-BHC | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Three organochlorine pesticide residues in µg/gm of chicken liver were detected in El-Dakahlia Governorate. These are PP-DDD with 0.04 and 0.02 µg/gm, while Δ-BHC is with 0.20 and 0.10 µg/gm, each at start and peak periods, of egg production, respectively. Endrin was detected at start and end periods of egg production, with 0.08 and 0.02 µg/gm, respectively.
In samples of chicken liver from El-Sharkia Governorate, PP-DDD was detected with 0.02 µg/gm at start period. Endrin was detected at start, peak and end of egg production period with 0.02, 0.02 and 0.04 µg/gm, respectively. Heptachlor at 0.02 and 0.04µg / gm in start and end periods of production, respectively.
In samples of chicken liver of El-Giza Governorate PP- DDD at 0.02, 0.04 and 0.02 and Endrin at 0.06, 0.08 and 0.08 µg/gm for start, peak and end of egg production period, respectively. Heptachlor was detected at 0.06 µg/gm in the peak period, while Δ-BHC is at 0.16 and 0.18±0.08 µg/gm in start and peak of egg production, respectively. ɤ-BHC (lindane) detected at 0.16 and 0.1 µg/gm in peak and end of egg production, respectively.
The obtained results were higher than those recorded in broiler tissues at El-Sharkia Governorate, Egypt16. This study detect ɤ-BHC, Heptachlore, Endrin, PP-DDD and PP-DDT residues in liver samples with the mean values of 0.0029, 0.03, 0.0006, 0.007 and 0.013 µg/gm respectively; while, the mean levels of the same organochlorine pesticides were 0.0013, 0.011, 0.002, 0.0 and 0.009 µg/gm, respectively, in the examined muscle samples. Moreover, in China, organochlorine pesticide residues were recorded17, lower than those in the present study. In that study of China, it detect ɤ-BHC residues in the examined chicken muscle, liver and skin samples in the mean values of 0.008, 0.010 and 0.49 ppb (ng/gm) respectively; while, PP-DDT residues were 0.035, 0.042 and 2.7 ppb (ng/gm) in the mentioned samples respectively; also, PP-DDD residues recorded were 0.026, 0.061 and 1.1 ppb (ng/gm) in the same examined samples, respectively. In another study3, it was recorded that the mean levels of PP-DDT, PP-DDD, Endosulfan, Heptachlor, Δ-BHC, ɤ-BHC and ɤ-chlordane were 6.3, 3.8, 3.4, 1.6, 11.0, 0.4 and 1.2 ppb (ng/ gm) in the examined imported chicken muscle samples in Egypt. These levels were obviously lower than our estimations. Furthermore, In Jordan, could not detect organochlorine pesticide residues in the examined local chicken samples18. On the other hand, the researchers estimated organochlorine residues in chicken samples in different localities in Tanzania19. They recorded PP-DDT in the mean values ranged between 0.6 to 3.3 ppm in liver samples; while, in muscle samples these values ranged from 0.4 to 1.4 ppm. Also, PP-DDD levels varied between 0.2 and 2.2 ppm in liver, but in muscle samples it ranged from 1.0 to 3.1 ppm. The mentioned concentrations were higher than our figures. In spite of prohibiting of organochlorine pesticide using from a long period, aforementioned results concluded relatively higher residues of these compounds in the current investigation comparing with the most of the previous studies including the Egyptian studies. This result may be explained by old age (24- 40 weeks) of the examined laying hens in our investigation comparing with the studies which examined broiler chickens (6 weeks of age) or local breed chickens. The long period of chicken breeding, gave opportunity for bioaccumulation of organochlorine residues because of their long persistent in the environment and animal tissues. On the other hand, the only mentioned study obtained on organochlorine residues more than our figures was conducted in Tanzania (Africa), since the current use of organochlorine pesticides until now8. The most probable source of organochlorine pesticide residues is the feed. The feed ingredients mainly imported and distributed all over the country. Thus, no considerable differences in organochlorine residues could be noticed between the different Governorates sampled in the present study (Tables 2 – 4).
Concerning the comparison between the organochlorine pesticide residues in the examined tissues during the different stages of egg production, no substantial differences could be noticed in pesticide values between the different stages of egg production. This result may be explained by the profuse egg production which leads to get rid of considerable levels of organochlorine pesticides via the produced eggs. So, the organochlorine accumulated slowly during the egg production period.
Because of fat solubility of organochlorine pesticides, the obtained results exhibited relatively higher levels of these residues in skin and liver samples comparing with those in the muscle samples. This result explained by higher fat levels in liver and skin samples than muscle. The obtained results in China17 and in Tanzania19 agreed with our findings.
The recorded results in the present study (Table 5), showed that the Endrin residues exceeded the Maximum Residual Limit (MRL) by 9 (20%), 18 (40%) and 20 (44.5%) out of the examined muscle, skin and liver samples respectively. Moreover, Δ-BHC residues exceeded MRL by 13 (28.8%), 26 (57.7%) and 7 (15.5%) from the examined muscle, skin and liver samples respectively. Also, Endosulfane, ɤ-BHC and ɤ-chlordane residues were above MRLs in 2 (4.4), 3 (6.6%) and 1 (2.2%) out of the examined skin and liver samples respectively. From the mentioned results, it could be notices that only 2 pesticides in muscle were above MRLs. Meanwhile, in skin and liver samples 4 and 3 pesticides were detected in levels exceeded MRLs. This result was explained by higher fat levels in skin followed by liver than those in muscle samples.
Table 5: Frequency distributions of detected organochlorine (O.C.) pesticide residues in the examined Tissues of laying hen laying hens (n = 45, ND = not detected).
| O.C. pesticide
Residues |
MRL*
(ppm) |
Muscle | Skin | Liver | ||||||||||||||||
|
ND |
Within
MRL |
Over
MRL |
ND |
Within
MRL |
Over
MRL |
ND |
Within
MRL |
Over
MRL |
||||||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | No | % | No | % | No | % | |||
| PP-DDT | 1 | 45 | 100 | 0 | 0 | 0 | 0 | 42 | 93.3 | 3 | 6.6 | 0 | 0 | 45 | 100 | 0 | 0 | 0 | 0 | |
| PP-DDD | 1 | 34 | 75.5 | 11 | 24.5 | 0 | 0 | 20 | 44.4 | 25 | 55.5 | 0 | 0 | 37 | 82.2 | 8 | 17.7 | 0 | 0 | |
| Endrin | 0.05 | 36 | 80 | 0 | 0 | 9 | 20 | 27 | 60 | 0 | 0 | 18 | 40 | 25 | 55.5 | 0 | 0 | 20 | 44.5 | |
| Endosulfan | 0.05 | 45 | 100 | 0 | 0 | 0 | 0 | 43 | 95.5 | 0 | 0 | 2 | 4.4 | 45 | 100 | 0 | 0 | 0 | 0 | |
| Heptachlor | 0.2 | 45 | 100 | 0 | 0 | 0 | 0 | 45 | 100 | 0 | 0 | 0 | 0 | 39 | 86.6 | 6 | 13.3 | 0 | 0 | |
| Δ-BHC | 0.02 | 32 | 71.1 | 0 | 0 | 13 | 28.8 | 19 | 42.2 | 0 | 0 | 26 | 57.7 | 38 | 84.5 | 0 | 0 | 7 | 15.5 | |
| ɤ-BHC (lindane) | 0.02 | 45 | 100 | 0 | 0 | 0 | 0 | 45 | 100 | 0 | 0 | 0 | 0 | 42 | 93.3 | 0 | 0 | 3 | 6.6 | |
| ɤ-chlordane | 0.05 | 45 | 100 | 0 | 0 | 0 | 0 | 44 | 97.7 | 0 | 0 | 1 | 2.2 | 45 | 100 | 0 | 0 | 0 | 0 | |
*: The Maximum Residual Limits were recommended (MRL) by Pesticide EU-MRLs20.
The obtained results of this study were coincided with previously obtained results16. On the other hand, although prohibiting of organochlorine compounds from long period ago endrin and Δ-BHC were detected in considerable levels exceeded MRLs in many samples, this result may be attributed to long life span of the examined laying hens as previously mentioned.
From aforementioned results it could be concluded that the tissues of laying hens contained a significant levels of organochlorine pesticide residues in spite of banning of these compound from approximately 40 years ago2. Therefore, this outcome indicates the very long persistence of organochlorine compounds especially in animal tissues of highly lipid contents. Moreover, the findings of the present investigation exhibited that the residues of organochlorine pesticides still dangerous in the environment until now.
In view of the achieved results of the present study, continuous monitoring organochlorine residues in animal products and highly fat content foods in addition to reduce the consumption of laying hen muscles and organs as much as possible are highly recommended.
Conclusions
No considerable difference in organochlorine residues could be noticed either between the different Governorates or between the different stages of egg production. The results exhibited relatively higher levels of these residues in skin and liver samples comparing with those in the muscle samples.
Conflict of Interest
The authors declared that the present study was performed in absence of any conflict of interest.
Acknowledment
The author would thank all participants and their parents
Author Contribution
All authors contributed equally in all parts of this study.
References
- Klaassen, CD editor (2013 ) Casarett and Doull’s Toxicology, The Basic Science of Poisons. 8th edition, McGraw-Hill Education, New York.
- Dogheim SM, Gad Alla SM, El-Sayes SM, Almaz MM and Salama EY (1996) Organochlorine and organophosphorus pesticide residues in food from Egyptian local markets. J. AOAC International, 79 (4): 949-952.
- Abou-Enein AM, Nasr IN, Abou-Elella FM, and Abdulla EM (2010) Monitoring of some organochlorines and organophosphorus residues in imported and locally raised chicken and bovine muscles in Egypt. Journal of Applied Sciences Research, 6 (6): 600-608.
- Salah El-Dien WM and Mahmoud HA (2011) Survey on some chemical pollutant residues in catfish at Sharkia Governorate, Egypt. Journal of American Science, 7(1): 286-293.
- Abdelfatah EN and Abu-Zeid EH (2016) Assessment of pesticide residues in edible and unhatched chicken eggs in Sharkia Province, Egypt. Advances in Animal and Veterinary Sciences, 4 (1): 25- 34.
- Salem DA and El-Saied MM (1997) Levels of organochlorine pesticides, lead and cadmium in mother’s milk and infant’s dairy intake in middle Egypt. J Egypt Soc. Toxicol., 18: 65-71.
- EL-Nemr A, Said TO, Khaled A, El-Sikaily A and Abd-Allah AMA (2003) Polychlorinated biphenyls and chlorinated pesticides in mussels collected from Egyptian Mediterranean coast. Bulletin of Environmental Contamination and Toxicology, 71(2): 290-297.
- Thompson LA, Darwish WS, Yoshinori-Ikenaka Y, Nakayama SMM, Mizukawa H and Ishizuka M (2017) Organochlorine pesticide contamination of foods in Africa: incidence and public health significance. The Journal of Veterinary Medical Science, 79(4): 751–764.
- Pearson AM and DutsonTR. editors, (1997) Production and Processing of Healthy Meat, Poultry and Fish Products. 1st edition, Blackie Academic and Professional, Chapman & Hall, London, UK: 385 pp.
- Tolosa I, Bayona JM and Albaiges J (1995) Spatial and temporal distribution, fluxes and budgets of organochlorinated compounds in Nortwest Mediterranean sediments. Environ. Sci. Technol., 29(10): 2519-2527.
- Lee KT, Tanabe S and Koh CH (2001) Distribution of organochlorine pesticides in sediment from Kyeonggi Bay and nearby areas, Korea. Environmental Pollution, 114: 207-213.
- Hong SH, Yim UH, Shim WJ, OH JR and Lee IS (2003) Horizontal and vertical distribution of PCBs and chlorinated pesticides in sediments from Massan Bay, Korea. Marine Pollution Bulletin, 46: 244-253.
- SANCO/12459/2011 Method validation and quality control procedure for pesticide residues analysis in food and feed.
- Prudnikov ED (1981) Theoretical calculation of the standard deviation in atomic emission spectroscopy. Spectrochimica Acta Part B: Atomic Spectroscopy, 36(4): 385-392.
- Petric A and Watson P (1999) Statistic for Veterinary and Animal Science. 3rd edition, Wiley-Blackwell, UK: 414 pp.
- Salah El-Dien WM (1997) Studies on Pesticide Pollution in Poultry Farms. MSc Thesis in Veterinary Medicine, Department of Animal Hygiene and Preventive Medicine, Faculty of Veterinary Medicine, Zagazig University.
- Tao S, Liu WX, Li XQ, Zhou DX, Li X, Yang YF, Yue DP and Coveney RM (2009) Organochlorine pesticide residues in chickens and eggs at a poultry farm in Beijing, China. Environmental Pollution, 157: 497-502.
- Al-Antry TM, Alawi MA, Abu Othman M and Haddad N (2018) Persistent organochlorine pesticide residues in foodstuff of animal origin from Southern Governorates of Jordan in 2016 and 2017. Fresenius Environmental Bulletin, 27(1): 9775-9781.
- Mahugija JAM, Chibura PE and Lugwish EHJ (2018) Residues of pesticides and metabolites in chicken kidney, liver and muscle samples from poultry farm in Dar es Salaam and Pwani, Tanzaniz. Chemosphere, 193: 869-874.
- Pesticide EU-MRLs Regulation (2013) EC No 212/2013. 11 March 2013; Official Journal of the European Union. Online, Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013R0212&from=EN

This work is licensed under a Creative Commons Attribution 4.0 International License.





