How to Cite | Publication History | PlumX Article Matrix
Mark Paul Rivarez1,2, * and Elizabeth Parac3
* and Elizabeth Parac3
1College of Agriculture and Food Science, University of the Philippines, Laguna, Philippines.
2Present Address: Department of Biotechnology and Systems Biology, National Institute of Biology and, Jožef Stefan International Postgraduate School, Ljubljana, Slovenia.
3College of Agriculture and Agri-Industries, Caraga State University, Butuan City, Philippines.
Corresponding Author E-mail: msrivarez@up.edu.ph
DOI : http://dx.doi.org/10.13005/bbra/2765
ABSTRACT: Xanthomonas oryzae pv. oryzicola (Xoc) is a seed-borne bacterial pathogen of rice. In this study, a rapid molecular detection method in seeds was developed to prevent unwanted movement of infected materials and greater yield loss. Crude DNA from ground seed extract supernatant was precipitated in ethanol and centrifuged. DNA pellets of homogenous subsamples were pooled and werepurified using SDS or a DNA extraction kit. Three pairs of primers previously designed on Xoc isolate BLS-256 genome sequence robustly amplify Xoc-specific DNA sequences. Primer T40 was found to efficiently detect Xoc but only in single primer reactions. While primers 3864 and 3866 can amplify Xoc DNA separately, however, their multiplex PCR reactions were unsuccessful, probably due to substrate competition in the reaction mixture. Optimized PCR mixture and conditions were able to detect as low as 10 cfu/mL Xoc cells and the direct extraction method and conditions were able to detect as low as 104 cfu/mL inoculated cells using primer T40. Lastly, Xoc transmission through seeds was observed only in plant samples which were heavily infected. Recovery from infection, at around 25-50% was also observed in some cultivars which also manifested good grain filling at 56 days after transplanting.
KEYWORDS: Molecular Diagnostics; Rice Seeds; Seed-Borne Pathogens; Xanthomonas; Xoc
Download this article as:| Copy the following to cite this article: Rivarez M. P, Parac E. Rapid Molecular Detection and Transmission of Bacterial Leaf Streak Pathogen, Xanthomonas Oryzae Pv. Oryzicola, in Rice Seeds.Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Rivarez M. P, Parac E. Rapid Molecular Detection and Transmission of Bacterial Leaf Streak Pathogen, Xanthomonas Oryzae Pv. Oryzicola, in Rice Seeds.Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2JnCPgB |
Introduction
Seeds play an important role in the sustainability of a civilization, more specifically in the sustainability of food production. Seeds serve as primary propagative and breeding materials, and certainly, for direct consumption as food by man (Schaad, 1982). However, seed-borne pathogens impose an immeasurable risk for food production, especially in terms of widespread transportation, emergence and outbreaks of diseases if introduced in new areas (Gitaitis and Walcott, 2007).
One of the most studied pathogen-harboring propagative materials is rice seed. It is known to have many viral, bacterial and fungal pathogens that are seed-borne and seed-transmitted (Agrawal et al., 1999). Two of these pathogens include the Xanthomonas oryzae pathovars oryzae (Xoo) and oryzicola (Xoc), which cause bacterial leaf blight (BLB) and bacterial leaf streak (BLS) diseases, respectively. Studies have been conducted with the leadership of the International Rice Research Institute (IRRI) that aimed to harmonize morphological, biochemical and molecular methods to efficiently detect both Xoo and Xoc in rice leaves and seeds. Several studies were also conducted to develop genomics-based diagnostic marker for Xoo and Xoc (Lang et al., 2010) and to develop molecular PCR-based methods to detect either Xoo or Xoc (Sakthivel et al., 2001; Adachi and Oku, 2000; Zhao et al., 2007). Recently, loop-mediated isothermal amplification (LAMP) was used to detect both Xoo and Xoc in leaf and seed samples (Lang et al., 2014). These studies also looked into the sensitivity of the PCR-based method based on the lowest colony forming units (cfu) or number of bacterial cells detected. Nevertheless, not all studies analyzed the sensitivity of PCR-based methods. Lastly, seed transmission test was given less attention in the aforementioned studies.
In many rice growing areas in the world, seed-borne bacterial diseases continue to be problematic and cause significant economic losses. In the Philippines, bacterial leaf streak disease caused by Xoc results to yield losses of epidemic proportions. Yield losses are more pronounced during wet seasons, which can reach up to 17%, than during dry seasons which can be only as low as 1.5% (Sparks, undated). Both Xoo and Xoc are seed-borne and seed-transmitted, thus, these pathogens are of quarantine importance. These bacteria are also endophytic in nature. Xoo causes blight by invading the vascular tissue, specifically the xylem vessels, while Xoc causes leaf streak by colonizing the parenchyma and apoplast. After harvest, the bacteria invaded the seeds through vascular tissues or direct exposure persist in the grain even during dry conditions or season. Thus, when the infected seed is used as a planting material for the next cropping season or simply for exchange of materials, the spread of the disease will be uncontrolled.
Due to this scenario, it is therefore pertinent to monitor seed health to save plants and prevent unwanted losses for the next cropping season. However, testing seeds for the presence of plant-pathogenic bacteria seems to receive little attention through the years (Schaad, 1982). Current seed testing units of the Bureau of Plant Industry (BPI) mostly tests only for fungal pathogens using the ‘blotter’ technique. Likewise, IRRI’s Seed Health Unit (SHU) primarily focuses on seed health with emphasis on seed-borne fungal pathogens. Detection of other seed-borne pathogens such as bacteria and viruses is not a routine work and will be done only upon special request by the person or institution concerned. Both Xoo and Xoc can be grown and detected using artificial media and distinguished using biochemical tests or even detected using serological methods. These methods consume much time and resources and the results are sometimes troublesome. In this study, the sensitivity of an optimized PCR-based detection method will be evaluated and the seeds tested positive for Xoc will be subjected to seed transmission or grow-out test.
This study primarily aimed to evaluate the sensitivity of an optimized PCR-based detection method for Xanthomonas oryzae pv. oryzicola (Xoc) in rice seeds, and evaluate the transmissibility of Xoc from infected seed to the mature plant. Moreover, this study primarily aimed to evaluate the sensitivity of an optimized PCR-based Xoc detection method in terms of lowest bacterial concentration that can be detected in artificially inoculated seeds; and to determine the transmissibility of Xoc in naturally-infected and artificially-infected seeds and observe for possible plant recovery to leaf streak disease upon maturity.
Materials and Methods
Seed transmission test of naturally- and artificially-infected rice seeds.
Seed samples (rice var. IR50) that tested positive for Xoc using the optimized PCR-based detection method were obtained. These samples were namely: (1) IRRI Seed Samples, (2) Seeds from inoculated plants (3) Isabela seed samples (varieties NSIC162, RC18, SL8). Fifty pieces of these naturally- infected rice seed samples were bagged in gauze cloth and soaked in running water in a 500-mL beaker for 24 hours. This is for the seeds to imbibe water and initiate root formation. The seeds were soaked on separate beakers to avoid cross-contamination. Pre-germinated seeds were sowed in a seedling pan containing moderately moistened clay-loam soil. The seeds were allowed to germinate for two weeks before it is transplanted to medium sized clay pots containing clay-loam soil, at three plants per pot. Sixteen plants per seed lot or sample were maintained as test plants for the entire experiment. On a daily basis, the plants were misted with sterile water to maintain high humidity levels favorable for the development of the characteristic BLS symptoms. Observation and photo documentation of symptoms were done on the major stages of growth of an IR50 rice variety, from seedling stage (14 days after transplanting (DAT)), tillering stage (28 DAT), stem elongation stage (42 DAT) and were terminated at the panicle initiation stage (56 DAT). Observation of the symptoms were done only at the early reproductive stage of the plant because most plants infected with Xoc could easily recover from bacterial infection from the early stages as it reach the flowering stage with only minimal grain reduction (Sparks, undated). Percent germination, percent survival, disease incidence and percent recovery were all noted throughout the course of observation. The seed transmission experiment were done twice.
Crude DNA Extraction from Rice Seeds.
Seed lot or samples used in this experiment were previously desiccated using silica gel and stored at 4°C. Fifteen (15) seeds were randomly picked aseptically and used as a subsample for each seed lot. At least five subsamples per seed lot were prepared. The seeds were placed in a 1.5 mL Eppendorf tube for easy handling and were disinfected with 70% ethanol for 30 seconds and washed thrice with sterile distilled water (sd water). The tubes were filled with 1.0 mL of 10% Tween-20 in phosphate-buffered saline (PBS-T) and incubated at 85°C water bath for 2 hours to soften the grain. After incubation, the seeds were ground using mortar and pestle and an additional 1.0 mL of PBS-T was added to re-suspend the ground extract. A 1.0 mL aliquot of the ground extract was transferred to a new set of 1.5 mL Eppendorf tubes and was centrifuged at 10000 rpm for 5 minutes to separate the seed debris. A 0.5 mL aliquot of the supernatant was collected and transferred to new tubes containing 1.0 mL of cold ethanol. To precipitate DNA, the tubes were incubated at -20°C for 30 min, after which the tubes were centrifuged at 12000 rpm for 10 min to pelletize DNA. The pellets were air dried under laminar flow hood for 30 min and re-suspended in 30.0 µL Tris-EDTA buffer (pH 8.0). Finally, extracts of the same seed lot (i.e. homogenous samples) were pooled.
SDS DNA Extraction.
The following method was adapted from Chen and Kuo (1993), with some modifications. The pooled crude DNA extracts were placed in a 1.5 mL Eppendorf tube and 100.0 µl of lysis buffer (SDS) was added thereafter. The solution was pipetted vigorously after which 33.0 µl of 5M NaCl solution was added. The contents of the tube were thoroughly mixed by inverting the tubes to remove most cell debris. The resulting viscous mixture was centrifuged at 12,000 rpm for 10 minutes. After transferring the clear supernatant into another clean 1.5 mL Eppendorf tube, an equal volume of chloroform was added, and the tube was gently inverted until a milky solution was formed. The mixture was centrifuged at 12,000 rpm for 10 minutes and the aqueous phase containing the DNA was then transferred to another clean 1.5 mL Eppendorf tube. Two volumes of chilled absolute ethanol were added to precipitate out DNA in the solution. This was mixed gently until a stringy white DNA precipitate was formed that condensed into a tight mass and let stand at -20°C freezer overnight. Centrifugation at 12,000 rpm for 10 minutes was done to obtain the pellet and the alcohol was discarded. The pellet was washed twice with 200.0 µl of 70% ethanol, air dried in the laminar flow hood for 30 min and then re-dissolved in 10.0 µl TE buffer (pH 8). DNA extracts were used directly for PCR or stored at 4°C for subsequent PCR assays.
Primers used for the PCR-based Detection Methods.
Xoc-specific DNA primers previously designed on Xoc isolate BLS-256 genome were used in this study. Primer design was done using Primer-BLAST online program, which is a combined Primer3 (www.bioinfo.ut.ee/primer3) and BLAST courtesy of the National Center for Biotechnology Information (NCBI, www.ncbi.nlm. nih.gov/tools/primer-blast). Primers 3864 and 3866 were based on the previous study by Lang et al. (2010) on the genomics-based approach in detecting Xoc in different plant materials. Primer sequence, expected amplicon size and percent detection efficiency of the primers are summarized in Table 1.
Table 1: DNA primers used for the PCR-based detection of X. oryzae pv. oryzicola.
| Primer ID | Gene Description | Nucleotide Sequence (5’-3’) | Expected Amplicon Size |
| T40 | ATP-binding cassette-2 | (F) GTTTTTCAATCCGCTCCTGA
(R) CGATATCGCGAACGAATACA |
422 bp |
| 3864 | Putative glucosyl transferase | (F) GTGCGTGAAAATGTCGGTTA
(R) GGGATGGATGAATACGGATG |
945 bp |
| 3866 | LPS O-antigen biosynthesis protein | (F) ATCTCCCAGCATGTTGATCG
(R) GCGTTCAATCTCCTCCATGT |
691 bp |
General Flow of the Detection Experiments.
Before coming up with an optimized protocol, several trials of optimization were done which includes culture enrichment and DNA extraction using an extraction kit. Finally, an optimized general workflow of the detection experiment was developed and illustrated in Figure 1 and the estimates of time and cost rates are presented in Figure 2. The workflow involves the direct detection of Xoc using the crude extraction and SDS extraction protocols described above. The protocol depicts the use of PCR as the main molecular detection technique used to determine the presence of Xoc in the seed samples.
Standardization of Xoc Population.
The population of Xoc isolate BLS-256 at 72 hours (room temperature) in peptone sucrose agar (PSA) in test tube slants was standardized. After growing the bacteria, 10 mL of sterile distilled water was added to the slant culture to suspend bacteria and make the stock suspension. Then a ten-fold serial dilution series was made starting at 10-1 up to 10-8. The last three dilutions, 10-6, 10-7 and 10-8 was used to grow colonies of Xoc in PSA culture plates. An aliquot of 0.1 mL of each dilution was spread onto the surface of the PSA culture plates. Five plates per dilution were prepared. The plates were incubated at room temperature for 72 hours after which distinct Xoc colonies were counted. Only triplicate plates of the same dilution with colony counts within 20-200 colonies were considered in computing for the number of colony forming units per mL of stock suspension, which is computed as follows:

In case two or more dilutions have acceptable colony counts, the average of their computed cfu/mL can be taken and used in subsequent calculations. Consequently, dilutions of 10%, 20%, 30%, up to 90% of the same stock suspension were prepared for spectrophotometric measurements. At least three absorbance readings of 2.0 mL aliquots of the suspensions were taken using a spectrophotometer set at wavelength of 600 nm, and the corresponding average absorbance reading was calculated. Percent transmittance values were also noted for each reading. Only the solutions that have average absorbance readings from 0.1 to 0.5 and percent transmittance values from 31% to 75% were considered in making the standard population curve. After getting the absorbance values, the dilution factor for each valid (i.e. average absorbance readings from 0.1 to 0.5 and percent transmittance values from 31% to 75%) solutions were also computed. A scatter graph was made thereafter. In the y-axis, the non-log absorbance data was plotted in average OD units. It was also carefully noted that the absorbance should correspondingly increase as the number of bacteria increases. On the other hand, the dilution factors (i.e. 0.5, 0.4, 0.3. 0.2 and 0.1) were plotted on the x-axis. The line of best fit was obtained from the scatter plot and served as a standard population curve for Xoc isolate BLS-256 of the same growing conditions. The regression equation can be used to estimate the number of viable cells present in a suspension (per mL) of a 72-hr Xoc PSA slant culture in sterile water by just getting the absorbance reading (0.1 to 0.5) and percent transmittance values (31% to 75%) of the suspension.
The OD (absorbance reading) value for the suspension can be obtained using the spectrophotometer and the corresponding dilution factor can be calculated using the regression equation of standard population curve. The computed dilution factor is then multiplied with the cfu/mL of the standard, thus giving the final value of the cfu/mL of the new culture obtained using similar growth conditions (Postgate, 1969)
Assay for Sensitivity of the Extraction Method and PCR Mixture and Conditions.
A 72-hour culture of Xoc isolate BLS-256 in PSA slant was used as source of bacterial cells for PCR detection limit test and as source of inoculum for the artificial inoculation of rice seeds. A stock suspension was made using 10 mL sterile distilled water and afterwards, a ten-fold serial dilution series was made up to 10-8 dilution. A 20% stock suspension was prepared and its absorbance and transmittance reading was taken at 600 nm. The number of viable cells in the 20% stock suspension was determined based on the standard population curve by just inputting the average absorbance reading of the sample. Based on the estimated concentration of viable cells, aliquots containing 100, 10 and 2 cells were obtained and used as template for the optimized conventional PCR mix to determine its detection limit. Consequently, seeds were sterilized twice using 70% ethanol for 5 minutes and washed thrice with sterile distilled water. Seeds were blot dried in sterile paper towels and air dried under laminar flow hood for 30 minutes before inoculation. Using the same serial dilution of the stock suspension, aliquots of 106, 104 and 102 cfu/mL were inoculated in sterilized seeds. Inoculated seeds were incubated at room temperature for 24 hours and blot dried in sterile paper towels and air dried under laminar flow hood for 30 minutes afterwards. Finally, the inoculated and dried seeds then underwent extraction following the aforementioned direct extraction method.
Results and Discussion
Globally, bacterial leaf streak (BLS) of rice is endemic to much of Asia countries (Mew, 1993). But due to frequent exchange of seed materials for breeding and direct planting, the disease was accidentally introduced to West Africa and Northern Australia (Niño-Liu et al., 2006). Reductions in grain yield due to BLS can reach up to 32% (Ou, 1985; Cottyn et al., 2001). In the Philippines, it is reported that BLS has affected more than 3000 hectares in Isabela and in nearby Northern and Central Luzon Provinces, namely Cagayan, Quirino and Nueva Vizcaya (news.agropages.com).
Xoc is transmitted through infected seeds and causes BLS on growing rice plants. Observation of rice plants for 56 days (see Figure 3) have gained insights as to whether the Xoc can be transmitted from seeds to growing plant. In terms of germination and survival (see Table 2), Xoc infection seems to have an influence on the growth of the germinating seeds. Seeds from BLS-infected rice plants from Isabela seems to have the lowest percent germination. SL8 variety had the lowest percent germination of 36.0%, while NSIC162 had only 42.00% germination. In contrast, seeds from greenhouse BLS-inoculated plants and seed samples from IRRI have high percent germination, 84.0% and 96.0% respectively. Nevertheless, all transplanted seedlings, sixteen of them, for all varieties were able to survive and sustain growth until flowering stage and even at 56 DAT.
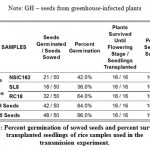 |
Table 2: Percent germination of sowed seeds and percent survival of transplanted seedlings of rice samples used in the transmission experiment. |
Note: GH – seeds from greenhouse-infected plants
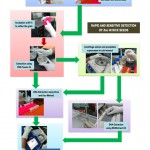 |
Figure 1: A rapid and sensitive detection PCR-based detection of Xanthomonas oryzae pv. oryzicola (Xoc) in rice seeds. |
Seed samples from Isabela, namely varieties NSIC162, SL8 and RC18 all showed symptoms of BLS as early as 14 days after transplanting (DAT). As shown in Table 3, most severe BLS symptoms were observed in SL8, while moderate symptoms only were observed on NSIC162. SL8 did not recover that much, with only 25.00% recovery, from BLS as it reached its reproductive stage at panicle initiation to flowering. Highest percent recovery was observed in RC18 variety (56.25%), although infections were already high at the beginning for these plants. In contrast, incidence of BLS in GH and IRRI seeds remained to be very low at 14 DAT (31.25% and 18.75%, respectively). Recovery from BLS in these plants was also observed at 56 DAT, when the plants start the grain filling stage. Finally, Xanthomonas oryzae pathovars oryzae and oryzicola has been shown to be borne on the husk and glume of the rice seed and as deep as the seed endosperm. Percent infection is also highly variable, from 1 to 100% (Kauffman and Reddy, 1974).
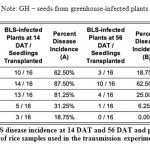 |
Table 3: Percent BLS disease incidence at 14 DAT and 56 DAT and percentage of at 56 DAT of rice samples used in the transmission experiment. |
Note: GH – seeds from greenhouse-infected plants
Detection of seed-borne bacterial pathogens received lesser attention as compared to detection of fungal or viral pathogens, which are part of routine seed health testing methods in many quarantine units worldwide. During the past decades, serological techniques were widely used to detect viruses and some bacterial pathogens in seeds. But these methods consume much time and resources especially in developing pathogen specific antibodies. A genomics-based computational study by Lang et al. (2010) have provided a suite of diagnostic primers for the specific detection of both X. oryzae pv. oryzae and X. oryzae pv. oryzicola. A few decades before the aforementioned study, 16S-23S rDNA spacer region was the most popular diagnostic marker for many bacterial pathogens (Adachi and Oku, 2000; Maes, et al., 1996). Finally, scientists in Korea lead by M.S. Cho (2011) have already developed a real-time Bio-PCR method for the sensitive and specific detection of Xoo based on the rearrangement hot spot (rhs) gene family, a family of evolutionarily conserved but dynamic DNA sequences in enterobacteria that first described in Escherichia coli. Development of diagnostic DNA markers for Xoo seemed to receive much attention and diagnostic tools for Xoc are lagged behind.
Xoc was not detected in newly obtained IR50 seeds from IRRI.
In order to perform evaluation of the sensitivity of the Xoc detection protocol developed in this study, clean or healthy seeds void of Xoc infection must be obtained. IR50 (IRGC 53433) seeds was requested at the Genebank of IRRI. Before further usage, the seeds was first tested for presence of Xoc using optimized protocol described above. Figure 4 shows the gel electrophoresis of the PCR detection reactions for Xoc in the new IR50 seed samples. The expected amplicon size is 422 bp (primer T40), 945 bp (primer 3864) and 691 bp (primer 3866). After three trials of detection, the seeds were found to be negative for the presence of Xoc. Since the seed lot is clean, it was further used for the sensitivity assay of the DNA extraction and PCR detection protocol for Xoc.
Optimized PCR conditions can detect as low as approximately ten Xoc cells using primer T40.
Based on the standard population curve of a 72-hour PSA slant culture of Xoc isolate BLS-256, aliquots of bacterial suspensions containing approximately 100, 10 and 2 cells were obtained and used as template for the PCR reaction.
 |
Figure 2: Estimates of time and fee rates of the rapid and sensitive detection PCR-based detection of Xanthomonas oryzae pv. oryzicola (Xoc) in rice seeds |
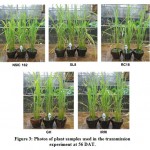 |
Figure 3: Photos of plant samples used in the transmission experiment at 56 DAT. |
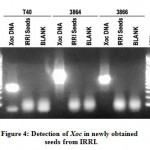 |
Figure 4: Detection of Xoc in newly obtained seeds from IRRI. |
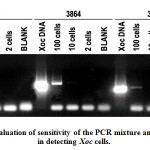 |
Figure 5: Evaluation of sensitivity of the PCR mixture and conditions in detecting Xoc cells. |
Based on the Figure 5 above, the optimized PCR conditions used in detecting Xoc was able to detect as low as approximately 100 cells that is suspended in the reaction mixture. This is true for primer T40. On the other hand, primers 3864 and 3866 can detect as low as 100 cells. The results clearly indicate the sensitivity of T40 AvrP1 in detecting Xoc cells in suspension. Finally, these results imply that the primers used can be used to evaluate irrigation water used in rice fields and determine whether it is contaminated by Xoc or other water-borne pathogens.
Optimized PCR conditions can detect as low as 104 artificially inoculated Xoc cells in rice seeds using primer T40.
Seeds obtained from IRRI and certified by their Seed Health Unit were further sterilized as previously described. Inoculation was done afterwards and mock inoculated (i.e. distilled water only) seeds were also prepared for comparison as negative control.
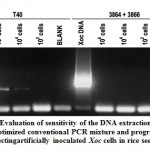 |
Figure 6: Evaluation of sensitivity of the DNA extraction protocol and optimized conventional PCR mixture and program in detecting artificially inoculated Xoc cells in rice seeds. |
With reference to the results shown in Figure 6 above, multiplex primer pair 3864+3866 seems to be not efficient enough to detect Xoc in seed extracts even at 106 inoculum load in rice seeds. T40 on the other hand, continued to efficiently detect Xoc for as low as 104 inoculated cells in the seeds. These results further supported the robustness of primer T40 and the optimized conditions in detecting Xoc in suspensions or in actual rice seeds.
Conclusion
Seed transmission of Xanthomonas oryzae pv. oryzicola (Xoc) and a rapid molecular detection method for Xoc in rice seeds were investigated and developed, respectively, in this study.
For the seed transmission test, SL8 variety (Isabela samples) had the lowest percent germination of 36.0%. In contrast, seeds from greenhouse BLS-inoculated plants and seed samples from IRRI have high percent germination, 84.0% and 96.0% respectively. Nevertheless, all transplanted seedlings, sixteen of them, for all varieties were able to survive and sustain growth until flowering stage and even at 56 DAT. Seed samples from Isabela, namely varieties NSIC162, SL8 and RC18 all showed symptoms of BLS as early as 14 days after transplanting (DAT). Most severe BLS symptoms were observed in SL8, while moderate symptoms only were observed on NSIC162. SL8 did not recover that much, with only 25.00% recovery, from BLS as it reached its reproductive stage at panicle initiation to flowering. Highest percent recovery was observed in RC18 variety (56.25%), although infections were already high at the beginning for these plants. Recovery from BLS in these plants was also observed at 56 DAT, when the plants start the grain filling stage.
For the direct extraction, seeds were first incubated at 85°C to soften the grain and then ground using mortar and pestle in 10% PBS-T. The crude seed extract was spun to separate the debris and supernatant was collected. DNA was precipitated by adding cold ethanol and storing at -20°C. Crude DNA was pelletized by centrifugation and re-suspended in 30 µL Tris-EDTA buffer. Homogenous subsamples were pooled together in a single tube to form a concentrated seed extract. For the DNA extraction using SDS, lysis buffer (SDS) was added to the pooled extract followed by 5M NaCl. The resulting viscous mixture was centrifuged and the supernatant was collected. An equal volume of chloroform was added to precipitate proteins. The mixture was centrifuged and the aqueous phase containing the DNA was transferred to another clean 1.5 mL Eppendorf tube with two volumes of chilled absolute ethanol. DNA was incubated at -20°C freezer overnight. Centrifugation was done to obtain the pellet and the alcohol was discarded. The pellet was washed with 70% ethanol, air dried and then re-dissolved in 10.0 µl TE buffer (pH 8).
PCR mixture and running conditions or program were also optimized in single and two-primer multiplex reactions. The concentration of all components per reaction mixture of 15.0 µL was all set at maximum: 3.0 mM MgCl2, 0.4 mM dNTPs, 0.4 µM for all the primers and 0.09 and 0.06 U/µL Taq DNA polymerase for single and multiplex reactions respectively. The optimum annealing temperature was found out to be 62°C and the cycle number was set 40 cycles to maximize amplification of bacterial DNA. The amplicons were separated at 1.2% agarose gel and stained using GelRed™.
The optimized conventional PCR mix using primer T40 can be used to detect as low as approximately 10 Xoc cells in suspension indicating that it is ultrasensitive. When cells were used as templated for PCR, 3864 and 3866 also gave reliable results and can detect 100 cells. This ultrasensitive property of PCR detection protocol can be utilized to test irrigation water, whether it is infected with Xoc or other water-borne pathogens. Finally, the optimized conditions can detect as low as 1000 inoculated cells in seeds. In single primer reactions, T40 gave the most robust results using seed extracts from the optimized protocol. While 3864 and 3866 in multiplex reactions were not able to detect even 106 inoculated Xoc cells.
Author Contributions and Acknowledgement
MPR conducted all activities in this research project, from conceptualization to data analysis and manuscript preparation. EPP contributed significantly in the seed transmission experiments in the preparation of the manuscript. The authors would like to thank Dr. Edna Ardales for the excellent supervision and Ms. Anna Rose Cunanan for her assistance during the counduct of this research.
Conflict of Interest
The authors state no conflict of interest.
Funding Source
This research is funded by the Biotechnology Program of the Department of Agriculture, Republic of the Philippines. The authors would like to thanks also the International Rice Research Institute for providing the seed materials.
References
- Adachi N., and T. Oku. (2000). PCR-mediated detection of Xanthomonas oryzae pv. oryzae by amplification of the 16S-23S rDNA spacer region sequence. Journal of General Plant Pathology, 66, 303-309.
CrossRef - Agarwal P.C., Mortensen C.N. and S.B. Mathur (1989). Seed-borne diseases and seed health testing of rice. In Phytopathological Papers (1989, No. 30), 106 pp.
- AgroNews. (2010). Bacterial leaf streak on rice in the Philippines. published online on Sep 3, 2010. URL: < http://news.agropages.com/News/NewsDetail—5483.htm
- Chen, W., and T. Kuo. (1993). A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Research, 21, 2260-2260. doi:10.1093/nar/21.9.2260.
CrossRef - Cho M.S., Kang M.J., Kim C.K., Seol Y.J., Hahn J.H., Park S.C., Hwang D.J., Ahn T.Y., Park D.H., Lim C.K., and Park D.S. (2011). Sensitive and specific detection of Xanthomonas oryzae pv. oryzae by real-time bio-PCR using pathovar-specific primers based on an rhs family gene. Plant Disease, 95, 589-594.
CrossRef - Cottyn, E. Regalado, B. Lanoot, M. De Cleene, T. W. Mew, and J. Swings (2001). Bacterial populations associated with rice seed in the tropical environment. Phytopathology, 91, 282-292.
CrossRef - Gitaitis, R. and R. Walcott. (2007). The epidemiology and management of seedborne bacterial diseases. Annual Reviews Phytopathology, 45, 371-397.
CrossRef - Gnanamanickam, S.S., Shikagi T., Medalla E.S., Mew T.W. and Alvarez A.M. (1994). Problems on detection of Xanthomonas oryzae pv. oryzae in rice seed and potential for improvement using monoclonal antibodies. Plant Disease, 78, 173-178.
CrossRef - Kauffman, H.E. and A.P.K. Reddy. (1974). Seed transmission studies of Xanthomonas oryzae in rice. Phytopathology, 65, 663-666.
CrossRef - Lang, J.M., Langlois P., Nguyen M.H.R., Triplett L.R., Purdie L., Holton L.A., Djikeng A., Vera Cruz C.M., Verdier V., and Leach J.E. (2014). Sensitive detection of Xanthomonas oryzae pathovars oryzae and oryzicola by loop-mediated isothermal amplification amplification. Applied Environmental Microbiology, 80, 4519-4530.
CrossRef - Lang, J.M., Hamilton J.P., Diaz M.G.Q., Van Sluys M.A., Burgos M.R.G., Vera Cruz C.M., Buell C.B., Tisserat N.A., and Leach J.E. (2010). Genomics-based diagnostic marker development for Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola. Plant Disease, 94, 311-319.
CrossRef - Maes, M., Garbeva P and C. Crepel (1996). Identification and sensitive endophytic detection of the fire blight pathogen Erwinia amylovora with 23S ribosomal DNA sequences and the polymerase chain reaction. Plant Pathology, 45, 1139-1149.
CrossRef - Mew, T.W., Alvarez A.M., Leach J.E., Swings J. (1993). Focus on bacterial blight of rice. Plant Disease, 77, 5-12.
CrossRef - Niño-Liu, D.O., Ronald P.C. and Bogdanove A.J. (2006). Xanthomonas oryzae pathovars: model pathogens of a model crop. Molecular Plant Pathology, 7, 303-324.
CrossRef - Ou, S.H. (1985). Rice Diseases. Kew, Surrey: Commonwealth Agricultural Bureau.
- Postgate, J.R. 1969. Viable counts and viability. Methods in Microbiology, 1, 611-628.
CrossRef - Sparks, A., Castilla N.P. and Vera Cruz C.M. (undated). IRRI Rice Knowledge Bank: Bacterial leaf streak. URL: < http://www.knowledgebank.irri.org/training/fact-sheets/pest-management/diseases/item/bacterial-leaf-streak?tmpl=component&print=1 >
- Sakthivel, N., Mortensen C. and Mathur S. (2001). Detection of Xanthomonas oryzae pv. oryzae in artificially inoculated and naturally infected rice seeds and plants by molecular techniques. Applied Microbiology and Biotechnology, 56, 435-441.
CrossRef - Schaad, N.W. (1982). Detection of seedborne bacterial pathogens. Plant Disease, 66, 885-890.
CrossRef - Zhao, W.-J., Zhu S.F., Liao X.L., Chen H.Y. and Tan T.W. (2007). Detection of Xanthomonas oryzae pv. oryzae in seeds using a specific TaqMan Probe. Microbial Biotechnology, 35, 119-127.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





