How to Cite | Publication History | PlumX Article Matrix
Adnan Yildiz1* , Tugba Gür2
, Tugba Gür2  and Şerafettin Alper1
and Şerafettin Alper1
1Yüzüncü Yıl University, Division of Chemistry
2Yüzüncü Yıl University, Division of Biochemistry
Corresponding Author: E-mail: adnanyildiz@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2797
ABSTRACT: In this investigation, the removing of some substances via adsorption onto tutmaç clay were performed in aqueous and organic solutions using batch system. It was aimed to remove the inorganic ions Mg2+ and Ca2+ (Excess of; Mg2+ and Ca2+ ions make water hard and cause to serious healt problems) organic substance cholest (its removing is important as by means of health and cosmetically) from the solution by adsorption method using the active surfaces of Tutmac clay was charactized by XRD. The amount of the substances adsorbed on the clay surface in the solutions were analyzed using AAS and UV spectroscopic methods. The experimental data have indicated that Tutmaç clay is good adsorbent and physical adsorption methot is quite suitable for the removing of Mg2+, Ca2+ and cholesterol from solutions. The adsorption data determined from experimental results best fitted Langmuir Isotherms. The obtained results also confirmed that the applicability of Tutmaç clay is an efficient supporter material for removing contaminations of inorganic and organic substances. And Tutmaç clay material can be used as a very effective adsorbent in removing of pollutants from organic and aqueous solutions.
KEYWORDS: Calcium; Cholesterol; Kinetic of Adsorption; Solutions; Magnesium
Download this article as:| Copy the following to cite this article: Yildiz A, Gür T, Alper S. A Study on Removıng of Some Organıc and Inorganıc Substances Usıng the Tutmac Clay Vıa Adsorptıon Method Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Yildiz A, Gür T, Alper S. A Study on Removıng of Some Organıc and Inorganıc Substances Usıng the Tutmac Clay Vıa Adsorptıon Method Biosci Biotech Res Asia 2019;16(4). Available from: https://bit.ly/35IceDu |
Introduction
Clay minerals formed by the erosion of volcanic rocks with strong air currents can be stored either in the place where they are or in the form of large beds elsewhere, carried by wind and water. Clay minerals are hydrated aluminum or magnesium silicates that are soft enough to be cut despite their hard appearance, can be easily shaped when wetted with water, and permanently hardened when heated. There are many clay minerals such as kaolinite, smectite, illite, chlorite, paligoskite and sepiolite in the killer as well as zeolite, feldspar, carbonate and silica polymorphs. In addition to the type of clay minerals, the abundance of minerals other than clay and clay is changing the economic value of the clay. It is very difficult to find a pure clay mineral in the country. Depending on the chemical composition of the minerals and minerals, the color of the killer can be in various shades of white, gray, green, pink and brown The clay formed by the erosion of volcanic tuffs is known as porous minerals with hydrophilic surfaces.3,4
The removal of contaminants by the natural clays via adsorption method are widely used in recent years, because natural clays are cheaper than other used materials such as activated carbon and zeolites. Owing to their high specific surface area, mechanical and chemical stability, highly variable surface and structural properties, clays are preferred as supporting material for removing contaminants from aqueous solutions. Clays are also used in solid waste storage areas, often forming ceilings and floor coverings, as well as in forming side girders.5
Due to its porous structure, the area of use of clays are changed from ceramic and cement production; paper, petrochemical and construction industry; bleaching vegetable oil, beer, wine and fruit juices; cleaning radioactive wastes and wastewater; ranging from cosmetics, pharmaceuticals, soaps, detergents, electrodes, catalysts, rubber and plastics to a wide range of products. Due to their high surface area, chemical and mechanical stability, surface and structural properties and diversity, the application areas are very high. Clays are mostly used in many areas such as ceramic painting, coating, cutting edge, oil regeneration, animal feed, animal litter, fertilizer and gas adsorption.6
As a result of the modification of the clay surfactants, organo-clay is formed and the surface area of the clay is changing and the adsorption capacities are increasing. As a result of the modification process, the hydrophilic clay has a hydrophobic structure by substitution of exchangeable metal ions between long chain quaternary ammonium cations and clay layers 7.Thus, the surface area of the clay increases with the organic cation having a long alkyl chain occupying the clay exchange zone. Organo-killer formed with cationic surfactant in this way finds wide applications 8-10.
The clays has specific mineral and small crystalline structures such as kaolin, illite and montmorillonite. In this crystal structure, there may be two different layers of silica tetrahedral and magnesium or aluminum octahedral. Silica consists of four oxygen atoms around a single tetrahedral silica atom. Mg or Al octahedral is composed of Al, Mg, Fe, or six H+ and OH– ions around a different atom. These two different layers of clay are different clay types with different atoms and different clay types are formed and clay types which provide necessary standards as coating material in solid waste storage areas are frequently used.11-16
The aim of this study was to determine the amount of adsorption of calcium, magnesium ions from aqueous solutions and cholesterol from the benzene solutions and to determine the adsorption isotherms of the adsorbates used in the experiments.
Experımental
The clay used in the experiments was provided from the village of Tutmaç/Van-Turkey and then charactized by XRD analysis.
AAS (Atomic Absorption Spectrophotometer, Perkin Elmer), UV-VIS (Ultraviolet Absorption Spectrophotometer) 400 mesh Sieve, Oven, Electronic balance, Experiment vessels, Glass materials, Magnetic stirrer, Tiller shaft, Clay mill, Pure benzene, CaCl2.2H2O salt, MgCl2.6H2O salt, 94% cholesterol (C27H46O), purified water, Centrifuge, AAS and UV were used.
| Chemicals | Molecular wt. (g/mol) | Elements | Molecular wt. (g/mol) |
| CaCI2.2H2O | 107.02 | Na | 23,00 |
| MgCI2.6H2O | 203,30 | Ca | 40,08 |
| Cholesterol | C27H46O | K | 39,10 |
| Mg | 24,31 | ||
| 386,65 | |||
The clay sample used in the experiment was taken from the clay in the vicinity of Tutmaç Village, Van Province, Gürpınar District. The receiving clay sample was dried. The dried clay was poured into a 400 mesh (0.038 mm) sieve after grinding the mill. The clay sample was then dried by heating at 100 ° C for 24 hours. Finally the clay sample was maintained in a desiccator to use in the adsorption process.
A stock solution of 0.1 M 50 mL each containing Ca+2, Mg+2 ions and cholesterol used as adsorbate was prepared. Pure water was used as solvent to prepare aqueous solutions for the compounds containing Ca+2, Mg+2 ions. Benzene was used as organic solvent to dissolve cholesterol. To use in adsorption processes from these stock solutions, 0.01 M, 0.02 M, 0.03 M, 0.04 M, 0.05 M and each individually 20 mL solutions were prepared, respectively. And then, 0.2 g of clay was added and the solutions were subjected to adsorption at room temperature with stirring with a magnetic stirrer so that the solution was equilibrated for 20 minutes. This process was performed for each sample and then the sample were centrifuged. The amounts of Ca+2 and Mg+2 ions in the filtrate were analyzed by Atomic Absorption Spectroscopy (AAS) instrument. In addition, for calibration, 0.1 M 50 mL cholesterol stock solution 0.01 M, 0.02 M, 0.03 M, 0.04 M, 0.05 M and 20 mL of standard cholesterol solutions were prepared and analyzed in an Ultraviolet Spectrophotometer (UV).
Then 0.01 M, 0.02 M, 0.03 M, 0.04 M, 0.05 M and 20 mL solutions were prepared for use in adsorption processes from cholesterol stock solution. Adsorption at room temperature was carried out by adding 0.2 g of clay to these solutions and stirring the solutions for 20 minutes with stirring by means of a magnetic stirrer so as to balance the solution. Then the samples were centrifuged. The amount of cholesterol in the filtrate was analyzed in an Ultraviolet Spectrophotometer (UV). And then the analysis of the experiment results were determined.
Results and Dıscussıon
Tutmaç Clay Analysis
The XRD analysis results we used in our study is given as follow
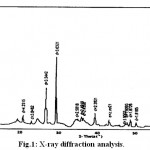 |
Figure 1: X-ray diffraction analysis. |
Mixed layered clay minerals was shown as follow:
According to the XRD analysis results (Fig. 1),
The following clay types were found as follow:
[Smectite +Chlorite] [İllite+ Smectite]Calcite
Quartz
Serpentine Group Mineral
Feldispate Group Mineral
 |
Figure 2: Untreated natural Tutmac Clay. |
 |
Figure 3: Tutmac Clay passed through a 400 mesh sieve. |
Langmuir Isotherms Calculated for Adsorption of Ca2+, Mg2+ and Cholesterol of on Tutmaç Clay
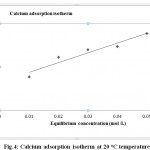 |
Figure 4: Calcium adsorption isotherm at 20 oC temperature |
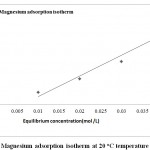 |
Figure 5: Magnesium adsorption isotherm at 20 oC temperature |
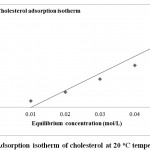 |
Figure 6: Adsorption isotherm of cholesterol at 20 oC temperature |
Conclusıons
Mg2+ and Ca2+ and cholesterol adsorption isotherms, the highest adsorption is Calcium, then mangnesium (Fig. 4,5) comes after magnesium comes from cholesterol (6) comes as the least adsorbed The Tutmaç Clay has performed better performance in the adsorption of inorganic cations. This is due to the hydrophilic negative charge centers on the clay surfaces, so the hydrophilic inorganic material adsorbed more. Because of its hydrophobic character, cholesterol is less adsorbed than inorganic materials due to their hydrophilic character. Furthermore, the adsorption of smaller molecules by the adsorption of smaller molecules and the adsorption of smaller cations by more adsorbed molecules are due to the smaller size of the clay pores. The concentrations of Mg 2+ and Ca 2+ ions were analyzed by AAS in the solution medium and they were eliminated from the analysis results. When the adsorption isotherms are examined, the concentration of adsorbate in the solution medium is increased in residual adsorption. Similar previous studies related to the removal of organic and inorganic materials by clay with solution support our work and he clay mineral we have used in our work as a good adsorbent 17-21.
Most of cosmetic preparations which are used cosmetically today are toxic, making big irritations on the skin of people and causing great harm to health.
Experts offer the using of clays, natural and dermatological. It also sheds light on the use of clays in the next advance appliciaple process.Finally, The adsorption data determined from experimental results, best fitted Langmuir Isotherms and showed that Tutmaç clay was good adsorbent and physical adsorption methot is quite suitable for the removing of Mg2+, Ca2+ and cholesterol from solutions. The data obtained from experimentals approve the applicability of Tutmaç clay as a efficient supporter material for removing wastewater and other organic pollutants.
Conflict of Interest
There is no conflict of interest
Acknowledgement
The author are thankful to the the laboratory members of Yuzuncu Yil University for techninal support.
Funding Source
This research was supported by the Science Laboratory, Yuzuncu Yil University, Van-TURKEY
References
- Kışlalıoğlu Kozmetoloji Bilimi. In: Kozmetik Bilimi. Editors: Yazan Y, Nobel Tıp Kitabevi, İstanbul. p. 3-9 2004.
- C. Loughnan, Chemical weathering on the silica minerals, Elsevier, New York. 1969.
- Önal, and Y. Sarıkaya, J. of Thermal Analysis and Calorimetry. 2007: 90(1); 167-172.
- İ. Goncaoğlu, , Ş. Yıldız Ö, Apaydın 10. Ulusal Kil Sempozyumu.19-22 Eylül, Konya, 2001.
- Bergaya, ve G. Lagaly, Surface modification of clay minerals Appl. Clay Sci., 2001; 19, 1-3.
- Yıldız, A. Gür, Adsorption of phenol and chlorophenols on pure and modified sepiolite J. of the Serbian Chemical Society, 2007: 72(5); 467-474.
- S. Özcan, B. Erdem, and A. Özcan, Adsorption of Acid Blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite J. Colloid Interface Sci., 2004: 280; 44-54.
- S. Özcan, B. Erdem, and A. Özcan, Adsorption of Acid Red 57 from aqueous solutions onto surfactant-modified sepiolite Colloids Surfaces A: Physicochem. Eng. Aspects, 2005; 266: 73-81.
- Li Z.H., Bowman, RS. Retention of inorganicoxyanions by organic-kaolinite, Water Res., 2001; 35:371-3776.
- Sharman, And S. Lewis, Waste Containment Systems, Waste Stabilization, and Landfiils-Desingand and Evaluation, John Wiley and Sons, New York, 1994.
- Yıldız and A. Gür, Adsorption of Phenol, Phenol Derivates on Pure and Modified Kaolinite, Asian journal of Chem., 2006; 18(4); 2650-2656.
- Şolpan, S. Duran, D. Saraydin and O. Güven, Radiation Physics and Chemistry, 2003; 66:117-127.
- Ilgin, H. Durak and A. Gür, A Novel pH-Responsive p(AAm-co-METAC) /MMT Composite Hydrogel: Synthesis, Characterization and Its Absorption Performance on Heavy Metal İons, Polymer-Plastics Tech. and Engineering, 2015: 54; 603-615.
- Tang, W., Zhou and L. Zhang, Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels, Journal of Hazardous Materials, 2012: (209-210); 218-225 2012.
- Ackacha and L. Elsharif, The use of Acacia tortilis leaves as a low-cost adsorbent environment, Conf. a Geo and Env. Sci. Jeju Island, South Korea, 2012.
- K. Prasod and S.N. Srivastova, Of distillery spent wash onto fly ash: Kinetics and Mass Transfer Studies, Chem. Eng. J., 2009: 146; 90.
- M. Smith and H.C. Van Ness, Introduction to chemical engineering thermodynamics, Mc Graw-Hill Publishing Company, New York, 1987.
- B. Wang and Z.H. Zhu, Characterisation and environmental application of an Australian natural zeolite for basle dye removal from aqueous solution, J. of Haz. Mat., 2006: 136; 946.
- Wang and E. Ariyonto, Competitive adsorption of malachite gren and Pb+2 ions on natural zeolite, J. of Col. and Int. Sci., 2007: 314; 25.
- P. Han, Y. Wang, Q. Sun, L. Wang, J. Song, X. He and C. Dou, Malachite gren adsorption onto natural zeolite and reuse by microwave irradiation, J. of Haz. Mat., 2010: 175; 1056.
- Yıldız, A. Gür, and H. Ceylan, Adsorption of aniline, phenol, and chlorophenols on pure and modified bentonite, Russian Journal of Physical Chemistry, 2006: 80 (1); 169-172.
- Demir, A. Gür, A. Yıldız and T. Gür, Immobilization of polyphenol oxidase purified from Igdir Apricot on bardakci-clay, Bioscience, Biotechnology Research Asia, 2008: 5(1); 93-98.
- Gür, A. Yıldız, E. Alkan and T. Gür, Sulu Çözeltiden Adsorpsiyonla Ağır Metal Uzaklaştırılması, Yuzuncu Yıl University, Journal of Sciences Institute, 2008: 13(2); 98-103.
- S. Nas, A. Gür, T. Gür and V. Yönten, Exploring thermodynamics and kinetic parameters of immobilized catalase enzyme via adsorption on krill clay, Desalination and Water Treatment, 2017: 67; 178-186.

This work is licensed under a Creative Commons Attribution 4.0 International License.





