How to Cite | Publication History | PlumX Article Matrix
Mathias T1, Asegbeloyin, J. N1, Oyeka, E. E1,2, Oji, E. O3,4 and Akpomie K. G1*
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Enugu State, Nigeria
2Department of Chemistry, Graduate School of Science, Tohoku University, 6-3 Aza-Aoba, Aoba-ku, Sendai, Miyagi, 980-8578 Japan
3Department of Chemistry, Michael Okpara University of Agriculture, Umudike, Nigeria
4Department of Chemistry, Gregory University Uturu, Nigeria
Corresponding Author E-mail: kovoakpmusic@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2802
ABSTRACT: The study evaluated the antimicrobial activity based on chromatographic analysis of ethanolic extract of Funtumia elastica leaves and stem bark. Funtumia elastica leaf and stem bark samples were air dried, grinded and extracted in ethanol by soxhlet extraction. The defatted ethanol extract was partitioned in dilute sodium hydroxide, hydrochloric acid and chloroform to obtain basic, neutral and acidic metabolites. The phytochemical test on the defatted crude extract of Funtumia elastica leaf and stem bark show the presence of tannins, alkaloids, flavonoids, saponins, terponoids, anthraquinone, phenolics and steroids. The crude extract, basic, neutral and acidic metabolites, and the fractions obtained by thin layer chromatography were screened for their antimicrobial activity at concentration of 100mg/ml against Streptococcus pneumoniae(sp), Staphylococcus aureus(sa), Salmonella typhi(st), Escherichia coli(ec) and Candida albicans(ca). The results show that the crude extract of stem with inhibition zone diameter(mm) of 22, 18, 20, 22 and 20 against (sp), (sa), (st), (ec) and (ca) respectively had better antimicrobial activity than the crude extract of leaf with inhibition zone diameter(mm) of 16,10,16,14 and 12 against (sp), (sa), (st), (ec) and (ca) respectively. Also the TLC fractions exhibited lower antimicrobial activity when compared to that of the basic metabolites. The fractions obtained by thin layer chromatography which showed promising antimicrobial activity were analysed by gas chromatography mass spectroscopy (GC/MS). The GC/MS spectra revealed thirty-nine peaks from which thirty nine compounds were identified. The identified compounds were mainly carboxylic acids and esters. The results of antimicrobial screening show that the crude extracts of the stem bark with inhibition zone diameter (mm) of 22, 18, 20, 22 and 20 were comparable to the standard drugs ciprofloxacin with inhibition zone diameter (mm) of 18, 20, 20, and 15 and gentamycin with inhibition zone diameter (mm) of 10, 20, 15, 12, and 18.
KEYWORDS: Chromatographic Analysis; Extract; Funtumia Elastica; Plant
Download this article as:| Copy the following to cite this article: Mathias T, Asegbeloyin J. N, Oyeka E. E, Oji E. O, Akpomie K. G. Chromatographic Analysis of Ethanol Extract of Funtumia Elastica Leaves and Stem Bark and their Antimicrobial Activity. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Mathias T, Asegbeloyin J. N, Oyeka E. E, Oji E. O, Akpomie K. G. Chromatographic Analysis of Ethanol Extract of Funtumia Elastica Leaves and Stem Bark and their Antimicrobial Activity. Biosci Biotech Res Asia 2019;16(4). Available from: https://www.biotech-asia.org/?p=34638 |
Introduction
Medicinal plants have been the mainstay of traditional herbal medicine amongst rural dwellers worldwide since antiquity to date. Medicinal plants are plants having their parts containing substances that have useful therapeutic functions or serve as precursors for the synthesis of antimicrobial, antivirus, antifungal, antitumour, antimalarial, anti-inflammatory, and analgesic, antitumour drugs, etc(WHO, 1977). There have been reports of multiple drug resistance and the persistence of this challenge has led to the development of more potent synthetic antibiotics and other drugs which are expensive, with their attendant and serious side effects. Therefore local medicinal plants provide the link for new possible antimicrobial as well as other drugs (World health organization 1993). Funtumia elastica which is also known as “Silk rubber” is a medicinal plant belonging to the Apocynaceae family. It is a medium-sized African rubber tree with glossy leaves, milky sap, and long woody seedpods. The bark is the medicinal portion. Scientists studied Funtumia extensively in the 1960s, but only recently have its medicinal properties recaptured the interest of science. Funtumia has important antioxidant, antifungal, anti-inflammatory, and antibiotic properties (Adekunle et al 2006).The extracts from the bark of the tree was found to kill free living amoebae and also kills entamoeba histolytica in the dysenteric stools of experimentally infected kittens. It is markedly lethal to the flagellate protozoon (Arias 1999). Also, Crude extracts of Funtumia elastica inhibit growth of many molds, including Aspergillus, Penicillium, and Candida, as well as the fungi that cause ringworm (Adekunle et al 2006). A medicinal plant can be defined as any plant used in order to prevent, relieve or cure a disease or to alter physiological and pathological process or any plant employed as a source of drugs or their precursors (Srivastava et al 1996). Plant derived medicines have made a large contribution to human health and well-being (Taylor et al 2001). Even today, plant materials remain an important source for combating illness, including infectious diseases (Matu and Van Staden, 2003). Dependence on plants as the source of medicine is more prevalent in developing countries where traditional medicine plays a major role in health care (World health organization 1993; Iwu et al 1999). Levels of sanitation, hygiene and living conditions for the majority of African people are not comparable to those of industrialized countries. This exposes African people to a wider array of microbial pathogens, which increase their susceptibility to bacterial infections. The problem is also exacerbated by lack of proper health care facilities and where these exist, the majority of the population cannot afford to pay for conventional medicines (Farnsworth 1994). The aim of this experiment was therefore to evaluated the antimicrobial activity based on chromatographic analysis of ethanolic extract of Funtumia elastica leaves and stem bark.
Material and Methods
Sample Collection and Identification
The sample was collected in the month of March 2016 from Oba area of Enugu state Nigeria and was identified by a renowned taxonomist Mr Alfred Ozioko of International center for ethnomedicine and drug development (interCEDD) located at no 110 Aku road, Nsukka, Enugu state Nigeria.
Sample Preparation
The sample was washed with distilled water air dried in a corner inside the laboratory in order to avoid direct contact of the samples with sun light. After that it was pulverized using Thomas–Wiley Laboratory Mill, Model 4.
Preparation of Extract
50g of the dried pulverized plant material was put in a soxhlet extractor fitted with a reflux condenser and extracted with 200ml of ethanol for 8h. The ethanol extract was allowed to evaporate completely at room temperature to give a gel, which was dissolved in ethanol/water mixture (4:1) and filtered. The filtrate was used without further purification. (Ejele et al 2012).
Preparation of basic metabolites
The basic metabolite was prepared according to the methods reported by (Ejele et al 2012). The filtrate of ethanol extract was treated with dilute HCl and extracted with chloroform in a separatory funnel. The lower chloroform layer was removed (and reserved for the preparation of neutral metabolite). The HCl layer was treated with dilute NaOH solution until the mixture becomes basic and was allowed to stand overnight. The precipitate formed was filtered and wash well with water and allowed to dry in air. The precipitate was then dissolved in 95% ethanol and used without further purification for antimicrobial experiments.
Preparation of second crop metabolites
The filtrate obtained from any of the metabolites is left to stay overnight. Water is added to the precipitate formed, if dissolved, glycoside is suspected and the content thrown away, if the precipitate did not dissolved, it is then filtered, labeled second crop and tested for antimicrobial
Preparation of acidic metabolites
The aqueous alkaline layer obtained during preparation was treated with dilute HCl until the solution became acidic. The precipitate formed was filtered and allowed to dry in air, dissolved in 95% ethanol and used without further purification for antimicrobial experiments.
Preparation of neutral metabolites
The chloroform extracts obtained above was placed in a separatory funnel and treated with dilute NaOH solution. After equilibrating, the aqueous NaOH layer was removed and reserved for the preparation of acidic metabolite. The chloroform layer was removed and allowed to evaporate completely at room temperature to produce a gel, which was dissolved in chloroform and filtered. The filtrate was used without further purification for antimicrobial experiments
Antimicrobial tests
Test microorganism used were Streptococcus spp, Staphylococcus aureus, Salmonella spp, Escherichia coli and Candida albicans and the method used was agar disc diffusion method. An inoculating loop was touched to isolate colonies of the pathogen on an agar plate and used to inoculate a tube culture broth, which was incubated at 35-370C until it becomes slightly turbid and was diluted to match the turbidity standard. Then a sterile cotton swab was dipped into the standardized bacterial test suspension and used to evenly inoculate the entire surface of the agar plate. After 5min, the appropriate antibiotic test disks were placed with a multiple applicator device. The agar plate was incubated at 35-370C for 18hours, after which the diameter of inhibition zones (areas showing little or no microbial growth) were measured to the nearest mm.
Qualitative Test for the Phyto-chemicals
Phytochemical analysis was done on the crude extract, acid, basic, and neutral metabolites to determine the presence of tannins, alkaloids, flavonoids, saponins, steroids, anthraquinone and terpenoids. Also chemical test to determine the presence of phenol was carried. All these were done using standard methods described by (Ejele et al 2012).
Test for tannins, alkaloids and flavonoids
Tannins: 5ml of distilled water was added to 1ml of the sample followed by addition of few drops of FeCl3 Formation of bluish/greenish precipitate shows the presence of simple tannins. Formation of dark-brown precipitate indicates presence of condensed tannins.
Alkaloids: 2ml of wagner’s reagent was added to 1ml of sample, dark-brown precipitate indicates the presence of alkaloids.
Flavonoids: 1ml of sample was dissolved in 1ml of dilute NaoH and filtrered. The filtrate was acidified by the addition of drops of conc HCl. Formation of precipitate shows presence of flavonoids.
Test for saponins, steroids, Terpenoids and phenols
Saponins: 1ml of distilled water was added to 1ml of the extract and shaken vigorously (in the presence or absence of olive oil).formation of persistent frothing (foaming) shows the presence of saponin.
Steroids: 5ml of sample was shaken with equal volume of chloroform, two layers were formed. The chloroform layer was removed into a clean test tube and 1ml of 5% H2SO4 was added cautiously down the side of the bent test tube. Formation of reddish brown ring indicates the presence of steroids. This test is salkowski test.
Terpenoids: 0.5ml of sample was dissolved in 2ml of acetic acid and allowed to cool, followed by slow and (cautious) addition of 1ml of conc H2SO4 along the line of the test tube, which is bent at an angle. Two layers are formed and reddish-brown ring at the interphase shows the presence of tepernoids. This test is called libermann’s test.
Phenols: A mixture of 0.5ml of sample and 4ml of water was heated and filtered, few drops of FeCl3 added to the filtrate. The formation of dark-brown precipitate shows presence of free phenols.
Chromatographic Purification
Both thin layer chromatography and preparative thin layer chromatography were used. The thin layer chromatography used was pre-coated aluminum plate used to test the solvent system that can separate the metabolites. While silica gel coated on glass was used for the preparative thin layer chromatography activated in an oven for 3h. The solvent system is ethanol, chloroform and acetic acid in the ratio of 1:1:1 and fifteen fractions were obtained
GC-MS Analysis
GC-MS analysis of selected antimicrobial active fractions was performed at the National Research institute for chemical technology Zaria (NARICT).
Results and Discussion
Table 1 shows the result of qualitative test of Funtumia elastica leaf and stem, Table 2, showed the antibacterial activity of crude extract, acidic, basic, second crop basic and neutral metabolites of Funtumia elastica stem and leaf, Table 3 shows the RF value of thin layer chromatography, Table 4 shows the antibacterial activity of the chromatographic fractions obtained from acidic metabolites of leaf, basic metabolites of leaf, second crop basic metabolites of leaf and neutral metabolites of stem, while Table 5 showed the metabolites with the best antibacterial activity.
Table 1: Results showing a phytochemical screening of the crude extract of leaf and stem of Funtumia elastica
| Phytochemicals | Leave extract | Stem extract |
| Tannins | + | + |
| Alkaloids | + | + |
| Flavonoids | + | + |
| Saponins | + | + |
| Terpenoids | + | + |
| Anthraquinone | + | + |
| Phenolics | + | + |
| Steroids | + | + |
Where (+) indicates present
Table 2: Antibacterial activity of crude extracts, acidic, basic, second crop basic metabolites Sand neutral metabolites of Funtumia elastica leaf and stem (zone of inhibition in mm) at concentration of 100mg/ml
| Test organism | A | B | C | D | E | F | G | H | I | J | K |
| Streptococcus pneumonia | 16 | 22 | 20 | 18 | 25 | 20 | 20 | 0 | 25 | 18 | 10 |
| Staphyloccus aureus | 10 | 18 | 16 | 20 | 20 | 20 | 22 | 0 | 18 | 20 | 20 |
| Salmonella spp | 16 | 20 | 18 | 0 | 20 | 22 | 25 | 0 | 23 | 20 | 15 |
| Escherichia coli | 14 | 22 | 18 | 16 | 23 | 23 | 24 | 0 | 25 | 22 | 12 |
| Candida albicans | 12 | 20 | 16 | 16 | 18 | 22 | 22 | 0 | 20 | 15 | 18 |
A=IZD of leaf extract, B=IZD of stem extract, C=IZD of acidic metabolites of leaf, D=IZD of acidic metabolites of stem, E=IZD of basic metabolites of leaf, F=IZD of basic metabolites of stem, G=IZD of second crop basic metabolites of leaf, H=IZD of neutral metabolites of leaf, I=IZD of neutral metabolites of stem, J=IZD of control drug ciprofloxacin, K=IZD of control drug gentamycin.
Table 3: RF values of thin layer chromatography with the solvent mixture of 1:1:1 of acetic acid, chloroform and ethanol.
| Metabolites | Distance moved by the sample | Distance moved by the solvent | RF values |
| Acidic metabolites in leaves | 5.2cm | 6cm | 0.87cm |
| Basic metabolites in leaves | 5.0cm | 6cm | 0.83cm |
| Second crop basic metabolites in leaves | 4.2cm | 6cm | 0.70cm |
| Neutral metabolites in stem | 5.4cm | 6cm | 0.90cm |
Table 4: Antimicrobial activity of fractions obtained from chromatographic separation of acidic metabolites of leaf, basic metabolites of leaf, second crop basic metabolites of leaf and neutral metabolites of stem (zone of inhibition in mm) at concentration of 100mg/ml.
| Test organism | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O |
| Streptococcus pneumoniae | 12 | 15 | 12 | 0 | 0 | 0 | 15 | 18 | 18 | 22 | 15 | 10 | 20 | 0 | 18 |
| Staphylococcus aureus | 0 | 22 | 15 | 0 | 0 | 0 | 20 | 23 | 15 | 20 | 20 | 22 | 16 | 12 | 14 |
| Salmonella spp | 13 | 14 | 15 | 0 | 0 | 0 | 15 | 20 | 10 | 22 | 20 | 20 | 15 | 0 | 10 |
| Escherichia coli | 0 | 18 | 12 | 0 | 0 | 0 | 10 | 13 | 0 | 20 | 18 | 18 | 12 | 0 | 18 |
| Candida albicans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 8 | 10 | 0 | 0 | 0 |
A=IZD of fraction one of acidic metabolites of leaf, B=IZD of fraction two of acidic metabolites of leaf, C=IZD of fraction three of acidic metabolites of leaf, D= IZD of fraction four of acidic metabolites of leaf, E=IZD of fraction one basic metabolites of leaf, F=IZD of fraction two of basic metabolites of leaf, G=IZD of fraction three of basic metabolites of leaf, H=IZD of fraction four of basic metabolites of leaf, I=IZD of fraction one of second crop basic metabolites of leaf, J=IZD of fraction two of second crop basic metabolites of leaf, K=IZD of fraction three of second crop basic metabolites of leaf, L=IZD of fraction four of second crop basic metabolites of leaf, M=IZD of fraction one of neutral metabolites of stem, N=IZD of fraction two of neutral metabolites of stem, O=IZD of fraction three of neutral metabolites of stem.
Table 5: Antibacterial activity of fractions with the best antibacterial activity (zone of inhibition in mm) at concentration of 100mg/ml.
| Test organism | A | B | C | D |
| Streptococcus pneumonia | 15 | 18 | 22 | 20 |
| Staphylococcus aureus | 22 | 23 | 20 | 16 |
| Salmonella spp | 14 | 20 | 22 | 15 |
| Escherichia coli | 18 | 13 | 20 | 12 |
| Candida albicans | 0 | 0 | 0 | 0 |
A=IZD of fraction two of acidic metabolites of leaf, B= IZD of fraction four of basic metabolites of leaf, C=IZD of fraction of two of second crop basic metabolites of leaf, D=IZD of fraction one of neutral metabolites of stem.
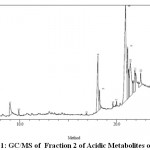 |
Figure 1: GC/MS of Fraction 2 of Acidic Metabolites of Leaf |
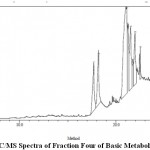 |
Figure 2: GC/MS Spectra of Fraction Four of Basic Metabolites of Leaf Click here to view figure |
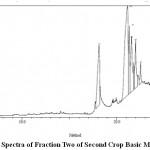 |
Figure 3: GC/MS Spectra of Fraction Two of Second Crop Basic Metabolites of Leaf. |
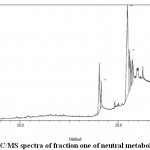 |
Figure 4: GC/MS spectra of fraction one of neutral metabolites of stem Click here to view figure |
Discussion
Preliminary phytochemical tests
Phytochemical studies of the ethanol crude extract of Funtumia elastica leaf and stem bark revealed that the crude extract contained tannins, alkaloids, flavonoids, saponins and Terpenoids, anthraquinone, phenolics and steroids as shown in Table 1. Phytochemicals like the flavonoids and phenolics have been found to be responsible for free radicals scavenging activity of many medicinal plants (WHO 1993) flavonoids specifically possess a natural tendency to modify body allergy interaction and have anti-microbial, anti-inflammatory and anti-cancer activities in many plant products (Iwu et 1999). For example presence of flavonoids and phenolics constituents in pepino fruits extract was discovered to be responsible for its antioxidant activity. Similarly tannins are reasonably abundant in leafy vegetables, traditionally used to protect against wounds and hemorrhoids (Akinyemi et al 2005). It inhibits cell wall formation in fungi and bacteria leading to death of organisms, some tannin exhibit and antiviral activity by selectively inhibit HIV replication. Therefore, suggesting the antifungal and antibacterial potential of the plant. Saponins on the other hand can act as detergent, a staining agent during histology, and used to manage conditions like hypercholesterolemia, hyper-glycaemia, antioxidant, anticancer, anti-inflammatory, weight loss and also known to have antifungal properties (Dahiru et al 2006).
Antimicrobial activity of crude extracts, acidic, basic, second crop basic and neutral metabolites and chromatographic fraction in mm at concentration of 100mg/ml
The results of antibacterial activity in table 2 shows that the crude extracts, the metabolites (acidic, basic and neutral) metabolites of Funtumia elastica leaf and stem, shows activity against the test organisms except neutral metabolite of leaf (H) which did not show any activity against any of the test organisms used. It can be seen from table 2 that neutral metabolites of stem (I) showed the highest activity against streptococcus and Escherichia coli with the IZD of 25mm respectively, second crop basic metabolites of leaf(G) showed its highest activity against Staphylococcus, Salmonella, and Candida albicans with the IZD of 22mm,25mm, and 22mm respectively. Basic metabolites of leaf showed its highest activity against streptococcus with IZD of 25mm, while basic metabolites of stem showed its highest activity against Candida albicans with IZD of 22mm. comparing the extracts from the Funtumia elastica leaf and stem with the two control drugs showed that the Funtumia elastica leaf and stem ( E=25mm, F=22mm, G=25) has a better activity Streptococcus spp, Staphylococcus aureus and Candida albicans than the two control drugs ciprofloxacin(J) with IZD of 18mm, for Streptococcus pneumoniae, 20mm for Staphylococcus aureus and Candida albicans has IZD of 15mm while gentamycin(K) has IZD of 10mm for streptococcus, pneumoniae, 20mm for Staphylococcus aureus and 18mm for Candida albicans. From Table 2 it was also discovered that the crude extract of stem had better activity than the crude extract of leaf, but after separation into acidic, basic and neutral metabolite, it was found that both acidic and basic metabolites of leaf showed higher activity than the acidic and basic metabolites of stem. This shows that some plants extract has better antibacterial activity in their crude state, while some with little activity in their crude state can perform better when separated into acidic, basic and neutral metabolites. Table 3 shows the RF value of the thin layer chromatography, Table 4 shows the IZD of the chromatographic fractions from which fractions were chosen for further antibacterial test while Table 5 shows the IZD of the four fractions with the best antibacterial activity which are acidic metabolites of leaf, basic metabolites of leaf, second crop basic metabolites of leaf and neutral metabolites of stem that were further subjected to G/C MS analysis.
Gas Chromatography – Mass Spectrometry (GC-MS)
A shimadzu Qp – 2010 plus GC-MS was used. The GC/MS was equipped with a split injector and an ion – trap mass spectrometer detector together with a fused – silica capillary column having a thickness of 1.00μm, dimensions of 20m x 0.22mm and temperature limits of 80oC to 25oC. The column temperature was programmed between 80oC and 250oC at a rate of 3.0ml/min. The temperature of the injector and detector were at 250oC and 200oC respectively. Helium gas was used as a carrier gas at a flow rate of 46.3 cm/sec.
GC-MS Analysis
The components present in the fraction two of acid metabolites of leaf, fraction four of basic metabolites of leaf, fraction two of second crop basic metabolites of leaf and fraction one of neutral metabolites of stem of Funtumia elastica were identified by GC-MS. The chromatogram is shown in (Fig. 1, 2, 3, 4). The active principles with their retention time (RT) molecular formula, molecular weight (MW) and percentage composition in the acidic metabolites of leaf, basic metabolites of leaf, second crop basic metabolites of leaf and neutral metabolites of stem of is presented in (Table 6,7,8 and 9).
GC-MS Analysis of fraction two (f2) Of Acidic Metabolites of Funtumia elastica Leaf
The components present in fraction two of acidic metabolites of Funtumia elastica were identified by GC-MS. The chromatogram is shown in (Fig. 1). The active principles with their retention time (RT) molecular formula, molecular weight (MW) and percentage composition in the fraction two of acidic metabolites of Funtumia elastic is presented in (Table 6). Fifteen components were identified in the fraction two Funtumia elastica leaf. The compounds were cis-9-octadecanoate (38.53%) as the major component followed by 9, 12-octadecanoic acid, methyl ester (14.62%), n-hexadecanoic acid, (14.12%)2-(2-hydroethoxy)ethylester(10.73%) , 9-octadecynoic acid (4.79%), 2-methoxy-3-(2-prenyl)(3.62%), ethyhexadecanoate(3.22%), 2, 3-dihydroxypropylester(3.13%), trans-13-docosenoic acid(2.58%), n-tetracosane(1.59), octacosane(1.19%), bicycle (7,2,0) undec-4-ene (0.62%), 2-propenylester (0.51%), methyl (13E) -13-Octadecanoate(0.51%), methyl-141methylpentadecanoate(0.24%) respectively. As shown in Table 6.
Table 6: Components detected in fraction two of acidic metabolites of leaf.
| Peaks | RT | Name of Compounds | Mol. Formula | MW | Area% |
| 1 | 8.867 | 2-methoxy-3-(2-propenyl) | C10H12O2 | 164 | 3.62 |
| 2 | 9.884 | 4,11,11-trimethyl-8-methylene | C15H24 | 204 | 0.62 |
| 3 | 16.833 | Methyl-14-methylpentadecanoate | C17H34O2 | 270 | 0.24 |
| 4 | 18.065 | n-hexadecanoic acid | C16H32O2 | 256 | 14.12 |
| 5 | 18.230 | Ethylhexadecanoate | C18H36O2 | 284 | 3.22 |
| 6 | 19.614 | 2-propenyl ester | C21H40O2 | 324 | 0.51 |
| 7 | 19.994 | Methyl(13E)-13-octadecanoate | C19H36O2 | 296 | 0.50 |
| 8 | 20.920 | Cis-9-octadecanoate | C19H36O2 | 282 | 38.53 |
| 9 | 21.141 | 2-(2-hydroethoxy)ethyl ester | C22H44O4 | 372 | 10.73 |
| 10 | 21.403 | 9,12-octadecanoic acid | C19H34O2 | 294 | 14.62 |
| 11 | 21.886 | 9-octadecynoic acid | C18H32O2 | 280 | 4.79 |
| 12 | 22.485 | 2,3-dihydroxypropyl ester | C19H38O4 | 330 | 3.13 |
| 13 | 24.302 | Trans-13-docosenoic acid | C22H42O2 | 338 | 2.58 |
| 14 | 26.066 | Octacosane | C28H58 | 394 | 1.19 |
| 15 | 26.418 | n-tetracosane | C24H50 | 338 | 1.59 |
GC-MS Analysis of fraction two (four) of basic Metabolites of Funtumia elastica
The components present in fraction four of basic metabolites of Funtumia elastica leaf were identified by GC-MS. The chromatogram is shown in (Fig. 2). The active principles with their retention time (RT) molecular formula, molecular weight (MW) and percentage composition in the fraction two of acidic metabolites of Funtumia elastic is presented in (Table 7). Seven components were identified in the fraction four of basic metabolites in Funtumia elastica leaf. The compounds were 9-octadecenoic acid (44.6%) as the major component followed by a compound with a molecular weight of 280 that was not suggested in the library (16.26%),2,6,10,14-tetramethyl-methylester(11.44%),n-hexadecanoic acid(11.22%) 9,12-octadecanoic acid(10.94%),9-octadecenal(3.92%),1,2-di-2-aminoethylhydrogen phospha (2.16%) respectively.
Table 7: components detected in fraction four of basic metabolites of leaf
| Peaks | RT | Name of Compounds | Mol. Formula | MW | Area% |
| 1 | 17.691 | n-hexadecanoic acid | C16H32O2 | 256 | 11.22 |
| 2 | 18.181 | 2,6,10,14-tetramethyl-methyl ester | C20H40O2 | 312 | 11.44 |
| 3 | 21.027 | Oleic acid | C18H34O2 | 282 | 44.06 |
| 4 | 21.503 | _ | _ | 280 | 16.26 |
| 5 | 21.956 | 9,12-octadecanoic acid | C19H34O2 | 294 | 10.94 |
| 6 | 22.499 | 1,2-di,2-aminoethylhydrogen phospha | C37H74NO3P | 691 | 2.16 |
| 7 | 24.297 | 9-octadecenal | C18H54O | 266 | 3.92 |
GC-MS Analysis of fraction two of second basic Metabolites of Funtumia elastica Leaf
The components present in fraction two of second crop basic metabolites of Funtumia elastica leaf were identified by GC-MS. The chromatogram is shown in (Fig. 3). The active principles with their retention time (RT) molecular formula, molecular weight (MW) and percentage composition in the fraction two of acidic metabolites of Funtumia elasticais presented in (Table 8). Eight components were identified in the fraction two of second crop basic metabolites in Funtumia elastica leaf. The compounds were 9-octadecenoic acid (41.92%) as the major component followed by n-hexadecanoic acid(18.61%),the target compound gave a molecular ion peak at m/z=279 and the molecular weight of compounds suggested in the NIST library is less this hence the compound is not suggested(13.61),n-octadecanoic acid(10.61%),9,12-octadecadienoic acid(9.58),2-hydroxy-1-(hydromethyl,ethyl ester(2.88%),trans-13-docosenoic acid(2.33%),z-17-nonadecen-1-ol-acetate(0.47%) respectively.
Table 8: Components detected in fraction two second crop basic metabolites of leaf
| Peaks | RT | Name of compounds | Mol. Formula | MW | Area% |
| 1 | 18.134 | n-hexadecanoic acid | C16H32O2 | 256 | 18.61 |
| 2 | 21.148 | 9-octadecanoic acid | C18H34O2 | 282 | 41.92 |
| 3 | 21.326 | n-octadecanoic acid | C18H36O2 | 284 | 10.61 |
| 4 | 21.571 | – | – | 13.61 | |
| 5 | 22.005 | 9,12-octadecadienoic acid | C19H34O2 | 294 | 9.58 |
| 6 | 22.511 | 2-hydroxy-1-(hydromethyl)ethyl ester | C19H38O4 | 330 | 2.88 |
| 7 | 23.860 | z-17-nonadecen-1-ol-acetate | C21H40O2 | 324 | 0.47 |
| 8 | 24.310 | Trans-13-docosenoic acid | C22H42O2 | 338 | 2.33 |
GC-MS Analysis of fraction one of neutral Metabolites of Funtumia elastica stem
The components present in fraction one of neutral metabolites of Funtumia elastica stem were identified by GC-MS. The chromatogram is shown in (Fig. 4). The active principles with their retention time (RT) molecular formula, molecular weight (MW) and percentage composition in the fraction two of acidic metabolites of Funtumia elastica is presented in (Table 9). Nine components were identified in the fraction one of neutral metabolites in Funtumia elastica stem. The compounds were 9-octadecenoic acid (51.17%) as the major component followed by n-hexadecanoic acid (17.24%), n-octadecanoic acid (11.71%), 9,12-octadecadienoic acid(6.87%), ethylhexadecanoate(5.46%), 9-octadecenal(2.93), eicosane(2.07%), 2,3-dihydroxypropyl ester(1.80%), alkyltetradecyl ester(0.75%).(13.61), respectively.
Table 9: Components detected from fraction one of neutral metabolites of stem
| peaks | RT | Name of compounds | Mol. Formula | MW | Area% |
| 1 | 18.073 | Pentadecane carboxylic acid | C16H32O2 | 256 | 17.24 |
| 2 | 18.249 | Ethylhexadecanoate | C18H36O2 | 284 | 5.46 |
| 3 | 19.626 | Alkyltetradecyl ester | C19H34O4 | 326 | 0.75 |
| 4 | 20.934 | Oleic acid | C18H34O2 | 282 | 51.17 |
| 5 | 21.155 | n-octadecanoic acid | C18H36O2 | 284 | 11.71 |
| 6 | 21.424 | 9,12-octadecadienoic acid | C19H34O2 | 294 | 6.87 |
| 7 | 22.494 | 2,3-dihydroxypropyl ester | C19H38O4 | 330 | 1.80 |
| 8 | 24.307 | 9-octadecenal | C18H34O | 266 | 2.93 |
| 9 | 25.130 | Eicosane | C20H42 | 282 | 2.07 |
Conclusion
The antibacterial properties of the crude extract, acidic, basic, neutral metabolites and the chromatographic fractions were assessed. The results obtained from crude extract of stem and the basic metabolites were comparable to that of the control drugs and the chromatographic fractions have promise as a source of potential antibiotic agent. Plant based antimicrobials are said to have enormous therapeutic potential, as they can serve the needed purposes with less side effect that are often associated with synthetic antimicrobials. The result of this investigation showed effective antimicrobial activity of the ethanolic extract of Funtumia elastica leaves and stem bark.
References
- Adekunle AA, Ikumapayi AM. (2006) Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J. Sep;55(4):219-23.
- Arias, T.D., (1999). Glosario de Medicamentos: desarollo, evaluaciõny uso. Washington: Organizaciõn Panamricana de la salud. Organizacõin mundial de lasalud, pp171.
- Akinyemi K O, Oladapo O, Okwara C E, Ibe C C, Fasure K A.( 2005) Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicilin resistant Staphylococcus aureus activity. BMC Complement Altern Med.; 5:6.
- Dahiru D, Onubiyi J A, Umaru H A.( 2006) Phytochemical screening and antiulcerogenic effect of Mornigo oleifera aqueous leaf extract. Afr J Trad CAM.; 3:70–75 Farnsworth, N.R., (1994). The role of medicinal plants in drug development. In: Krogsgaard Larsen, S., Brogger- Christensen, S., Kofod, H. (Eds), Natural Products and Drug development. Munksgaard, Copenhagen.
- Ejele A.E, Iwu I.C, Enenebeaku C.K., Ukiwe L.N and Okolue B.N (2012). Bioassay-guided isolation, purification and partial characterization of antimicrobial compound from basic metabolites. Journal of emerging trends in engineering and applied sciences (JETEAS) 3(4):668-672
- Farnsworth N. R. (1994). Ethnopharmacology and drug Development, Novartis Foundation Symposium, Ciba Foundation, Wiley p42-59.
- Iwu, M.M., Duncan, A.R., Okunji, C.O., (1999). New antimicrobial of plant origin. Janick, J. (Eds), ASHS Press, Alexandria, VA.
- Matu, E.N, Van Staden J. ( 2003). Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. Journal of Ethnopharmacology 81 (1), 35-41.
- Srivastava, J., Lambert, J. and Vietmeyer, N. (1996). Medicinal plants: An expanding role in development. World Bank Technical Paper. No. 320
- Taylor J. L. S, Rabe T, McGaw L. J, Jager A. K, Van-Staden J. (2001). Towards the scientific validation of traditional medicinal plants. Plant Growth Regulation 34(1): 23-37.
- World Health Organizations, (1993). Summary of WHO guidelines for the assessment of herbal medicines. Herbal Gram 28, 13-14.
- World Health Organization (WHO) (1977). Resolution-promotion and development of training and research in traditional medicine, WHO document 30: 49.

This work is licensed under a Creative Commons Attribution 4.0 International License.





