How to Cite | Publication History | PlumX Article Matrix
In Vitro Anti-Candida Activity of Different Saudi Honeys and Honey Mixed with Taifi Rose Oil
Anan Kalakattawi1, Sana G. Al Attas1, Sherif Edris1,2,3*
![]() , Ahmed Z. Abdel Azeiz4, Ahmad F. AlGuthami5, Ahmed G. Hegazi6
, Ahmed Z. Abdel Azeiz4, Ahmad F. AlGuthami5, Ahmed G. Hegazi6  , Saad B. Almasaudi1, Rashad R. Al-Hindi1, Ahmed Bahieldin1,2
, Saad B. Almasaudi1, Rashad R. Al-Hindi1, Ahmed Bahieldin1,2 
1Department of Biological Sciences, Faculty of Science, King Abdulaziz University (KAU), Jeddah, Saudi Arabia
2Department of Genetics, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
3Princess Al-Jawhara Al-Brahim Centre of Excellence in Research of Hereditary Disorders (PACER-HD), Faculty of Medicine, King Abdulaziz University (KAU), Jeddah, Saudi Arabia
4College of Biotechnology, Misr University for Science and Technology (MUST), Egypt
5AlGuthami Foundation, Jeddah, Saudi Arabia.
6National Research Centre, Giza, Egypt
Corresponding Author E-mail: sedris@aucegypt.edu
DOI : http://dx.doi.org/10.13005/bbra/2794
ABSTRACT: Candida albicans is a common human yeast that infect several epithelial tissues including vagina. The increase of drug-resisting C. albicans encouraged the researchers to find alternative treatment. Honey medical signatures such as bactericidal, antifungal and anti-candida made it a possible candidate for disease treatment. In addition, rose essential oil possesses a wide range of biochemical activities in folkloric medicine including anti-microbial activities. The present research utilizes honey alone or in conjunction with Taifi rose (Rosa damascena) oil as anti-candida agent to treat vaginal candidiasis. Three local monof oral honeys from different flower sources and/or geographic origins were tested with four concentrations (50, 80 and 95%), while two concentrations of the Taifi rose oil (1 and 2%). anti-candida activity of honey alone or in conjunction with Taifi rose oil was determined as well as phenolic and flavonoids contents were determined. Also, GC-MS analysis of volatile oils and alkaloids were evaluated. The results of this study indicated that acidity is within the allowed range for commercialization and long-lasting storage. All honeys tested inhibited completely the C. albicans growth at concentrations 80% and 95% either incubation after 48 or 72 h. Also, only Markh and Manuka honeys were completely inhibited C. albicans growth at 50% concentration. Also, C. albicans growth inhibited completely at 2% Taifi rose oil after the incubation periods of 48 and 72 h. The phenolic compounds and flavonoids were analysed by mass spectrometry analysis which revealed the Markh honey showed the presence of gallic acid and quercetin that proved to have antifungal activity. It could be concluded that mixed Markh honey and Taifi rose oil treatment was capable to inhibit C. albicans growth completely. Further research is required to determine the anti-candida activity of the mixture of Markh honey and Taifi rose oil in the human body as a new therapeutic drug to treat vaginal candidiasis.
KEYWORDS: Flavonoids; Phenols; Vaginal Candidiasis
Download this article as:| Copy the following to cite this article: Kalakattawi A, Attas S. G. A, Edris S, Azeiz A. Z. A, Al-Guthami A. F, Hegazi A. G, Almasaudi S. B, Al-Hindi R. R, Bahieldin A. In Vitro Anti-Candida Activity of Different Saudi Honeys and Honey Mixed with Taifi Rose Oil. Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Kalakattawi A, Attas S. G. A, Edris S, Azeiz A. Z. A, Al-Guthami A. F, Hegazi A. G, Almasaudi S. B, Al-Hindi R. R, Bahieldin A. In Vitro Anti-Candida Activity of Different Saudi Honeys and Honey Mixed with Taifi Rose Oil. Biotech Res Asia 2019;16(4). Available from: https://bit.ly/2QDb6g7 |
Introduction
Candida albicans is among the most common human yeast that adheres and colonizes epithelial tissues of the mucosal membranes including the oral, gastrointestinal tract, bladder and genitalia especially vagina (Rodrigues et al., 2019). Candida infection was raised dramatically in terms of severity mainly due to the increase of disorder incidences – such as cancer and AIDS-, chemotherapy, etc. (WHO, 2014 and Darvishi et al. 2015). The incidence of fungal infections by Candida spp. is increasing in both the community and hospital environments. C. albicans causes over 50% of oral infections, while as high as > 90% of vaginal candidiasis (Irish et al. 2006; Bahadoran et al., 2010 and Cortegiani et al., 2018). Vaginal candidiasis is common in Saudi Arabia in which 70.2% is yeast vaginitis caused by C. albicans (Al-Aali 2013). Overgrowth of yeast can result from pregnancy, high-dose oral estrogen use, contraceptives tablets, over usage of broad-spectrum antibiotics, uncontrolled diabetes, corticosteroids and obesity (Darvishi et al. 2015 and Yokoyama et al., 2019). The increase of drug-resisting C. albicans encouraged the researchers to find alternative treatment.
Over thousands of years, the old Egyptian, Greek and Muslims have used honey as a folkloric medicine for many diseases (Hegazi, 2012). Honey has biological activity as enhance immune response (Hegazi et al., 2015 and 2017a), potential antibacterial activity (Hegazi et al.,2014a, 2014b and 2017b), antitumor and antioxidant ((Hegazi et al.,2014c) and antioxidant (Hörmet-Öz et al. 2009). However, the differences between honey types are due to maturation of the honey itself inside the honey bees and the derivation from different kinds of flower sources (Michalkiewicz et al. 2008). Moreover, seasonal climatic, geographic origin, harvesting time, storage and processing conditions all play significant roles of honey bioactive effects (Michalkiewicz et al. 2008). In Arab cultures, honey is extremely used for both nutrition and therapy, however, there is a limited number of research investigations on the antimicrobial activity of honey published in Arabian region (Hegazi 2011 and Hegazi et al.,2017b).
Other alternative suggestion for candida treatment is the rose oil. Rosa species members of the Rosacea family are important decorative plants with pink flowers and have been referred to as the “King of Flowers” and the “Sambal of Love” (Mahboubi 2016). Rosa damascena is one of the most important Rosa species whose products are rose concrete, rose water, rose oil, rose absolute, and dried petals (Abdel-Hameed et al. 2013). These products have long been used in cosmetics, perfumes, medicinal purposes and food industries (Mahboubi 2016). Rose essential oil in addition to its perfuming effects was reported to possess a wide range of biochemical activities in folkloric medicine such as hypnotic, analgesic, antispasmodic, anticonvulsant, anti-inflammatory, anti-oxidants and anti-microbial activities (Ulusoy et al. 2009). Taifi rose (Ward Taifi, e.g., Rosa damascena trigintipetala Dieck) is one of the most important commercially propagated rose in Taif, Saudi Arabia. Taifi rose essential oil is very expensive and is known for its good perfumery applications and the use in cosmetic products (Hagag et al. 2014).
Up to our knowledge, no research on the influence of Taifi rose oil alone or in conjunction with honey as anti-microbial agent was reported. In this study, the anti-candidia effects of different kinds of Saudi honey in addition to Taifi rose essential oil was investigated individually and in combination.
Materials and Methods
Microorganism and culture conditions
One local C. albicans isolate used in this study was kindly provided by the Microbiology Unit, East Jeddah Hospital, Jeddah, Saudi Arabia. The C. albicans isolate was previously identified by VITEK microbial identification system. The isolate was originally cultured on Sabouraud dextrorse agar (SDA) media (Oxoid, UK) and incubated at 37°C for 48-72 h. One single colony was subcultured in three replicates on Nutrient Broth (NB) (Himedia). Rate of candida growth was evaluated in suspension cultures by spectrophotometer at OD 600 and diluted to 1.0 OD by nutrient broth media (NB).
Preparation of honey and Taifi rose’s essential oil samples
Four kinds of monof oral honey from different flower source and/or geographic origin, were used. Three of them (Markh, Qatad and Sider) were obtained from a commercial Al Nahl Al Gawal Aprifarm (AlGuthami Foundation, Jeddah, Saudi Arabia), while the fourth is Manuka honey (New Zealand). All honey types were stored in sterile bottles in the dark at 4oC temperature until used. Four different concentrations (50, 80 and 95%) of honey were used in addition to the control (nutrient broth media without honey). Dilutions were prepared just before use. Essential oil extracted from Taifi rose (Rosa damascena trigintipetala) was kindly provided by farms of Taif rose. Two different concentrations of the Taifi rose oil (1 and 2%) were used in addition to the control (nutrient broth media without oil).
Physiochemical analysis of honey
The physiochemical analysis of honey samples (Hegazi et al., 2018). The physicochemical properties in terms of glucose, fructose, and sucrose contents and parameters of identity and quality of local honeys in terms of moisture content and acidity. The Codex Alimentarius Committee (SASO, 1990) and Hegazi et al., 2018 ) permitted a maximum value in commercialized honey.
Estimation of anti-candida activity
A freshly grown C. albicans suspension was cultured in Falcon tubes (50 ml) with different honey (50, 80 and 95%) or Taifi rose oil (1 and 2%) dilutions in nutrient broth (Himedia) in three replicates. Based on the results, an extra culture of C. albicans was prepared to include the lowest concentration of a selected local honey that showed the highest anti-candida activity mixed with Taifi rose oil at the low concentration (1%). All tubes were incubated at 37oC for 48 and 72 h incubation periods with continuous shaking at 150 rpm. For control, microbe-free media was used. Each broth culture (100 µl) was serially diluted by sterile water to the dilution factors 102, 103 or 104. Then, cultures were spread into SDA agar plates and incubated at 37°C for 72 h. The colony forming unit (CFU) was measured by open CFU 3.9.0 sof tware (Geissmann 2013).
Determination of total phenols and flavonoids in Markh honey
For the determination of free phenols and flavonoids, the Markh honey sample was diluted 10 times with (80%) ethanol solution. For determination of total phenols and flavonoids, the honey samples were further diluted 10 times by 2M HCl and heated at 95°C for 30 min to liberate the ether binding phenols and flavonoids. The total phenols were determined by Lowry method (Lowry et al. 1951) , while the total flavonoids were determined in both extracts by the aluminum chloride method (Akbay et al. 2003; Barker 2019)
The phenolic compounds and flavonoids were detected in hydrolysate solution of honey by mass spectrometry using Xivo TQD mass unit: Waters, after electrospray ionization (ESI) using daughter scan mode and negative ion detection [M-H]. The capillary and cone volts were 2.7 KV and 30 V, respectively, while the desolvation gas flow was 600 L/h. The MW and daughter ions for the different compounds were detected. Gallic acid has MW of 170, while daughter ions of 150.6, 125 and 97. Caffeic acid has MW of 180, while daughter ions of 161, 107.1 and 88.7. Vanillic acid has MW of 182, while daughter ions of 59.2, 67.1, 78.8, 93.0, 106.4 and 120.8. Quercetin has MW o f302, while daughter ions of 273, 178.9 and 151.
GC-MS analysis of volatile oils and alkaloids
The analysis was performed using Trace GC1310-ISQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a direct capillary column TG–5MS (30 m x 0.25 mm x 0.25 µm film thickness). The column oven temperature was initially held at 50°C, then increased by 5°C/min to 230°C and held for 2 min, then increased again to the final temperature of 290°C by 30°C/min and held for 2 min. The injector and MS transfer line temperatures were kept at 250 and 260°C, respectively. Helium was used as a carrier gas at a constant flow rate of 1 ml/min. The solvent delay was 3 min and diluted samples of 1 µl were injected automatically using Autosampler AS1300 coupled with GC in the split mode. EI mass spectra were collected at 70 eV ionization voltages over the range of m/z 40–1000 in full scan mode. The ion source temperature was set at 200°C. The components were identified by comparison of their retention times and mass spectra with those of WILEY 09 and NIST 11 mass spectral database.
Results
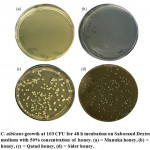
General description of the different honey types used in the present study is shown in Figure 1. It included the physicochemical properties in terms of glucose, fructose, and sucrose contents and parameters of identity and quality of local honeys in terms of moisture content and acidity. The results indicated that contents of glucose and fructose are almost the same in the three used local honey. Sucrose content was much less in the three types of honey especially Qatad. Moisture content is almost similar for the three types of honey averaging ~15%. Acidity was much higher in Markh (40 meq/kg) as compared with the other two types of honey (Figure 1).
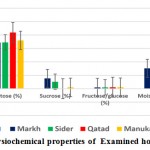 |
Figure 1: Physiochemical properties of Examined honey samples |
As shown in Table 1and Figure 2, all four honey types completely inhibited the C. albicans growth at both concentrations 95% and 80% after either incubation period, e.g., 48 or 72 h. On the other hands, Markh and Manuka honey continued to completely inhibit C. albicans growth at 50% concentration, while Qatad and Sider honey gave counted growth at both incubation periods of 48 and 72 h (Figure 2). Much heavier growth was shown for the latter two types of honey as compared with Markh and Manuka honey at 50% concentration. Overall, Sider seemed to have the least anti-candida effects, while Manuka had the highest effects at both incubation periods as compared to the other types of honey. The data of Taifi rose oil indicated that 2% had completely inhibited C. albicans growth at the incubation periods of 48 and 72 h, while 1% concentration caused a declined C. albicans growth (Table 2). Based on the above-mentioned results, Markh was selected for further analysis in order to detect the accumulative effects of mixing honey at either 30 or 50% with rose oil at 1% for either incubation period of 48 or 72 h, respectively. The results indicated that the two mixed honey/oil treatments were capable to completely inhibit C. albicans growth (Table 2).
Table 1: Influence of different kinds of honey dilutions (at 95, 80, 50 and 30%) on in vitro growth of C. albicans.
| 48 h | 72 h | |||||||
| 95% | 80% | 50% | 30% | 95% | 80% | 50% | 30% | |
| Manuka | 0 | 0 | 0 | 256 | 0 | 0 | 0 | 258 |
| Markh | 0 | 0 | 0 | 799 | 0 | 0 | 0 | 642 |
| Qatad | 0 | 0 | 225 | N/A* | 0 | 0 | 82 | N/A |
| Sider | 0 | 0 | 1879 | N/A | 0 | 0 | 1989 | N/A |
| Candida Control | 27900 | 128400 | ||||||
Table 2: Influence of Markh honey and combinations with Taifi rose oil (at 1 and 2%) on in vitro growth of C. albicans.
| Time | 48 h | 72 h | ||
| Concentration | 1% | 2% | 1% | 2% |
| Taifi rose oil | 1.81 | 0 | 2.25 | 0 |
| Mixed treatment (50% Markh + 1% rose oil) | 0 | 0 | ||
| Candida Control | 27900 | 128400 | ||
The analysis of phenols showed the presence of 0.1 % free phenols and 0.96% of total phenols (free and ether bind phenols). The mass spectrometry analysis for detection of phenolic compounds and flavonoids showed the presence of caffeic acid, gallic acid and quercetin (Figure 3). The mass spectrum of gallic acid clearly showed the [M-H] ion at m/z 169 and all daughter ions of 150.6, 125 and 97 were detected (Figure 2a). The mass spectrum of caffeic acid clearly showed the [M-H] ion at m/z 178.9 and all daughter ions 161, 107.1 and 88.7 were detected (Figure 2b). The mass spectrum of quercetin showed the [M-H] ion at m/z 301.1 and all daughter ions of 273, 178.9 and 151were detected (Figure 2c).
Discussion
The mechanism of the anti-candida effect of honey is not completely understood, however, several honey characteristics have been proposed. They include acidity and osmolarity due to high sugar content up to a certain threshold (Hegazi et al. 2017; Zam et al. 2018 and Hegazi et al., 2018 ). In this study, Markh honey had the highest free acidity as compared with the other two kinds of local honey (Table 1), thus, had the highest anti-candida effect (Table 2). Free acidity is an important parameter related to the deterioration of honey. Then, we speculate that Markh is potent to more storage periods. Free acidity is characterized by the presence of organic acids in equilibrium with several other elements such as lactone, internal esters and some inorganic ions such as phosphates, sulfates and chlorides (Moreira et al. 2010 and Hegazi et al. 2017and Hegazi et al., 2018 ). The free acidity in Markh honey was 40. The Codex Alimentarius Committee on Sugars stated that values higher than 50 may be indicative of fermentation of sugars into organic acids especially in monof oral honeys (Fett et al. 2015). Therefore, many organic acids have influence on honeys’ free acidity (SOS, 1999, Alves et al. 2013; Tornuk et al. 2013; Hegazi et al. 2018).
The antifungal activity of honey also relies on several other factors such as activity of glucose oxidase to retard ripening of nectar, hence, reduce the level of hydrogen peroxide (AL-Waili et al. 2013; Hegazi 2011; Hegazi et al. 2017; Zam et al. 2018). Moreover, geographic origin, harvest season and flower source contribute to antibacterial mechanism of honey Hegazi et al. 2017; Zam et al. 2018). In the current study, four different kinds of honey from different flower source in different geographic origins were studied. Markh and Qatad are monof loural types of honey from Leptadenia pyrotechnica and Astragalus spinosus flowers, respectively, growing in Tihama, Madinah Almonawarah region, Saudi Arabia. They have ambergris and light ambergris colours and are grown at spring and autumn seasons, respectively. Sider is also a monof loural honey from Ziziphus nimmularia flowers growing in Fakhrah region in Saudi Arabia. It has an ambergris colour and is grown at autumn season. The fourth kind of honey is Manuka New Zealand honey, from the flowers of native Manuka tree. Manuka has a light ambergris colour.
Prior results indicated that the flower source of Markh honey (Leptadenia pyrotechnica) has components such as phytols, cardenoids, flavonoids, alkaloids, steroidal glycosides and terpenes (Ghaneian et al. 2015; Verma et al. 2014). Phytol (3,7,11,15-tetramethylhexadec-2-EN-1-OL) is an important member of branched chain unsaturated terpene, and is a product of chlorophyll metabolism in plants. Interestingly, phytol can inhibit microbes (Ghaneian et al. 2015). Manuka honey has been also shown to contain high levels of methylglyoxal (MGO) produced by the non-enzymatic conversion of dihydroxyacetone that presents at high concentrations in the nectar of Leptospermum scoparium flowers. Reports indicate that neutralization of MGO in Manuka honey abolished the antimicrobial activity of the honey against Staphylococcus aureus, but did not abolish the antimicrobial activity against Escherichia coli and Pseudomonas aeruginosa (Kwakman et al. 2011; McLoone et al. 2016; Matzen et al. 2018). The authors concluded that MGO is not fully responsible for Manuka honey’s non-peroxide antimicrobial activity and that other components, possibly polyphenols, may be responsible.
In contrary to our results, Hegazi and others indicated that Qatad honey has the highest antibacterial activity on Klebsiella pneumoniae as compared with other Saudi honey (Hegazi et al. 2017). However, the latter study was concentrated on the anti-bacterial, rather than anti-candida, influence. Al-Waili et al. (2013) indicated that the minimum inhibitory concentration to inhibit candida growth is 70%. They also indicated that the rate of growth after 72 h was similar to that after 24 h when candida was cultured on the same honey concentration. Other reports indicated that honey at 80% could completely inhibit the candida growth when incubated for 2-6 hour (Khosravi-Darani et al. 2013). Our results recommend the further use of either 30 or 50% in treating vaginitis. Higher concentration might be sticky enough to prevent penetration of the honey within the tissues, thus, retards the anti-candida effect.
The detected phenolic compounds are among those of the aerial part of Leptadenia pyrotechnica plant (Khasawneh et al. 2011). The quercetin is a flavonoid exists in the form of quercetin-3-β-D-glucoside in the plant (Moustafa et al.2009). This compound is well known as an antioxidant and antimicrobial agent. Quercetin also showed antifungal activity against Cryptococcus spp. (Oliveira et al. 2016). It was the most active flavonoid tested against dermatophytes (Bitencourt et al. 2014). The alcoholic extract of Sebastiania commersoniana exhibited significant antifungal activity against the dermatophytes Microsporum gypseum, Trichophyton mentagrophytes and Trichophyton rubrum. The active components in this extract include quercetin and gallic acid that are among flavonoid and phenolic compounds (Hnatyszyn et al. 2007). Among the tested phenolic compounds against Candida sp., gallic acid showed the highest MIC value (Alves et al. 2014). The GC/MS analysis of volatile oils and alkaloids showed absence of any volatile oil compounds, while the alkaloid 5-Heptyl-2-pyrrolidinone was detected at retention time 6.2 min. Pyrrolidin derivatives have been extensively studied as antifungal compounds (Bharose and Gajera 2018; Moradi et al. 2013). Interestingly, previous investigations of the chemical composition of Manuka honey also showed the presence of gallic acid, caffeic acid, and quercetin (Alvarez-Suarez et al. 2014).. Therefore, the antimicrobial activity of Manuka honey can also be attributed to the presence of these compounds.
In the present study, the possible accumulative impact of Taifi rose oil mixed with Markh honey on the candida growth, rather than the separate impact of either treatment (1% rose oil or 30% Markh honey), was suggested. Prior reports showed that honey and ginger extract had a more significant inhibition of Candida albicans growth (Khosravi-Darani et al. 2013). Darvishi et al. (2015) also indicated that honey mixed by yogurt is more effective on treating vaginal candidiasis than clotrimazole vaginal cream. The present study its kind to provide a substantial in vitro investigation of the anti-candida effects of Markh honey and Taifi rose oil, either separately and mixed. Further research is required to determine the anti-candida activity of the mixture when treating vaginal candidiasis in the human body. Successful results might suggest the use of the new mixture as a therapeutic drug for patients with vaginal candidiasis.
Conclusion
From the current results, it is concluded that a substantial in vitro investigation of the anti-candida effects of Markh honey and Taifi rose oil, either separately or mixed, indicates the possible use as complementary anti-candida agent.
Acknowledgments
The authors are grateful for the financial support by the Al Guthami Foundation, Saudi Arabia.
Conflicts Interests
The authors declare no competing interests.
References
- Abdel-Hameed, El Sayed S., Salih A. Bazaid, and Mahmood S. Salman. 2013. “Characterization of the Phytochemical Constituents of Taif Rose and Its Antioxidant and Anticancer Activities.” BioMed Research International 2013:13.
- Akbay, Pinar, A. Ahmet Basaran, Ulkü Undeger, and Nursen Basaran. 2003. “In Vitro Immunomodulatory Activity of Flavonoid Glycosides from Urtica Dioica L.” Phytotherapy Research 17(1):34–37.
- Al-Aali, Khadijah Yosif. 2013. Prevalence of Vaginal Candidiasis among Pregnant Women Attending Al-Hada Military Hospital, Western Region, Taif, Saudi Arabia.
- AL-Waili, Noori et al. 2013. “Differences in Composition of Honey Samples and Their Impact on the Antimicrobial Activities against Drug Multiresistant Bacteria and Pathogenic Fungi.” Archives of Medical Research 44(4):307–16.
- Alvarez-Suarez, José, Massimiliano Gasparrini, Tamara Forbes-Hernández, Luca Mazzoni, and Francesca Giampieri. 2014. “The Composition and Biological Activity of Honey: A Focus on Manuka Honey.” Foods 3(3):420–32.
- Alves, Andreia, Adalgiza Ramos, Maria Margarida Gonçalves, Maria Bernardo, and Benilde Mendes. 2013. “Antioxidant Activity, Quality Parameters and Mineral Content of Portuguese Monof loral Honeys.” Journal of Food Composition and Analysis 30(2):130–38.
- Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni , Giampieri
- The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods. 2014 Jul 21;3(3):420-432. doi: 10.3390/foods3030420.
- Alves, Carlos Tiago et al. 2014. “Antifungal Activity of Phenolic Compounds Identified in Flowers from North Eastern Portugal against Candida Species.” Future Microbiology 9(2):139–46.
- Bahadoran, Parvin et al. “Investigating the therapeutic effect of vaginal cream containing garlic and thyme compared to clotrimazole cream for the treatment of mycotic vaginitis.” Iranian journal of nursing and midwifery research vol. 15,Suppl 1 (2010): 343-9.
- Barker, Allen V. 2019. Natural Products from Plants, Second Edition.
- Bharose, AA and HP Gajera. 2018. “Antifungal Activity and Metabolites Study of Bacillus Strain Against Aflatoxin Producing Aspergillus.” Journal of Applied Microbiology and Biochemistry 02(02).
- Bitencourt, Tamires Aparecida, Tatiana TakahasiKomoto, Mozart Marins, and Ana Lúcia Fachin. 2014. “Antifungal Activity of Flavonoids and Modulation of Expression of Genes of Fatty Acid Synthesis in the Dermatophyte Trichophyton Rubrum.” BMC Proceedings 8(S4):P53.
- Cortegiani A., Misseri G., FascianaT., Giammanco A.,Giarratano A.,and Chowdhary A. (2018): Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 6: 69. doi: 10.1186/s40560-018-0342-4
- Darvishi, Maryam, Freshte Jahdi, Zeinab Hamzehgardeshi, Saeed Goodarzi, and Mohsen Vahedi. 2015. “The Comparison of Vaginal Cream of Mixing Yogurt, Honey and Clotrimazole on Symptoms of Vaginal Candidiasis.” Global Journal of Health Science 7(6):108–16.
- Fett, Roseane, Priscila Missio da Silva, Ana Carolina Oliveira Costa, Cony Gauche, and Luciano Valdemiro Gonzaga. 2015. “Honey: Chemical Composition, Stability and Authenticity.” Food Chemistry 196:309–23.
- Geissmann, Quentin. 2013. “OpenCFU, a New Free and Open-Source Sof tware to Count Cell Colonies and Other Circular Objects” edited by R. M. Merks. PLoS ONE 8(2):e54072.
- Hagag, Heba A., Salih A. Bazaid, El Sayed S. Abdel-Hameed, and Mahmood Salman. 2014. “Cytogenetic, Cytotoxic and GC–MS Studies on Concrete and Absolute Oils from Taif Rose, Saudi Arabia.” Cytotechnology 66(6):913–23.
- Hegazi, Ahmed G. 2011. “Antimicrobial Activity of Different Egyptian Honeys as Comparison of Saudi Arabia Honey.” Research Journal of Microbiology 6(5):488–95.
- Hegazi AG., Al Guthami FM. and Al Gethami AF. M. (2018): Physiochemical Analysis of Some Saudi Arabia Honey. Int.J.Curr.Microbiol.App.Sci. 7(2): 1441-1448.
- Hegazi A. G. Al Guthami, F. M, Al Gethami AF. M. and El Fadaly H Ali. (2017a): Beneficial Effects of Capparis Spinosa Honey on the Immune Response of Rats Infected with Toxoplasma gondii, Journal of Pharmacopuncture; 20 (2):112-118.
- Hegazi A. G., Al Guthami, F. M, Al Gethami AF. M., Abd Allah F M., Saleh A A. and Fouad E A. (2017b): Potential antibacterial activity of some Saudi Arabia honey. Veterinary World, 10 (2): 233-237.
- Hegazi A. G.; Abdel- Rahman E. H.; Abd Allah F. and Abdou A.M. (2015): Influence of Honey on Immune Status in Mice-Bearing Ehrlich Carcinoma. Journal of Clinical & Cellular Immunology. 6:1.
- Hegazi A.G, Abd El-Moez S. I., Abdou A. M. and Abd Allah F. (2014a): Antibacterial activity of some types of monof loral honey against Clostridium acetobutylicum and Clostridium perfringens. Int.JCurMicobl.ApSci 3(9) 552-565
- Hegazi A. G, Abd El-Moez S. I., Abdou A. M. and Abd Allah F. (2014b): Synergistic antibacterial activity of Egyptian honey and common antibiotics against Clostridium Reference strains, Int.J.Curr.Microbiol.App.Sci.3(8): 312-325.
- Hegazi A.G., Al Tahtawy R. H.M., Abd Allah F. and Abdou A. M. (2014c): Antitumor and antioxidant activity of honey in mice bearing Ehrlich Ascites Carcinoma. Academic Journal of Cancer Research 7 (3): 208-214.
- Hnatyszyn, Oksana et al. 2007. “Phytochemical Analysis and Antifungal Evaluation of Sebastiania Commersoniana . Extracts.” Pharmaceutical Biology 45(5):404–6.
- Hörmet-Öz, Hatice Tuna et al. 2009. “Antifungal Activity of Turkish Honey against Candida Spp. and Trichosporon Spp: An in Vitro Evaluation.” Medical Mycology 47(7):707–12.
- Irish, Julie, Dee A. Carter, Tahereh Shokohi, and Shona E. Blair. 2006. “Honey Has an Antifungal Effect against Candida Species.” Medical Mycology 44(3):289–91.
- Khasawneh, Mohammad A., Hanan M. Elwy, Alaaeldin A. Hamza, Nael M. Fawzi, and Ahmed H. Hassan. 2011. “Antioxidant, Anti-Lipoxygenase and Cytotoxic Activity of Leptadenia Pyrotechnica (Forssk.) Decne Polyphenolic Constituents.” Molecules 16(9):7510–21.
- Khosravi-Darani, Kianoush et al. 2013. “Antifungal and Anti-Bacterial Synergistic Effects of Mixture of Honey and Herbal Extracts.” Zahedan Journal of Research in Medical Sciences 15(8):30–33.
- Kwakman, Paulus H. S., Anje A. te Velde, Leonie de Boer,et al. . 2011. “Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity” edited by P.-J. Cardona. PLoS ONE 6(3):e17709.
- Lowry, O. H., N. J. ROSEBROUGH, A. L. FARR, and R. J. RANDALL. 1951. “Protein Measurement with the Folin Phenol Reagent.” The Journal of Biological Chemistry 193(1):265–75.
- Mahboubi, Mohaddese. 2016. “Rosa Damascena as Holy Ancient Herb with Novel Applications.” Journal of Traditional and Complementary Medicine 6(1):10–16.
- Matzen, Reem Dina et al. 2018. “The Antibacterial Effect in Vitro of Honey Derived from Various Danish Flora.” Dermatology Research and Practice 2018.
- McLoone, Pauline, Mary Warnock, and Lorna Fyfe. 2016. “Honey: A Realistic Antimicrobial for Disorders of the Skin.” Journal of Microbiology, Immunology and Infection 49(2):161–67.
- Michalkiewicz, Anna, Magdalena Biesaga, and Krystyna Pyrzynska. 2008. “Solid-Phase Extraction Procedure for Determination of Phenolic Acids and Some Flavonols in Honey.” Journal of Chromatography A 1187(1–2):18–24.
- Moradi, Shoeib, Parisa Azerang, Vahid Khalaj, and Soroush Sardari. 2013. “Antifungal Indole and Pyrrolidine-2,4-Dione Derivative Peptidomimetic Lead Design Based on in Silico Study of Bioactive Peptide Families.” Avicenna Journal of Medical Biotechnology 5(1):42–53.
- Moustafa, Amal., Ahmed .. Khodair, and Mahmoud. Saleh. 2009. “Isolation, Structural Elucidation of Flavonoid Constituents from Leptadenia Pyrotechnica and Evaluation of Their Toxicity and Antitumor Activity.” Pharmaceutical Biology 47(6):539–52.
- Oliveira, V. M., E. Carraro, M. E. Auler, and N. M. Khalil. 2016. “Quercetin and Rutin as Potential Agents Antifungal against Cryptococcus Spp.” Brazilian Journal of Biology 76(4):1029–34.
- Rodrigues CF. , Rodrigues ME. And Henriques M. (2019): Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 8(1), 76; https:// doi.org/ 10.3390 /jcm8010076.
- Saudi Arabian Standards Organization, SASO (1990). Saudi Arabian standards, test methods of honey No. 102. Saudi Arabia: Ministry of
- Tornuk, Fatih et al. 2013. “Quality Characterization of Artisanal and Retail Turkish Blossom Honeys: Determination of Physicochemical, Microbiological, Bioactive Properties and Aroma Prof ile.” Industrial Crops and Products 46(46):124–31.
- Ulusoy, Seyhan, Gülgün Boşgelmez-TInaz, and Hale Seçilmiş-Canbay. 2009. “Tocopherol, Carotene, Phenolic Contents and Antibacterial Properties of Rose Essential Oil, Hydrosol and Absolute.” Current Microbiology 59(5):554–58.
- World Health Organization . World Cancer Report. International Agency for Research on Cancer; Lyon, France: 2014.
- Yokoyama H, Nagao A, Watanabe S, Honjo J. (2019): Incidence and risk of vaginal candidiasis associated with sodium-glucose cotransporter 2 inhibitors in real-world practice for women with type 2 diabetes. J Diabetes Investig. 10(2):439-445. doi: 10.1111/jdi.12912. Epub 2018 Oct 19.
- Zam, Wissam, Rim Harfouch, Al Dwiri Mais, and Khwanda Rand. 2018. “Anti- Staphylococcus Aureus Efficacy of Six Natural Honey Samples Originated from Syria.” Research Journal of Pharmacognosy and Phytochemistry 10(1):23.

This work is licensed under a Creative Commons Attribution 4.0 International License.