How to Cite | Publication History | PlumX Article Matrix
Investigation of Some Antioxidant Enzyme Activities in Cherry Fruit
Tuğba Gür1*, Fatih Karahan2, Halit Demir2 and Canan Demir1
1Yüzüncü Yil University, Vocational School of Health Services, Van, Turkey
2Van Yüzüncü Yil University, Faculty of Science, Department of Biochemistry
Corresponding Author Email: tugbagur80@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2788
ABSTRACT: Superoxide dismutase (SOD) and catalase enzyme (CAT) activities with strong antioxidant properties were determined in cherry fruits obtained from different regions such as Aegean, Mediterranean and Marmara. The cherry fruit extract was prepared and some antioxidant activities were determined. Cherry (prunus avium) is a fruit belonging to the family of rosaceae. Its homeland is asia minor. Many varieties are grown in Turkey. There are more than a hundred culture forms grown in north america with temperate regions of europe and asia. Its body is in the form of a flat-shell tree. Cherry is a fruit rich in vitamin C. They do not contain fat and cholesterol. It contains essential minerals such as fiber, vitamin A, iron, calcium, protein as well as abundant potassium. Red cherries also contain melatonin, which helps combat harmful toxins. Due to its antioxidant properties, it has many benefits such as prevention of some types of cancer, reduction of inflammation, prevention of gout and removal of muscle pain. For this purpose, it is aimed to determine some enzyme activities which are thought to be found in cherry fruit. In this study, antioxidant enzyme activities in cherry fruit were determined by spectrophotometric method. Additonaly the findings were analyzed by using multidimensional statistical methods and the results were discussed in a multidimensional manner. It is obtained that the highest catalase enzyme activity was determined in the Aegean region (4.330 U/L), while the highest superoxide dismutase enzyme activity was found in the Mediterranean region (7.176 U/L).
KEYWORDS: Antioxidant Enzymes Cherry; Extract; Statistical Analysis
Download this article as:| Copy the following to cite this article: Gür T, Karahan F, Demir H, Demir C. Investigation of Some Antioxidant Enzyme Activities in Cherry Fruit. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Gür T, Karahan F, Demir H, Demir C. Investigation of Some Antioxidant Enzyme Activities in Cherry Fruit. Biosci Biotech Res Asia 2019;16(4). Available from: https://www.biotech-asia.org/?p=34652 |
Cherry (Prunus avium L.) has become one of the most important non-climatic fruits worldwide because of its quality characteristics1. The homeland of the cherry (Prunus avium, L.) is south caucasia, the Caspian Sea and North-East Anatolia. This gene has spread to the east and west to cover a wide area on the world. Cherries are also known as sourccherry fruits.The leaves of cherry tree are larger than that of sourcherry, oval shaped, leaf crinkle, lower face is feathery, with tip spikes and edges as tooth saws. The cherry is round, shaped, succulent, juicy, low-fiber, delicious and pleasant. In sourccherry fruits, the sweetness is mainly caused by glucose and fructose, while the tartness is mainly caused by organic acid (malic acid)2,3.The cherries structure contains both water soluble (C, B) and fat soluble (A, E and K) vitamins. It also contains beta-carotene, lutein, zeaxanthin and some carotenoids. Cherry, which has a very low fat content (which can be considered as a fat-free fruit, has a saturated fat content but does not contain cholesterol. At the same time, the calorie value of cherry, which is a fruit that does not contain sodium salt, is also low. As with other red fruits, cherries also turn green to red in the process of maturation by breakdown of polyphenolic compounds, anthocyanins and chlorophyll. Phenolic compounds condense on the skin and contribute to the sensory and organoleptic properties of fruits such as taste and shrinkage4.It has a very strong antioxidant property. Because of its antioxidant properties, it prevents alzheimer’s disease, reduces the risk of stroke, and prevents gout and inflammation. Cherries contain plenty of anthocyanins and melatonin. Cyanide 3-glucoside, cyanide 3-rutinoside, cyanidine 3-sophorocyte, pelargonidine 3-glucoside, pelargonidine 3-rutinoside, 3-glucoside and peonidine 3-rutinoside are defined as anthocyanins found in sweet and sour cherries5,6,7. Cherries, biological activities have recently been investigated in different experimental models and antioxidant, anti-inflammatory and anticancer properties were seen. Among the anthocyanins tested, it was determined that cyanide 3-glucoside exhibited stronger antioxidant8. In vitro studies using several cell culture systems including colon, endothelial, liver, breast and leukemic cells, and keratinocytes have indicated that anthocyanins have multiple anticancer effects 9.
In this study, it was aimed to investigate the antioxidant enzyme activities of catalase (CAT) and superoxide dismutase (SOD) in cherry fruit.
Materials and Methods
Preparation of catalase (CAT) and superoxide dismutase (SOD) enzyme solutions for cherry fruit
In this study, a certain amount of fresh cherry fruits were taken. These parts were thoroughly shredded with the help of the blender. Phosphate buffer (pH = 7.4) with 50 mM was used during the shredded process.
Determination of cherry fruit catalase (CAT) and superoxide dismutase (SOD) enzyme activities
In this study, antioxidant catalase and superoxide dismutase enzyme activities of cherry species in Aegean, Mediterranean and Marmara regions were brought to determine. Then, the cherry fruits obtained were shredded to determine catalase and superoxide dismutase enzyme activity and centrifuged at 8000 rpm for 5 minutes. The liquid at the top of the centrifuge tube was then receipted. One empty tube and one sample tube were achieved for measurement of catalase activity. 2.8 ml of 30 mM hydrogen peroxide (H2O2) was placed into the empty tube and 0.2 mL of phosphate buffer was adding. The blend was shaken quickly and spectrophotometrically measured (Ati Unicam UV/VIS-UV2-100, England) two times at 240 nm with thirty-second intervals. Then again, 2.8 ml of 30 mM hydrogen peroxide (H2O2) was placed into the sample tube and 0.2 mL of test tube was adding. The combination was shaken quickly and absorbance was read at 240 nm in Hitachi U-290010.
Activity = (2.3 / ∆x) x [(log A1 / log A2)];
Activity; Calculated as in U / L.
Δx = 30 seconds
2.3 = 1 μmol optical density of H2O2 in 1 cm light path
Determination of Cherry Fruit Superoxide Dismutase
SOD activity was determined by using the proposed method of 11. SOD accelerates the dismutation of hydrogen peroxide and molecular oxygen of superoxide radicals (O2•) formed during the oxidative energy production. This method is based on the reading of optic density resulted from using of xanthine and xanthine oxidase in which superoxide radicals that generated from the blue colored formazan dye of the nitro blue tetrazolium (N.B.T) in the optical density wavelength of 560 nm. The SOD that exists in the sample serum inhibits the formazan reaction by excluding superoxide radicals from the environment. Under the experimental conditions, 1 unit of SOD is the %50 inhibition of N.B.T reduction rate11.
% inhibition = (blank OD – sample OD) /blank OD ×100
Statistical analysis
Descriptive statistics for the features discussed; Mean, standard deviation, minimum and maximum values are expressed. One Way Analysis of Variance was used for normal distribution conditions and Kruskal Wallis test statistic was used for cases where normal distribution condition was not provided. Statistical significance level was taken as 5% in the calculations and SPSS statistical package program was used for the calculations.
Results
Descriptive statistics and comparison results for CAT and SOD are given in Table 1. When Table 1 was examined, the difference between the averages in terms of CAT and SOD levels was found to be statistically significant for cherry fruit grown in Marmara, Aegean and Mediterranean regions (p <0.05). CAT levels were 2,643 for the cherry fruit grown in Marmara region; 4,330 in the Aegean cherry fruit and 4,077 in the Mediterranean region. SOD level was found to be 3,045 for the cherry fruit grown in Marmara region, 5,215 for the cherry fruit grown in the Aegean region, and 7,176 in the cherry fruit grown in the Mediterranean region (Graph 1).
Table 1: Descriptive statistics and comparison results of catalase and superoxide dismutase enzyme activity of cherry fruit.
| n | Mean ± standard deviation | Minimum | Maximum | p | ||
| CAT (U/L) | Marmara Region | 10 | 2.643±0.074 | 2.55 | 2.74 | 0.001 |
| Aegean Region | 10 | 4.330±0.235 | 3.94 | 4.65 | ||
| The Mediterranean Region | 10 | 4.077±0.134 | 3.88 | 4.28 | ||
| SOD (U/L) | Marmara Region | 10 | 3.045±0.047 | 2.96 | 3.09 | 0.001 |
| Aegean Region | 10 | 5.215±0.185 | 5.05 | 5.54 | ||
| The Mediterranean Region | 10 | 7.176±0.095 | 7.05 | 7.33 | ||
n=10, sample number , ± standard deviation, p≤0.001, meaningfulness
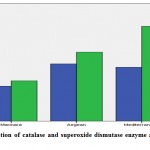 |
Figure 1: Distribution of catalase and superoxide dismutase enzyme activities of cherry fruit. |
Discussıon
In our study, superoxide dismutase (SOD) and catalase enzyme (CAT) activities with strong antioxidant properties were determined in cherry fruits obtained from different regions such as Aegean, Mediterranean and Marmara. Cherry is a fruit with strong antioxidant properties. Moreover, cherry is becoming an increasingly popular fruit because of its bioactive compound with antioxidant properties, including polyphenols, vitamins, anthocyanins and carotenoids12-14. Studies with cherry fruit have shown that consumption of antioxidant cherry fruit contributes to the healing of many diseases including cardiovascular, diabetes and inflammatory diseases15.
The catalase enzyme is a powerful antioxidant enzyme that catalyzes the decomposition of hydrogen peroxide into water and oxygen, thereby reducing the harmful effects caused by free radicals16. At the same time, superoxide dismutase enzyme plays an important role in protecting cells against many diseases such as cancer, heart attack caused by superoxide radicals 17.In our study, catalase enzyme and superoxide dismutase enzyme activities of cherry fruit in Marmara, Aegean and Mediterranean regions were determined. Descriptive statistics and comparison results for catalase and superoxide dismutase enzyme are given in Table 1.
Accordingly, catalase enzyme activity values were calculated as 2.643 U/L in cherry fruit grown in Marmara region, 4.330 U/L in cherry fruit grown in Aegean region and 4.077 U/L in cherry fruit grown in Mediterranean region. In addition, superoxide dimutase enzyme activity in cherry fruits was found to be 3.045 U/L in the Marmara region, 5.215 U/L in the Aegean region and 7.176 U/L in the Mediterranean region.
In a study, antioxidant enzyme activities of catalase and superoxide dismutase were determined in edible fruits in Odisha region. Antioxidant catalase enzyme activities in these edible fruits were calculated as 4.2×104, 4.08×104, 3.77×104 μmol. Superoxide dismutase enzyme activities in edible fruits were found to be 2.66 and 2.15 U/L18. In another study, antioxidant activities of cherry fruit were determined. Antioxidant activities were calculated as 1.145 and 1.916 U/L19. It was found that the activity values obtained were compatible with other studies.
Conclusıon
As a result, antioxidant enzyme activities in cherry fruit taken from three different regions were compared statistically. Antioxidant catalase enzyme activities in Aegean, Mediterranean and Marmara regions were found to be 4.330, 4.077 and 2.643 U/L respectively, while superoxide dismutase enzyme activities were found to be 5.215, 7.176 and 3.045 U/L, respectively, These results showed that antioxidant enzyme activities of cherry fruit were different in different regions.
References
- Habib M., Bhat B. N., Dar, A. A. WaniSweet cherries from farm to table: a review. Rev. Food Technol. 2015; 57: 1638-1649.
- Bernalte M. J., Sabio E., Herna´ndez M.T., Gervasini C. Influence of storage delay on quality of “Van” sweet cherry. Biol. Technol. 2003; 28: 303- 312.
- Esti M., Cinquante L., Sinesio F., Moneta E., Matteo M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002; 76: 399-405.
- Serrano M., Guillen F., Romero D. M., Castillo S., Valero D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. Agric. Food Chem. 2005; 53: 2741-2745.
- Mozetic B., Trebse P., Hribar J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica Region (Slovenia). Food Technol. Biotechnol. 2002; 40: 207-212.
- Chaovanalikit A., Wrolstad R. E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. Food Sci. 2001; 69: 67- 72.
- Chandra A., Rana J., Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. Agric. Food Chem. 2001; 49: 3515-3521.
- Kim D. O., Heo H. J., Kim Y. J., Yang H. S., Lee C. Y. Sweet and sour cherries phenolics and their protective effects on neuronal cells. Agric. Food Chem. 2005; 53: 9921-9927.
- Wang L. S., Stoner G. D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008; 269: 281-290.\
- Aebi H. Catalase. In methods of enzymatic analysis. Academic press. 1974; 673-684.
- Popov B., Gadjeva V., Valkanov P., Popova S., Tolekova A. Lipid peroxidation, superoxide dismutase and catalase activities in brain tumor tissues. Archives of physiology and biochemistry. 2003; 111(5): 455-459.
- Gonçalves B., Landbo A. K., Knudsen D., Silva A. P., Pereira J. M., Rosa E., Meyer A. S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J Agr. Food Chem. 2004; 52: 523.
- Serradilla M. J., Martín A., Ruizmoyano S., Hernández A., Lópezcorrales M., Mdg C. Physicochemical and Sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012;133: 1551-1559.
- Usenik V., Fabcic J., Stampar F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008; 107: 185-192.
- Mccune L. M., Kubota C., Stendellhollis N. R., Thomson C. A. Cherries and health: a review. Rev. Food Sci. Nutr. 2011;51: 1-12.
- Olson K. R., Straub K. D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. 2016; 31: 60–72.
- Castro A. J., Alche J. D. D. Identification of novel superoxide dismutase isoenzymes in the olive (Olea europaea L.) pollen. BMC Plant Biol. 2018; 18: 114.
- Patnaik M., Basak U. C. Enzymatic antioxidant activities in eight wild edible fruits of Odisha. Tropical Plant Research. 2014; 1: 36–42.
- Ferretti G., Bacchetti T., Belleggia A., Eri D. Cherry Antioxidants: From Farm to Table. 2010; 15: 6993-7005.

This work is licensed under a Creative Commons Attribution 4.0 International License.





