How to Cite | Publication History | PlumX Article Matrix
Madhubanti Chaudhuri1 , A.K. Paul1 and Arundhati Pal2*
, A.K. Paul1 and Arundhati Pal2*
1Microbiology Laboratory, Department of Botany, University of Calcutta 35, Ballygunge Circular Road, Kolkata 700 019
2Department of Botany, Serampore College, William Carey Road, Serampore, Hooghly, West Bengal 712201
Corresponding Author E-mail: arundhatipalcu@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2789
ABSTRACT: Carnivorous plants with unique mode of nutrition and physiology have attracted the attention of the microbiologists in studying the microbial diversity inherent in their internal environment. This work is aimed to study the culturable endophytic diversity of the carnivorous plants Drosera burmannii Vahl., Utricularia stellaris L. f. and U. exoleta R. Br. collected from different districts of West Bengal, India. During the study, a total of 168 phenotypically distinct endophytic bacteria were isolated and their colonization frequency, isolation rate, Shanon-Weaver, Gleason and Simpson diversity indices were analyzed. The metabolic activities of these endophytic isolates have been evaluated following standard microbiological methods. A preliminary screening have led to the selection of nineteen bacterial isolates having potent antimicrobial, antioxidant, proteolytic and plant growth promoting activities involving IAA and siderophore production as well as phosphate solubilization. Detailed phenotypic characterization followed by the determination of simple matching coefficient has tentatively assigned these potent endophytic bacterial isolates to the genera Bacillus, Acetobacterium, Pseudomonas, Klebsiella, Alcaligens and Xanthomonas. The metabolic attributes of these bacterial endophytes leading to the production of bioactive compounds therefore deserve special attention in understanding the survival and growth strategies of the carnivorous hosts in nutrient deficient environment as well as exploring their biosynthetic products in human health and hygiene.
KEYWORDS: Antioxidants; Antimicrobial Activity; Bacterial Endophytes; Carnivorous Plants; Drosera Burmannii; Plant Growth Promotion; Proteolytic Activity; U. Exoleta; Utricularia Stellaris.
Download this article as:| Copy the following to cite this article: Chaudhuri M, Paul A. K, Pal A. Isolation and Assessment of Metabolic Potentials of Bacteria Endophytic to Carnivorous Plants Drosera Burmannii and Utricularia Spp. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Chaudhuri M, Paul A. K, Pal A, Isolation and Assessment of Metabolic Potentials of Bacteria Endophytic to Carnivorous Plants Drosera Burmannii and Utricularia Spp. Biosci Biotech Res Asia 2019;16(4). Available from: https://bit.ly/2uxHEzs |
Introduction
Carnivorous plants characterized by a cluster of morphological and physio-biochemical features commonly known as “carnivorous syndrome” have evolved independently during evolution and are distributed in five orders and ten families of angiosperms (1, 2). These unique features enable the photoautotrophic plants in attracting, capturing and digesting the prey for acquisition of nitrogen, phosphorus, potassium, magnesium and sulphur from the insects by secreting a plethora of hydrolytic enzymes. In many of them the digestion of prey is aided by the enzymes of bacteria and/or fungi (1, 3) while others are entirely dependent on the enzymes secreted by the plant-associated microbiota (4, 5).
Preliminary analysis by Lindquist (1975) for the first time documented the occurrence of proteolytic, chitinolytic and pectin hydrolyzing bacterial species in the pitcher fluid of Sarracenia purpurea. Bacterial and fungal communities present in the trap fluid of Byblis, Brocchinia, Darlingtonia and Heliamphora also played the key role in prey degradation in complete absence of plant enzymes (5, 7). In Utricularia foliosa, U. purpurea and U. vulgaris bacteria constituted nearly 58% of the trap microbial biomass and secreted extracellular phosphatases which benefit the rootless aquatic plant with acquisition of phosphate instead of nitrogen (8).
Bacterial diversity comprising firmicutes (46.8%), proteobacteria (33.9%), acidobacteria, actinobacteria, bacteriodetes, chloroflexi, cyanobacteria, chlamydiae, and tenericutes were observed in the traps of U. hydrocarpa and Genlesia filiformis (9). Recent whole-genome shotgun metagenomics approach (10) has unraveled the taxonomic and functional diversity of the trap microbiome in U. gibba and suggested that trap bacteria play a significant role both in nutrient scavenging and assimilation. While Glen and Bodri (6) have isolated bacterial and fungal endophytes from glandular and digestion/absorption zone of the pitcher in Sarracenia orephila, endophytes of stem and leaf tissues of Nepenthes spp. were predominated by Bacillus (59.4%) followed by beta- and gamma-proteobacteria (11).
The endophytic microcosm within carnivorous hosts has been speculated to produce a large number of bioactive metabolites which not only contribute to nutrient utilization of their host but also ensure protection and survival against pathogen infections (12, 13). Assemblage of diazotrophic endophytes showing the presence of nif H gene were recorded from root and leaf segments of Drosera villosa var. villosa growing on oligotrophic habitat (14). Likewise, root endophytic actinobacterial and pseudomonad populations from D. latifolia showed amylolytic, cellulolytic, proteolytic and nitrogen fixing potentials (15). Endophytic Bacillus spp. of U. brevicapsa in particular were found to be inhibitory to several phytopathogenic fungi like Alternaria, Fusarium, Phytophthora, Rhizoctonia, and Cryphonectria (16).
In India, although only five genera of carnivorous plants have been reported from different phytogeographical regions, majority of the widely available species belonged to Drosera and Utricularia as against the monogeneric Aldrovanda, Pinguicula and Nepenthes (17). However till date, little attention has been paid to unveil the microbial association of Indian carnivorous species. Only recently, Naseem and Kayang (18) have highlighted the diversity of endophytic fungi of Nepenthes khasiana, endemic to eastern Himalayan zone. In this study we made an attempt to elucidate the bacterial endophytic colonization in Drosera burmanii Vahl, Utricularia exoleta R. Br. and U. stellaris L. f. which commonly grow in West Bengal and also to evaluate their metabolic potentials for biotechnological exploitation.
Materials and methods
Collection of plant samples
Carnivorous plants Drosera burmannii Vahl., Utricularia stellaris L. f. and U. exoleta R. Br. used in this study were collected from the districts of East and West Medinipur, Birbhum and North 24 parganas of West Bengal, India in sterile zip-lock polythene bags and immediately brought to the laboratory, stored at 4°C until used for microbial analysis. The taxonomic identity of plants were duly authenticated and deposited to Calcutta University Herbarium (CUH) with proper accession numbers.
Isolation of endophytic bacteria
Endophytic bacteria were isolated from different organs of D. burmannii, U. stellaris and U. exoleta following the modified surface sterilization method of Panchal and Ingle (19). The plant organs were washed thoroughly with tap water, surface sterilized in 70% ethanol (2 mins) and 0.05% sodium hypochlorite (1 min), washed thoroughly in sterile distilled water, aseptically cut into small segments and placed on Nutrient agar, Tryptic Soy agar and Lindenbein Synthetic agar plates. Plates were incubated at 32°C for 2-4 days and observed for growth of bacterial colonies around the plant segments. Colonization frequency of the bacterial endophytes was calculated as the total number of plant segments yielding the bacteria divided by the total number of segments incubated. Isolation rate was determined as the number of bacterial isolates obtained from the plant samples divided by the total number of samples incubated. The Shannon-Weaver diversity index was calculated as H= -Σ Pi ln Pi where Pi is the species abundance. Simpson diversity index was calculated as D= 1- [ Ʃ n(n-1)/ N(N-1)] where n is the species abundance and N is the total number of organisms of all species. Gleason diversity index was calculated as K= Np-1/ ln Ni where Np is the number of species identified among isolates and Ni is the total number of individuals. Pure cultures of bacterial endophytes were developed following dilution-streaking on the same agar media and maintained on nutrient agar slants by regular sub-culturing.
Assessment of Metabolic Potentials
Antimicrobial activity: Antimicrobial activity of the endophytic bacterial isolates were determined following the cross-streak method (20) using Bacillus subtilis MTCC 441, Staphylococcus aureus MTCC 2943, Escherichia coli MTCC 1687, Pseudomonas cepacia MTCC 4684, Penicillium citrinum MTCC 1256, Aspergillus niger MTCC 281and Saccharomyces cerevisiae MTCC 170 as test organisms. The endophytic isolates were allowed to grow in form of a single streak in the center of the tryptic soy agar plates and incubated at 32°C for 2-3 days. The test organisms were then inoculated in form of narrow streaks at right angles to the growth of the endophyte and incubated further at 32°C for 24-48 h. Length of inhibition of growth of test organisms were recorded in nearest millimeter to express their sensitivity.
Antioxidant activity: Antioxidant activity of the endophytic bacteria was determined by % DPPH (2,2-diphenyl picrylhydrazyl) scavenging following the modified method of Liu et al. (21). The bacterial isolates were grown in tryptic soy broth for 48 h and the cell mass was harvested by centrifugation at 10,000 rpm for 10 min. The washed cell pellets were extracted in methanol (5 ml) for 24 h under continuous shaking and centrifuged to separate the supernatant. Freshly prepared methanolic extract (0.5 ml) was mixed with 0.2 ml of 0.4 mM DPPH and 2.5 ml distilled water and incubated for 30 min at room temperature. Optical density was measured at 517 nm and the percentage of free radical scavenging was calculated as follows:
% scavenging activity = {1- (A1-A2/A0)} X 100
Where, A1 = O.D. of reaction mixture
A2 = O.D. of reaction mixture without DPPH
A0 = O.D. of reaction mixture with DPPH but without sample
Proteolytic activity: Proteolytic activity of the endophytes was determined by growing the isolates in form of streaks on nutrient agar supplemented with 1% (w/v) casein for 48 h at 32°C. The plates were then flooded with protein precipitating reagent and observed for the formation of clear zone surrounding the growth (22). Protease index was expressed as the ratio of the width of the clear zone including growth to that of the width of the bacterial growth only.
Plant growth promoting traits: Plant growth promoting traits of the endophytes including indole acetic acid (IAA) production, phosphate solubilization and siderophore production were evaluated following standard methods. Indole-3-acetic acid (IAA) production was assessed following Salkowski colorimetric assay (23). Isolates were grown in tryptophan broth at 32º C for 5 days and to 1 ml of the cell-free culture filtrate obtained by centrifugation (10,000xg for 10 min), 3 ml of Salkowski reagent and 2 ml of distilled water were mixed and incubated for 30 min in the dark. Optical density of the pink colour developed was measured at 540 nm and the amount of IAA produced was quantified from the standard curve prepared in the same way. Ability of endophytes to solubilise insoluble phosphate was determined in Pikovskya’s agar medium (24). The inoculated plates were incubated at 32º C for 5-7 days and observed for development of clear zone surrounding the bacterial growth. Production of siderophore was tested following the modified protocol of Schwyn and Neilands (25). Isolates were grown in succinate agar medium at 32º C for 96 h, flooded with Fe-CAS indicator solution and observed for the change of colour from blue to orange around the bacterial growth.
Characterization and taxonomic considerations of the endophytic bacteria
The morphological and physio-biochemical characters were determined following standard microbiological methods (22). Antibiotic sensitivity of the endophyte was determined following the Kirby Bauer disc-diffusion method (26) using commercially available antibiotic impregnated discs (HiMedia 6 mm dia.). The characters were compared with the standard descriptions in Bergey’s Manual of Determinative Bacteriology (27) and tentative generic identity of the isolates was ascertained.
Dendogram of the endophytic bacterial isolates were prepared based on the analysis of a set of phenotypic characters of each of the isolates. The clustering was done using sequential, agglomerative, hierarchial and nested method (SAHN). Different and pairwise coefficient of similarity (Dice) was used for clustering with the algorithm using the software NTSYSPc version 2.11f (28). The similarity indices were expressed as simple matching coefficient calculated as the ratio of number of similar characters between two bacterial isolates (both positive and negative matches) and the total number of characters studied.
Statistical analysis
The results are expressed as mean of triplicate readings ± standard deviation. One way analysis of variance (ANOVA) was implemented for evaluating the interactions among different metabolic activities of the endophytic isolates. Tukey’s adjustment was used to adjust the p values (p <0.05) for multiple comparisons. All data management and analysis were done using Microsoft Excel and SPSS software version 19.
Results and Discussion
Diversity of the endophytic bacteria
Studies associated with the culturable diversity of endophytic microorganisms of carnivorous plants Drosera and Utricularia species have reported the occurrence of nitrogen-fixing bacteria (14), heterotrophic bacteria, actinobacteria and fluorescent pseudomonads from roots and leaves as well as endorhizosphere as a whole (15). Analysis of the trap-associated microorganisms of Utricularia spp. revealed that >50% of their microbial content is comprised of bacteria (8) and are dominated by members of Aeromonas and Acidomonas spp. (9). More recently, Lima et al. (16) have established that stolon and trap-associated bacterial diversity of U. breviscapa collected from different geographical locations of Brazil are colonized by Acidobacteria, Sphingomonas, Bacillus, Aquitalea, Pelomonas and Microbacterium many of which are already reported to be beneficial to host plants (29).
Drosera burmannii Vahl. (CUH 20029), Utricularia stellaris L. f. (CUH 20030) and U. exoleta R. Br. (CUH 20035) collected from the districts of East and West Medinipur, Birbhum and North 24 parganas of West Bengal, India were analyzed microbiologically for their endophytic bacterial population. Surface sterilized segments of these plants yielded phenotypically distinguishable bacterial colonies surrounding them after 48-96 h of incubation at 32°C on nutrient agar, tryptic soy agar and Lindenbein synthetic agar media. A total of 37, 68 and 63 endophytic bacteria were isolated from D. burmannii, U. stellaris and U. exoleta respectively. Colonization frequency (%) of the endophytic isolates in different plant organs ranged from 12.5 to 95.38, the highest being represented in leaves of U. stellaris collected from East Medinipur. Isolation rate was very poor (0.09) in the flower of D. burmannii from Birbhum while the reverse was true for flower (0.58) of U. stellaris collected from north 24 parganas (Table 1).
Diversity indices of the endophytic bacteria isolated from different plant parts showed wide degree of variations. While both Shanon-Weaver (2.99) and Gleason (5.69) diversity indices were highest in the leaf segments of U. stellaris, Simpson diversity index was maximum in stem and flower segments of D. burmannii (0.97) (Fig. 1). Such variations of endophyte population in terms of colonization frequencies, isolation rates and diversity indices however, have not been correlated with their geological locations, seasonal variations and environmental conditions. Culturable fungal endophytes demonstrated a colonization frequency of 45.2% in roots of Drosera rotundifolia which also illustrated seasonal differences reflecting endophytic life cycle or developmental stage of the host plant (30).
Table 1: Isolation of bacteria endophytic to different parts of selected carnivorous plants collected from the districts of East and West Medinipur, Birbhum and North 24 parganas, West Bengal
| Plant (CUH accession no.) | Locality | Parts used | Number of segments incubated | Number of segments yielding endophytes | Number of bacteria isolated | Colonization frequency (%) | Isolation rate |
| Drosera burmannii (20029) | West Medinipur | Leaf | 65 | 32 | 08 | 49.23 | 0.12 |
| Stem | 07 | 02 | 02 | 28.57 | 0.28 | ||
| Root | 04 | 02 | 01 | 50.00 | 0.50 | ||
| Flower | 08 | 01 | 01 | 12.50 | 0.12 | ||
| Total | 84 | 37 | 12 | 44.04 | 0.14 | ||
| Birbhum | Leaf | 47 | 40 | 11 | 85.11 | 0.23 | |
| Stem | 33 | 07 | 02 | 21.21 | 0.06 | ||
| Root | 40 | 14 | 08 | 35.00 | 0.20 | ||
| Flower | 43 | 27 | 04 | 62.79 | 0.09 | ||
| Total | 163 | 88 | 25 | 53.99 | 0.15 | ||
| Utricularia stellaris (20030) | East Medinipur | Leaf | 65 | 62 | 12 | 95.38 | 0.18 |
| Stem | 17 | 15 | 07 | 88.23 | 0.41 | ||
| Bladder | 37 | 32 | 09 | 86.49 | 0.24 | ||
| Total | 119 | 109 | 28 | 91.60 | 0.23 | ||
| North 24pgns | Leaf | 52 | 41 | 13 | 78.85 | 0.25 | |
| Stem | 32 | 29 | 10 | 90.62 | 0.31 | ||
| Bladder | 38 | 30 | 10 | 78.95 | 0.26 | ||
| Flower | 12 | 10 | 07 | 83.33 | 0.58 | ||
| Total | 134 | 110 | 40 | 82.09 | 0.30 | ||
| Utricularia exoleta (20035) | East Medinipur | Leaf | 58 | 48 | 14 | 82.76 | 0.24 |
| Bladder | 32 | 25 | 08 | 78.12 | 0.25 | ||
| Stem | 67 | 56 | 09 | 83.58 | 0.13 | ||
| Flower | 52 | 46 | 05 | 88.46 | 0.10 | ||
| Total | 209 | 175 | 36 | 83.73 | 0.17 | ||
| West Medinipur | Leaf | 40 | 32 | 07 | 80.00 | 0.17 | |
| Bladder | 42 | 35 | 09 | 83.33 | 0.21 | ||
| Stem | 35 | 25 | 06 | 71.43 | 0.17 | ||
| Flower | 23 | 18 | 05 | 78.26 | 0.21 | ||
| Total | 140 | 110 | 27 | 78.57 | 0.19 | ||
| Total | 849 | 639 | 168 | 75.26 | 0.20 |
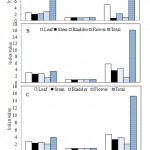 |
Figure 1: Diversity indices of endophytic bacteria isolated from different parts of (A) D. Burmannii (B) U. Stellaris and (C) U. Exoleta |
Screening of metabolic potentials of bacterial endophytes
Pure cultures of all 168 endophytic isolates were screened for their metabolic potentials such as antimicrobial, proteolytic, antioxidant and plant growth promoting activities including production of IAA, siderophore and phosphate solubilisation. While there is a worldwide thrust in exploring the functional metabolites of the endophytes (11, 12, 31-33), the present study reports on the primary screening of selected metabolic attributes (Table 2) of the endophytes isolated from D. burmannii, U. stellaris and U. exoleta collected from different locations of West Bengal.
Antimicrobial activity: The antimicrobial activity of the isolated endophytic bacteria as determined by cross-streak method against the test bacterial (04) and fungal (03) strains revealed that 72.62 % of 168 endophytes were unable to show any antibiosis. The antimicrobially active isolates (46) however, showed variation in terms of the number of test organisms inhibited and the length of inhibition. Five isolates showing inhibition to more than 3 test strains and with a length of inhibition zone >6.5 mm were considered as potent (Table 2, 3) and they include DF03 and DL06 from D. burmannii, UL10 and US02 from U. stellaris and UEF01 representing U. exoleta. Among these, isolates DL06 and UEF01 appeared to be the most effective as they inhibited S. aureus, B. subtilis, E. coli, A. niger and S. cerevisiae with the lengths of inhibition ranging from 7.0 to 16 mm (Table 2). None of the endophytes however, were inhibitory to Pseudomonas cepacia and Penicillium citrinum.
Antioxidant activity: Endophytic metabolites are potent sources of natural antioxidants (34-36). Freshly prepared methanolic extract of the bacterial biomass was tested for DPPH scavenging activities. Nearly 70% of the endophytic isolates showed antioxidant activity. Only 3 isolates, one from D. burmannii (DF01) and two from U. stellaris (US01 and US04) showed 65% scavenging of DPPH free radicals and were selected as the potent isolates (Table 2 and 3).
Proteolytic activity: The proteolytic activity of the endophytic bacteria was tested on casein supplemented nutrient agar medium and the enzymatic index was measured as the ratio of width of growth of the bacterial isolate including the clear zone to width of the growth. Majority of the isolates (75.6%) showed proteolytic activity with enzymatic index ranging from 1.0-6.0. The endophytic isolates (DR04, DS01, UB02 and UL04) with high enzymatic indices (4.1-6) (Table 2) were in conformity with the production of hydrolytic enzymes by endophytic fungi from Nepenthes spp. (33). The proteolytic property of the endophytes might contribute to the digestion of insect prey by the host plant (16). Four potent proteolytic isolates were derived from D. burmannii (DR04, DS01) and U. stellaris (UB02, UL04) (Table 3).
Plant growth promoting traits: Promotion of plant growth by endophytes was assessed by screening them for production of IAA and siderophore as well as solubilization of phosphate. More than 50% of the endophytic isolates produced IAA in tryptophan broth with an amount ranging from 500-1000 µg/ml after 5 days of incubation. The endophytes UL05, UEB08 and UEF04 were exceptional in producing 867, 1015 and 965 µg/ml of IAA respectively (Table 2 and 3). Phosphate solubilization in Pikovskya medium after incubation at 32°C for 5-7 days was measured as the ratio of width of growth of the bacterial isolate including the clear zone to width of the growth. More than 65% of 168 isolates could not solubilize the tricalcium phosphate in the medium. The isolates DR07, DR09, UEB08, and UEL12 were identified as the potent phosphate solubilizers showing an index value ranging from 1.75-2 (Table 2 and 3). Siderophore producing ability of the endophytes was assessed by Schwyn and Neilands method (25) and it was revealed 72.02% of the isolates demonstrated negative response. The potent siderophore producing isolates UL10, UEL12 and DF04 showed index values >1.54 (Table 2). Association of these potent endophytic IAA (UL05, UEB08 and UEF04) and siderophore producers (DF04, UL10, UEL12) and phosphate solubilizers (DR07, DR09, UEB08, UEL12) (Table 3) with carnivorous plants might contribute to the growth and survival of the carnivorous plants in nutrient deficient environment, the usual habitat of these plants (8, 12).
Selection of Potent Endophytes
Based on the overall primary screening of metabolic attributes, a total of 22 endophytic bacterial isolates appeared to be potent in terms of antimicrobial (05), antioxidant (03), proteolytic (04), IAA synthesis (03), phosphate solubilisation (04) and siderophore (03) producing activities (Table 3). Further, it was revealed that the isolates UL10 and UEB08 were common among the potent antimicrobial and siderophore producing isolates and for IAA producing and phosphate solubilizing isolates respectively. Similarly, isolate UEL12 was common for selected phosphate solubilizers and siderophore producers. Eliminating these three common isolates from among the six groups, all 19 primarily selected potent isolates were considered for determination of their taxonomic status.
Table 2: Metabolic diversity of selected endophytic bacteria isolated from carnivorous plants of West Bengal
| Host | Isolates | Antimicrobial activity
Length of inhibition zone (mm) against test isolates |
Antioxidant activitya | Protease productionb | Indole acetic acid production (µg/ml) | Phosphate solubilizationc | Siderophore productiond | ||||
| B. subtilis | S. aureus | E. coli | A. niger | S. cerevisiae | |||||||
| Drosera burmannii | DF01 | 5.5±0.5 | 5.5±0.76 | 6.0±1 | NI | NI | 65±3.2 | 0 | 90±3.2 | 0 | 0 |
| DF03 | 9.5±1.5 | 11.5±0.5 | 8.0±1 | NI | NI | 30±3.7 | 3.16±0.95 | 175±15 | 1.33±0.34 | 0 | |
| DF04 | NI | NI | NI | NI | NI | 18±2.6 | 2.62±0.61 | 775±15 | 1.12±0.33 | 1.55±0.4 | |
| DL06 | 8.0±2 | 6.5±0.5 | 10.0±2 | 6.5±0.5 | 7.0±1 | 60±2 | 2.14±0.44 | 32.5±2.6 | 0 | 1.2±0.13 | |
| DR04 | NI | NI | NI | NI | NI | 18±1.8 | 5.25±0.93 | 55±3 | 0 | 1.5±0.39 | |
| DR07 | NI | NI | NI | NI | NI | 12±1.8 | 3.57±0.54 | 0 | 1.76±0.13 | 1.5±0.48 | |
| DR09 | NI | NI | NI | NI | NI | 12±2.4 | 0 | 35±2.6 | 1.75±0.23 | 1.2±0.47 | |
| DS01 | NI | 14.0±1 | NI | 7.5±0.5 | NI | 50±4.7 | 4.4±1.4 | 32.5±2.3 | 1.33±0.21 | 1.4±0.29 | |
| Utricularia stellaris | UB02 | NI | NI | NI | NI | NI | 30±2.1 | 5.2±1.6 | 615±64 | 0 | 1.36±0.51 |
| UL04 | 6.0±1 | 5.5±0.5 | NI | 5.5±0.76 | NI | 30±3.5 | 4.6±1.38 | 525±53 | 0 | 1.29±0.47 | |
| UL05 | NI | NI | NI | NI | NI | 59±4.3 | 1.14±0.17 | 867±44 | 1.44±0.12 | 0 | |
| UL10 | NI | 7.0±1 | 6.5±0.5 | 11.7±1.3 | NI | 41±2.8 | 2.18±0.58 | 745±33 | 0 | 2.37±0.3 | |
| US01 | NI | NI | NI | NI | NI | 65±2.4 | 2.16±1.05 | 55±7 | 0 | 1.37±0.3 | |
| US02 | 6.5±0.5 | 10.0±2 | 6.5±0.5 | 8.0±1 | NI | 59±5.1 | 2.15±0.61 | 0 | 1.22±0.17 | 1.24±0.19 | |
| US04 | NI | NI | NI | NI | NI | 65±1.3 | 1.71±0.16 | 15±2.6 | 1.42±0.27 | 0 | |
| Utricularia exoleta | UEB08 | NI | NI | 6.0±1 | NI | NI | 47±1.6 | 1.5±0.37 | 965±33 | 2±0.34 | 0 |
| UEF01 | 16.0±2 | 15.0±2 | 10.0±2 | 10.5±1.5 | 10±1 | 47±1.1 | 2.33±0.46 | 25±2.7 | 0 | 1.2±0.25 | |
| UEF04 | NI | NI | NI | NI | NI | 30±2.7 | 0 | 1015±87 | 1.57±±0.32 | 0 | |
| UEL12 | NI | NI | NI | NI | NI | 47±2.1 | 1.12±0.33 | 775±37 | 1.75±0.36 | 1.54±0.25 | |
| F value
p< 0.05 |
102.18 | 142.90 | 75.08 | 165.16 | 211.83 | 120.97 | 13.59 | 423.19 | 44.99 | 18.13 | |
NI= no inhibition; aExpressed as the % DPPH scavenging activity; bValues represent the protease enzymatic index; cValues represent phosphate solubilization index; dValues represent siderophore production index
All values represent mean of triplicate experiments ± SD; p-values are adjusted for comparisons using Tukey’s method
Table 3: Summary of potent endophytic bacteria from carnivorous host plants showing antimicrobial, antioxidant, proteolytic and plant growth promoting activities
| Host plant | Total number of isolates tested | Number of potent endophytic bacteria showing specific activities | |||||
| Antimicrobial activity | Antioxidant activity | Proteolytic activity | Plant growth promoting activities | ||||
| Indole acetic acid production | Phosphate solubilisation | Siderophore production | |||||
| Drosera burmannii | 37 | 2 | 1 | 2 | 0 | 2 | 1 |
| Utricularia stellaris | 68 | 2 | 2 | 2 | 1 | 0 | 1 |
| Utricularia exoleta | 63 | 1 | 0 | 0 | 2 | 2 | 1 |
| Total | 168 | 5 | 3 | 4 | 3 | 4 | 3 |
| Isolates | DF03, DL06, UL10, US02, UEF01 | DF01, US01,
US04 |
DR04, DS01 UB02,
UL04 |
UL05, UEB08, UEF04 | DR07, DR09, UEB08, UEL12, | DF04,
UL10, UEL12 |
|
Characterization and taxonomic consideration of endophytes
All selected potent endophytic bacterial isolates (19) were analyzed for their morphological, physiological, biochemical features and antibiotic sensitivity. The individual isolates were compared based on a total of 91 phenotypic features and similarity was calculated according to simple matching coefficient. Similarity matrix (Fig. 2) and dendogram (Fig. 3) were constructed using the software NTSYSPc version 2.11f.
Majority (12) of the isolates (DF03, DF04, DL06, DR04, DR07, DR09, UB02, US01, US04, UEB08, UEF01 and UEL12) were Gram-positive, aerobic, endospore forming rods and belonged to the genus Bacillus as reported earlier from D. villosa var villosa (14) and U. breviscapa (16). The only Gram-positive non-sporing isolate DF01 was assigned to Acetobacterium. The remaining Gram-negative isolates were categorized as less diverse forms of Gram-positive and negative bacteria such as Xanthomonas (DS01 and US02), Pseudomonas (UL04 and UL10), Klebsiella (UEF04) and Alcaligenes (UL05) as a part of the endophyte microbial community of D. burmanii, U. stellaris and U. exoleta.
Conclusions
Survey of endophytic bacteria isolated from the carnivorous plants D. burmannii, U. stellaris and U. exoleta of West Bengal represented the predominance of the members of the genus Bacillus along with a variety of Gram-negative bacteria like Pseudomonas, Xanthomonas, Alcaligenes, Acetobacterium and Klebsiella. Metabolic potentials of these endophytes ranging from production of antimicrobials, antioxidants, proteolytic enzymes and plant growth promoting substances are no doubt fascinating. Exploitation of these metabolically potent endophytes may lead to large scale production of several bioactive compounds having novel activity. Moreover, these endophytes can serve as plant growth promoting bacteria (PGPB) for use as biofertilizers in agriculture and crop productivity. An in-depth study of these potential areas will unravel the unique host-endophyte relationship in carnivorous plants and facilitate their utilization in the field of agriculture, medicine and pharmaceutical biotechnology.
We express our sincere gratitude to Prof. G. G. Maiti, Department of Botany, University of Kalyani and Prof. N. D. Paria, Department of Botany, University of Calcutta for collection and taxonomic identification of the plant materials.
Funding source
Financial assistance to Madhubanti Chaudhuri from University Grants Commission, New Delhi, India (UGC/1261/RFSMS/Appointment; dated 24.11. 2014) is duly acknowledged.
Conflict of interest
Authors have declared that no conflict of interests exists.
References
- Ellison A. M and Gotelli N. J. Evolutionary ecology of carnivorous plants. Trends. Ecol. Evol., 2001; 16: 623-629.
- Krol E, Plachno B. J, Adamec L, Stolarz M, Dziubinska H and Trebacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Bot., 2011; 109 (1): 47-64.
- Ellison A. M and Gotelli N. J. Energetics and the evolution of carnivorous plants-Darwin’s ‘most wonderful plants in the world’. Expt. Bot., 2009; 60 (1): 19–42.
- Chase M. W, Christenhusz M. J. M, Sanders D and Fay M. F. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Linn. Soc. Bot., 2009; 161 (4): 329–356.
- Givnish T. J. New evidence on the origin of carnivorous plants. PNAS, 2015; 112 (1): 10–11
- Glenn A and Bodri M. S. Fungal endophyte diversity in PLOS One, 2012; 7 (3): e32980
- Adlassnig W, Peroutka M and Lendl T. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Ann. Bot., 2011; 107 (1): 181-194.
- Sirova D, Borovec J, Cerna B, Rejmankova E and Adamec L. Microbial community development in the traps of aquatic Utricularia Aquat. Bot., 2009; 90 (2): 129-36.
- Caravieri F. A, Ferreira A. J, Ferreira A, Clivati D, de Miranda V. F. O and Araujo W. L. Bacterial community associated with traps of the carnivorous plants Utricularia hydrocarpa and Genlisea filiformis. Bot., 2014; 116: 8-12.
- Alcaraz L. D, Martínez-Sánchez S, Torres I, Ibarra-Laclette E and Herrera-Estrella L. The metagenome of Utricularia gibba’s traps: into the microbial input to a carnivorous plant. PLOS One, 2016; 11:
- Bhore S. J, Komathi V and Kandasamy K. I. Diversity of endophytic bacteria in medicinally important Nepenthes J. Nat. Sc. Biol. Med., 2013; 4 (2): 431-434.
- Hardoim P. R, van Overbeek L. S and Elsas J. D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol., 2008; 16 (10): 463-471.
- Strobel G. A. Endophytes as sources of bioactive products. Microbes. Infec., 2003; 5 (6): 535-544.
- Albino U, Saridakis D. P, Ferreira M. C, Hungria M, Vimuesa P and Andrade G. High diversity of diazotrophic bacteria associated with the carnivorous plant Drosera villosa villosa growing in oligotrophic habitats in Brazil. Plant Soil, 2006; 287 (2): 199-207.
- Saridakis D. P, Nogueira M. A, Filho G. A and Galvao F. An appraisal on saprophytic and functional microbial communities associated to the carnivorous plant Drosera latifolia (Eichler) Gonella and Rivadavia (Droseraceae). Ciencias Biologicas e da Saude, 2014; 35 (1): 3-14.
- Lima F. R, Ferreira A. J, Menezes C. G, Miranda V. F. O, Dourado M. N and Araujo W. L. Cultivated bacterial diversity associated with the carnivorous plant Utricularia breviscapa (Lentibulariaceae) from floodplains in Brazil. Braz. J. Microbiol., 2018; 49: 714-722.
- Jayaram K and Prasad M. N. V. Drosera indica and D. burmanii Vahl., medicinally important insectivorous plants in Andhra Pradesh– regional threats and conservation. Curr. Sci., 2006; 91 (7): 943-946.
- Naseem F and Kayang H. Fungal endophytes associated with Nepenthes khasianaF., an endemic plant of Meghalaya, India. In.t J. Curr. Res. Life Sci., 2018; 7 (4): 1907-1912.
- Panchal H and Ingle S. Isolation and characterization of endophytes from the root of medicinal plant Chlorophytum borivilianum (Safed Musli). Adv. Dev. Res., 2011; 2 (2): 205-209.
- Velho-Pereira S and Kamat N. M. Antimicrobial screening of Actinobacteria using a modified cross-streak method. Ind. J. Pharm. Sci., 2011; 73 (2): 223-228.
- Liu R, Wanq M, Duan J, Guo J. M and Tang Y. P. Purification and identification of three novel antioxidant peptides from Cornu bubali (water buffalo horn). Peptides, 2010; 31(5): 5-11.
- Gerhardt P. Methods for General and Molecular Bacteriology, Washington D. C.: American Society for Microbiology, 1994.
- Miliute I and Buzaite O. IAA production and other plant growth promoting traits of endophytic bacteria from apple tree. Biologija, 2011; 57 (2): 98-102.
- Pikovskya R. I. Mobilization of phosphorous in soil connection with the vital activity of some microbial species. Microbiologiya, 1948; 17: 362- 370.
- Schwyn B and Neilands J. B. Universal chemical assay for the detection and determination of siderophore. Anal. Biochem., 1987; 160 (1): 47-56.
- Bauer A. W, Kirby W. M, Sherris J. C and Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol., 1966; 45 (4): 493.
- Buchanan R. E and Gibbons N. E. Bergey’s Manual of Determinative Bacteriology, Baltimore : Williams and Wilkins. 1975.
- Rohlf F. J. NTSYS-pc, Numerical taxonomy and multivariate analysis system, New York: Applied Biostatistics Inc. 2000.
- Lau H, Faryna J and Triplett E. W. Aquitalea magnusonii nov., sp. nov., a novel Gram-negative bacterium isolated from a humic lake. Int. J. Syst. Evol. Microbiol., 2006; 56 (4): 867–871.
- Quilliam R. S and Jones D. L. Fungal root endophytes of the carnivorous plant Drosera rotundifolia. Mycorrhiza, 2010; 20 (5): 341-348.
- Strobel G and Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev., 2003; 67 (4): 491-502.
- Manganyi M. C, Tchatchouanga C. K, Regnierb T, Bezuidenhoutc C. C and Ateba C. N. Bioactive compound produced by endophytic fungi isolated from Pelargonium sidoides against selected bacteria of clinical importance. Mycobiol., 2019; 47 (3): 335-339.
- Lee J. M, Tan W. S and Ting A. S. Y. Revealing the antimicrobial and enzymatic potentials of culturable fungal endophytes from tropical pitcher plants (Nepenthes). Mycosphere, 2014; 5 (2): 364-377.
- Strobel G. A, Ford E, Worapong J, Grant D. M and Fung P. C. Isopestacin, an isobenzofuranone from Pestaliopsis microspora possessing antifungal and antioxidant activities. Phytochemistry, 2002; 60 (2): 179-183.
- Liu X, Chen X, Lv X, Dong M, Jiang M and Yan G. Antioxidant activity and phenolics of an endophytic Xylaria from Ginkgo biloba. Food Chem., 2007; 105 (2): 548-554.
- Danagoudar A, Joshi C. G, Ravi S. K, Rohit Kumar H. G, Ramesh B. N. Antioxidant and cytotoxic potential of endophytic fungi isolated from medicinal plant Tragia involucrata Phcog. Res., 2018; 10 (2): 188-194.

This work is licensed under a Creative Commons Attribution 4.0 International License.







