How to Cite | Publication History | PlumX Article Matrix
Rajmahammad Rasul Tamboli
Department of Microbiology, Maharashtra Udayagiri Mahavidyalaya, Udgir- 413517 India
Corresponding author E-mail: raju_tamboli2005@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2796
ABSTRACT: Rhizosphere soil samples from Wheat crop were collected from the 10 different locations in Latur district of Marathwada region with the objective to isolate the zinc solubilizing bacteria, their screening and characterization. Zinc carbonate was used as insoluble zinc source. Out of 10 Zn solubilizers, 3 most outstanding isolates were maintained for further screening for mineral solubilization (Zn and K). Among these RRT19, RRT34 and RRT13 which was identified as Bacillus cereus and Bacillus subtilis by morphological and biochemical test. These isolates showed maximum zone of solubilization with 34, 31 to 30 mm on liquid salt agar medium after 48 hrs of incubation respectively. The bacterial species isolated from the Rhizosphere soil can be use in soils that are deficient in Zinc or where insoluble zinc is abundant. The present study concluded that the use of zinc solubilising bacteria in the zinc deficient soil will help to enhance the growth and yield of wheat crop.
KEYWORDS: Bacillus Cereus; Bacillus Subtilis; Wheat; Zinc Solubilizing Bacteria
Download this article as:| Copy the following to cite this article: Tamboli R. R. Isolation and Characterization of Zinc Solubilizing Bacteria from Rhizosphere Soil of Latur District, Marathwada, India . Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Tamboli R. R. Isolation and Characterization of Zinc Solubilizing Bacteria from Rhizosphere Soil of Latur District, Marathwada, India . Biosci Biotech Res Asia 2019;16(4). Available from: https://bit.ly/2T6pwqw |
Introduction
Zinc is an important mineral and essential for plants, animals, and man (Kabata-Pendias, 2000). It is plays and important role as a component of enzymes that drive and increase the rate of many important metabolic reactions involved in crop growth and yield of the crop. It gives best effect on basic plant life and involved in the nitrogen metabolism and uptake of nitrogen and protein quality; photosynthesis and chlorophyll synthesis etc. (Potarzycki and Grzebisz, 2009). The proper use of zinc in soil for the growth of plant is adversely affected the crop yields and growth development of various plants. So, for proper growth and development of crop plants ae well as for increasing the yielding capacity of crop a supply of certain minimum level of Zn is essential. (Saeed and Fox, 1977).
In India, the deficiency of zinc is increased because of intensive cultivation, imbalanced nutrient application and use of organic manure without zinc dispensing, others reasons are soil with imbalanced pH, calcareous and low organic matter content (Behera et al., 2011). Now a days the use of microorganisms can overcome these problems (Anthoni Raj, 2002). Microorganisms can play a important role in the solubilization, transport and deposition of metals and minerals in the environment. Thus, microorganisms play a major role in the transformation of unavailable form of metal to available form depending upon the reactions involved and the products formed (Lovely, 1991). Several researchers have also reported about the solubilization of insoluble Zn compounds by bacteria (Di simine et al., 1998 and Fasim et al., 2002). The complex zinc can be converted into available form by applying a microorganism having ability of solubilizing the insoluble zinc (Saravanan et al., 2003). Among these microorganisms, a group of soil bacteria referred to as plant growth promoting Rhizobacteria (PGPR) are having their role in nutrient cycling and therefore deserve special attention for using the same as bio inoculants in agriculture (Weller and Thomashow, 1993; Glick, 1995). In this context, the uses of beneficial rhizosphere microorganisms to make insoluble zinc into soluble form for crop assimilation and achieve the objective of low-input is highly essential for sustainable agriculture (Hughes et al., 1989). Zinc solubilizing ability of some bacterial genera has been already studied by Hutchins et al., (1986). The present study deals on zinc solubilization by rhizosphere bacteria which has great importance in growth of the plants as well as increasing the yield capacity.
Materials and Methods
The present study was taken up to isolate potential zinc solubilizing bacteria (ZSB) from wheat rhizosphere. The screened efficient ZSB isolates were subjected to biochemical and cultural characterization. Laboratory studies were done to find out effective zinc solubilizing bacteria from selected bacterial isolates.
Collection of soil samples
A total of 10 rhizosphere soil samples were collected from wheat rhizosphere from 10 locations of Latur District. After collection, a portion of each sample was immediately transferred to laboratory and stored at 40C for microbial analysis while as the rest part of soil samples was shade dried and powdered and stored physical and chemical parameters.
Isolation of Zinc Solubilizing Bacteria From Soil Samples
Serial dilution pour plate technique was used for isolation of zinc solubilizing bacteria and 1gm of rhizosphere soil from each sample was used for serial dilution. The samples were serially diluted upto 10-5 dilution factor. The modified Pikovaskya’s agar media (Bapiri et al., 2012). Sterilized medium was poured in to sterilized petri plates under aseptic conditions after solidification of medium 0.5 ml of diluted sample suspension from 10-5 dilution was poured on these plates which were incubated at 28± 20C for 72 hours in BOD incubator. A total of 38 isolates showed zinc solubilization. Pure cultures of these isolates were obtained by repeated streaking and were preserved for further studies (Bunt and Rovira, 1955).
Characterization of Isolated Zinc Solubilizing Bacteria
Preliminary characterization of some outstanding zinc solubilizers was performed on the basis of colony features, morphological characteristics and biochemical tests like Gram’s staining, Hydrogen sulphide test, catalase test, starch hydrolysis test, methyl red test, urease test, Voges-Proskauer test, casein hydrolysis test, gelatine liquification, growth at 7% NaCl, citrate utilization. The morphological and biochemical characterization the selected isolates were identified according to the Bergey’s Manual of Systematic Bacteriology
Screening of Isolates for Zinc Solubilization by Plate Assay
A loop full of bacterial culture of isolates were diluted in sterile distilled water using serial dilution method individually and spread on to petri plates containing liquid salt agar medium having insoluble sources of ZnO and ZnCO3 separately. After incubation, the diameter of bacterial colony and halozone around colony were measured and the values were calculated using solubilizing index formula SI= (Colony 54 diameter + Halozone diameter / colony diameter) (Edi-Premono et al., 1996). The ZSB isolates showed maximum value of solubilizing index named as RRT with serial number 1 to 38 and used for further study.
Morphological Characterization of ZSB
Colony morphological futures of bacterial isolates were observed by culturing zinc solubilizing bacterial isolates in liquid mineral salts agar medium after incubating for 48 hours. The capsule staining, cell shape, grams staining, cell motility and spore formations were observed.
Results and Discussion
The present study was taken to isolate potential Zinc solubilizing bacteria (ZSB) from wheat rhizosphere. Screening was done to identify two effectives efficient ZSB and the isolates were subjected to biochemical and cultural characterization. Laboratory studies were done to find out zinc solubilization potentiality of selected bacterial isolate by using insoluble form of zinc (Table 1).
Table 1: List of Obtained ZSB Isolates from Rhizosphere at Different Area of Latur District
| Sr.No | Location | No. of isolates |
| 1. | Latur | 6 |
| 2. | Ahmadpur | 5 |
| 3. | Ausa | 3 |
| 4. | Nilanga | 3 |
| 5. | Renapur | 3 |
| 6. | Chakur | 5 |
| 7. | Deoni | 2 |
| 8. | Shirur Anantpal | 4 |
| 9. | Udgir | 4 |
| 10. | Jalkot | 3 |
| Total | 38 | |
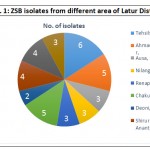 |
Figure 1: ZSB isolates from different area of Latur District |
Table 2: Zinc solubilization different bacterial isolates
| Sr.No | Culture number | Name of the isolates | Zone diameter in mm |
| 1. | RRT4 | Pseudomonas aeruginosa | 22mm |
| 2. | RRT7 | Pseudomonas fluroscence | 26 mm |
| 3. | RRT11 | Pseudomonas aeruginosa | 23 mm |
| 4. | RRT13 | Bacillus subtilis | 30 mm |
| 5. | RRT14 | Bacillus megaterium | 21mm |
| 6. | RRT19 | Bacillus cereus | 34 mm |
| 7. | RRT24 | Bacillus thuringenesis | 17 mm |
| 8. | RRT26 | Bacillus pumilis | 20 mm |
| 9. | RRT34 | Bacillus subtilis | 31 mm |
| 10. | RRT37 | Bacillus thuringenesis | 20 mm |
Zinc Solubilization Efficiency of Isolates
Isolation of zinc solubilizing bacteria (ZSB) wheat rhizosphere soil sample from ten randomly selected location in Udgir of Latur district. There were totally 38 zinc solubilizing bacterial isolates obtained using liquid salts medium supplemented with two insoluble zinc sources such as ZnO and ZnCO3. Among the different location, 4 number of ZSB isolates were obtained from rhizosphere soil sample of from Udgir. The lowest number of 2 ZSB isolates obtained from Deoni sampling site. There were 3 ZSB (RRT19, RRT34 and RRT13) isolates selected which showed high solubilization halao zone on liquid salt agar medium during isolation (Table: 3) Among the total 10 ZSB isolates, the maximum number of isolates four was from Latur tahsil site.
Table 3: List of obtained ZSB isolates from wheat rhizosphere at different area of Latur district
| Sr.No | Location | No. of isolates | Name of the isolates |
| 1. | Latur Tehsils | 6 | RRT1 |
| 2. | RRT2 | ||
| 3. | RRT3 | ||
| 4. | RRT4 | ||
| 5. | RRT5 | ||
| 6. | RRT6 | ||
| 7. | Ahmadpur | 5 | RRT7 |
| 8. | RRT8 | ||
| 9. | RRT9 | ||
| 10. | RRT10 | ||
| 11. | RRT11 | ||
| 12. | Ausa | 3 | RRT12 |
| 13. | RRT13 | ||
| 14. | RRT14 | ||
| 15. | Nilanga | 3 | RRT15 |
| 16. | RRT16 | ||
| 17. | RRT17 | ||
| 18. | Renapur | 3 | RRT18 |
| 19. | RRT19 | ||
| 20. | RRT20 | ||
| 21. | Chakur | 5 | RRT21 |
| 22. | RRT22 | ||
| 23. | RRT23 | ||
| 24. | RRT24 | ||
| 25. | RRT25 | ||
| 26. | Deoni | 2 | RRT26 |
| 27. | RRT27 | ||
| 28. | Shirur Anantpal | 4 | RRT28 |
| 29. | RRT29 | ||
| 30. | RRT30 | ||
| 31. | RRT31 | ||
| 32. | Udgir | 4 | RRT32 |
| 33. | RRT33 | ||
| 34. | RRT34 | ||
| 35. | RRT35 | ||
| 36. | Jalkot | 3 | RRT36 |
| 37. | RRT37 | ||
| 38. | RRT38 | ||
| Total | 38 |
Identification of Zinc Solubilizing Bacteria
Morphological characteristics of zinc solubilizing bacteria namely Pseudomonas aeruginosa (2), Pseudomonas fluroscence (1), Bacillus subtilis (2), Bacillus megaterium (1), Bacillus cereus (1), Bacillus thuringenesis (2) and Bacillus pumilis (1). The potent zinc solubilization bacterial isolates, Bacillus cereus (RRT19), Bacillus subtilis (RRT34) and Bacillus subtilis (RRT13) were selected for further identification. The culture characteristics of the isolates on broth media in the form surface growth and gloomy of broth were studied. Both the isolates were aerobic, non fermentive, gram negative, and rod cocci. The details of the characters are given in (Table: 4).
Table 4: Biochemical characteristics of Zinc Solubilizing Bacteria
| Sr. No. | Code of Isolate | Indole | MR | VP | Citrate | Catalase | Oxidase | Nitrate reduction | Gelatin hydrolysis | Starch hydrolysis | Glucose | Lactose | Maltose | Fructose | Sucrose | Mannitol | Arabinose | Xylose | Sorbitol | Ribose | Protease | Probable Identified isolate |
| 1. | RRT4 | – | – | – | + | + | + | + | + | – | – | – | + | + | – | – | + | – | – | – | + | Pseudomonas aeruginosa |
| 2. | RRT7 | – | + | + | – | + | + | – | + | + | + | – | + | + | – | + | + | – | – | – | – | Pseudomonas fluroscence |
| 3. | RRT11 | – | – | – | + | + | + | + | + | – | – | – | + | + | – | – | + | – | – | – | + | Pseudomonas aeruginosa |
| 4. | RRT13 | – | – | + | – | – | – | + | + | + | – | + | + | + | + | – | – | + | + | + | + | Bacillus subtilis |
| 5. | RRT14 | – | – | – | + | + | – | – | – | – | – | – | – | + | + | – | – | – | + | + | + | Bacillus megaterium |
| 6. | RRT19 | – | + | – | – | + | + | – | + | – | + | + | + | – | – | – | – | – | – | – | + | Bacillus cereus |
| 7. | RRT24 | – | + | – | – | + | – | – | + | – | – | – | + | + | – | – | – | – | + | – | + | Bacillus thuringenesis |
| 8. | RRT26 | – | – | + | – | + | + | – | – | + | + | – | – | + | + | + | – | – | – | + | + | Bacillus pumilis |
| 9. | RRT34 | – | – | + | – | – | – | + | + | + | – | + | + | + | + | – | – | + | + | + | + | Bacillus subtilis |
| 10. | RRT37 | – | + | – | – | + | – | – | + | – | – | – | + | + | – | – | – | – | + | – | + | Bacillus thuringenesis |
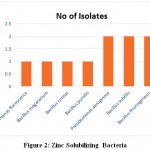 |
Figure 2: Zinc Solubilizing Bacteria |
In the present study, two ZSB isolates namely, RRT19 and RRT34 produced maximum zone of solubilization. It was observed that RRT19 is potent zinc solubilizing bacteria which showed 34 mm zone of solubilization than RRT34 on liquid salts medium amended with ZnO and ZnCO3 respectively. Similarly, Goteti et al., (2013.) reported that the maximum halozone in zinc oxide and zinc carbonate were observed by Bacillus sp. Saravanan et al., (2003) also reported that the solubilization potentiality of bacteria isolates Pseudomonas sp was high in zinc oxide than zinc sulphate supplemented medium. They have also recorded maximum of 20 cm halozone in zinc oxide and 14. cm in zinc carbonate medium. The present study has a similar result of previous study and the study is support that, both RRT19 and RRT34 can significantly solubilised the insoluble zinc in liquid salt medium and can be use in the soil for the growth and development of the plant.
Conclusion
It is concluded that both the strains, RRT19, RRT34 and RRT13 either alone or dual along with or without insoluble source of zinc showed better performance on growth and yield of wheat compared to control. In short, results from all these experiments suggest that the isolates RRT19, RRT34 and RRT13 has the promising PGPR attributes to be developed as a biofertilizer to enhance the availability of zinc in soil for better growth and development of wheat crop. The bacterial species isolated from the Rhizosphere soil can be use in soils that are deficient in Zinc or where insoluble zinc is abundant. The present study concluded that the use of zinc solubilising bacteria in the zinc deficient soil will help to enhance the growth and yield of wheat crop.
References
- Anthoni Raj, 2002. Biofertilizers for micronutrients. Biofert. Newslett. 10: 8-10.
- Bapiri, A., Asgharzadeh, A., Mujallali, H. and E. Pazira, 2012. Evaluation of Zinc solubilization potential by different strains of Fluorescent Pseudomonads. J. Appl. Sci. Environ. 16(3): 295 -298.
- Behera S.K., Singh M.V., Singh K.N., Sandeep Todwal. 2011. Distribution variability of total and extractable zinc in cultivated acid soils of India and their relationship with some selected soil properties, Geoderma 162, 242–
- Bunt, J.S. and Rovira, A.D., 1955. Microbiological studies of some sub antartic soils. Journal of Soil Science 6:119-128.
- Di Simine C.D., Sayer J.A., Gadd G.M. 1998. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol. Fertil, Soils 28, 87–
- Edi Premono, M., Moawad, A.M. and P.L.G. Vlek, 1996. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 11: 13-23.
- Fasim F., Ahmed N., Parsons, R., Gadd G.M. 2002. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery, FEMS Microbiol. Lett, 213, 1–
- Glick, B.R., 1995. The enhancement of plant growth by free living bacteria. Can. J. Microbiol. 41: 109-117.
- Goteti, P.K., Emmanuel, L.D.A., Desai, S. and M.H.A. Shaik, 2013. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays ). Int. J. Microbiol. Article ID 869697, 7 pages.
- Hughes, M.N. and R.K. Poole, 1989. Metals and microorganisms. Chapman and Hall, London. pp: 412.
- Hutchins, S.R., Davidson, M.S., Brierey, J.A. and C.L. Brierley, 1986 Microorganisms in reclamation of metals. Ann. Rev. Microbiol. 40: 311-336.
- Kabata-Pendias, A., 2000. Trace elements in soils and plants. 3rd ed. CRC Press, Boca Raton, FL.
- Lovely, D.R., 1991. Dissimilatory Fe (III) and Mn (IV) reduction. Microbial. Rev. 55: 259-287.
- Potarzycki, J. and W. Grzebisz, 2009. Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environ. 55(12): 519-527.
- Saeed, M. and R. L. Fox, 1977. Relation between suspension pH and Zn solubility in acid and calcareous soils. Soil sci. 124: 199- 204.
- Saravanan, V.S., Subramoniam, S.R. and S.A. Raj, 2003. Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Brazil. J. Microbiol. 34: 121–125.
- Saravanan, V.S., Subramoniam, S.R. and S.A. Raj, 2003. Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Brazil. J. Microbiol. 34: 121–125.
- Weller, D.G. and L.S. Thomashow, 1993. Use of rhizobacteria for biocontrol. Curr. Opin. Biotechnol. 4: 306-311.

This work is licensed under a Creative Commons Attribution 4.0 International License.





