How to Cite | Publication History | PlumX Article Matrix
Essa Ali1, Aqib Iqbal2, Sayed Hussain3*, Jawad Munawar Shah4 , Fazal Said3, Muhammad Imtiaz3, Fazal Jalal3 and Muhammad Ali Khan3
, Fazal Said3, Muhammad Imtiaz3, Fazal Jalal3 and Muhammad Ali Khan3
1Ocean College, Zhejiang University of Technology, Hangzhou, Zhejiang, 310014, China
2Institute of Biotechnology and Genetic Engineering, KPK Agricultural University, Peshawar, Pakistan
3Department of Agriculture, Abdul Wali Khan University Mardan, KPK, Pakistan
4College of Agriculture Layyah Campus, BZU Multan
Corresponding Authors E-mail: sayedhussain@awkum.edu.pk
DOI : http://dx.doi.org/10.13005/bbra/2791
ABSTRACT: Wheat is an important “rabi” (post-rainy season) crops cultivated on more than 8.0 million hectares in Pakistan. Selection based on different secondary traits enhances the progress and accuracy by which drought tolerant genotypes can be identified. In a glass house experiment, different physical and biochemical characteristics associated with drought adaptation were assessed in twelve (12) wheat genotypes. Drought tolerance index, calculated based on seedling dry weight, exhibited that Pirsabak-2004 was the most drought tolerant genotype. Minimum reduction in dry weight (14.32 %), RWC (14.15 %) and leaf area (5.59%) as well as least increase in H2O2 content (104.9%) was noted in Pirsabak-2004. However, Pirsabak-2008 has suffered minimum reduction in fresh weight (28%) and cellular membranes stability (10.89%). Maximum increase in proline (7.75 fold) and sugar content (163.51 %) was noted in PR-97 and PR-90, respectively. Similarly, Saleem-2000 has incurred the minimum reduction in chlorophyll content (32.27%) under drought stress conditions. Furthermore, correlation coefficient calculated between the dry weight and different parameters exhibited seedlings fresh weight, relative water content, H2O2 concentration and membrane stability (r = 0.654, 0.796, -0.824, 0.812, respectively) as the most important secondary traits for selection of wheat genotypes under drought stress conditions.
KEYWORDS: Chlorophyll; Drought stress; Membrane stability; Proline; Protein content; Wheat genotypes
Download this article as:| Copy the following to cite this article: Ali E, Iqbal A, Hussain S, Shah J. M, Said F, Imtiaz M, Jalal F, Khan M. A. Selection Criteria to Assess Drought Stress Tolerance in Wheat Genotypes Using Physiological and Biochemical Parameters. Biosci Biotech Res Asia 2019;16(4). |
| Copy the following to cite this URL: Ali E, Iqbal A, Hussain S, Shah J. M, Said F, Imtiaz M, Jalal F, Khan M. A.Selection Criteria to Assess Drought Stress Tolerance in Wheat Genotypes Using Physiological and Biochemical Parameters. Biosci Biotech Res Asia 2019;16(4). Available from: https://www.biotech-asia.org/?p=35010 |
Introduction
Plant responds to water deficit at diverse levels including physiological, molecular, biochemical and at cellular level. At physiological and biochemical level, it can induce changes like stomatal closure, reduction in cell growth, change in the rate of transpiration and photosynthesis rate, and modulation in antioxidant enzymes. Similarly, the expression of drought inducible genes can be affected either by ABA dependent or independent signal transduction pathway. The products of these gene-regulated processes could be osmoprotectants such as proline and glycine betain Chen, Murata (2002), biomolecular protection factors including molecular chaperon and LEA proteins, Membrane proteins (aquaporins and transporter proteins), Detoxifying enzymes such as GST and SOD, and Transcription factors including MYC, MYB and BZIP (Wang et al., 2003).
Drought stress has very crucial effect on different physiological growth and development of the crops such as emergence, plant height, stem diameter, number of leaves, leaf size and area, dry weight of the crops, flowering, fruit quantity and quality, and maturity (Anjum et al., 2017). Among the many physiological responses, increased production of Reactive Oxygen Species (ROS) formation is one of the primary effects of drought stress. The ROS are highly reactive and can cause cellular damage. ROS have the potential to encourage synthesis and degradation of various important biological molecules. A change in the proportion of photosynthesis pigment is considered as one of the first indication of drought stress caused by ROS Darrel, Jager (1984).
In order to elevate the osmotic pressure plants are adapted to accumulate different types of solutes, which may be organic or inorganic Rhodes, Samaras (1994). Proline is a well known and well-studied organic solute that accumulates as a result of drought stress Delauney, Verma (1993). Besides proline, polyols and glycine betaine have also been reported as osmoprotectents (Kishor et al., 1995; Bajji et al., 2000). All these solutes are produced in huge quantity in drought stressed plants without interfering in the metabolism (Yancey,1994).
From the existing data of global climatic changes, it can be easily concluded that changes in the abiotic factors of the atmosphere will be continuous during 21st century, and drought will be one of the most detrimental effect of these changes. To combat these effects, researchers are trying to develop new cultivars with the ability to cope with such climatic changes (Waggoner, 1993). Hypothetically, indirect selection based on a given secondary traits leads to the greater progress for grain yield than direct selection (Falconer,1989). The use of secondary traits generally improves accuracy by which drought tolerant genotypes are identified, compared to measuring grain yield only (Bolaňos, Edmeades,1996). The aim of this work is to assess the relative effectiveness of various secondary traits for selecting wheat genotypes under drought stress conditions.
Materials and Methods
Plant Materials
The experiment was conducted at Institute of Biotechnology and Genetic Engineering, Khyber Pakhtunkhwa Agricultural University Peshawar, Pakistan in a glasshouse with CRD arrangement. Each treatment was replicated three times. Seeds from different wheat (Triticum aestivum L.) genotypes (Table 1) were planted in plastic pots. Each pot was filled with 5.5 kg of silt and well rotten Farm Yard Manure (1:1). The saturation percentage of the soil mixture was calculated to be 35%, thus 1.9 liter of water was added to each pot until the imposition of drought stress. Fifteen days after germination 5 uniform plants were maintained in each pot which was regularly irrigated for further 15 days. Water stress condition was imposed by withholding water from half the pots of each genotype for 10 days.
Table 1: Wheat Genotypes Used During the Experiment
| S/No. | Name of Genotypes | S/No. | Name of Genotypes |
| 1 | PR-97 | 7 | FAKHR-E-SARHAD |
| 2 | PR-98 | 8 | SALEEM-2000 |
| 3 | PR-100 | 9 | PIRSABAK-2004 |
| 4 | PR-101 | 10 | PIRSABAK-2005 |
| 5 | PR-102 | 11 | PIRSABAK-2008 |
| 6 | PR-90 | 12 | NOWSEHRA-96 |
Sample Collection
Ten days after imposition of drought stress, samples were collected from the well-watered and stressed seedlings and data was recorded on the following biochemical and morphological characters to assess the tolerance potential of each genotype.
Seedling Fresh Weight (FW) and Dry Weight (DW)
Whole plant weight was determined twice by measuring its fresh weight and dry weight by analytical balance. Fresh weight was measured immediately after collection and dry weight was measured after placing it in incubator for 24 h. Drought tolerance index was calculated by the method of Fischer & Maurer (1978).
Leaf Area (LA)
Leaf area was determined according to Kemp (1960). Briefly, three leaves from each genotype were excised and plotted on a graph paper. The leaf area of each genotype was estimated by counting the total number of small squares. After calculating the leaf area of each genotype, the constant factor (f) was estimated from the formula A = fLB, where L is length of leaf; B is the breadth at a point midway along the length; and A is the area. After determination of the constant, leaf area of each genotype was estimated by the above formula after measuring the length and breadth.
Relative Water Content (RWC)
Leaf samples (about 5 cm2 each) were obtained from WW and WS seedlings in 15 ml tubes and directly weighed with analytical balance to obtain fresh weight (FW). The leaf samples were then entirely immersed in double distilled water and placed at 4°C for 24 h in dark. After 24 h, the samples were blotted dry on filter paper and weighed again to obtain the turgid weight (TW). The samples were finally dried in oven at 70°C for 48 h and dry weights were obtained (DW). RWC was calculated using the following formula.
RWC = [(FW – DW) / (TW – DW)] x 100
Membrane Stability Index (MSI)
The membrane stability index (%) was calculated by determining the electrolyte leakage from the leaf disks with a conductivity meter (Consort C-931, USA). The initial conductivity (Ci) was measured after subjecting the samples from controlled and drought stressed seedlings after incubation at 25°C in 5 ml de-ionized water for about 3 h with continuous shaking. Then the samples were subjected for autoclavation at 121°C for 20 min at 120 psi. Final conductivity (Cf) was measured after the samples had cooled down to 25°C.The MSI for each sample was determined as follows; MSI = [1 – (Ci / Cf)] × 100
Chlorophyll Content
A known weight (usually ~100 mg) of leaf samples was immediately placed in liquid nitrogen and homogenized in 3 ml of 80 % acetone. The homogenate was centrifuged at 15,000 rpm at 4°C for 10 min. The supernatant was collected after acetone extraction and chlorophyll content was determined by spectrophotometer (Biorad SmartSpecTM Plus, USA) according to the method described by Arnon (1949).
Proline Content (PC)
Hundred (100) mg of frozen plant material was cooled in ice and immersed in 2 ml of sterilized ion-free water; boiled for 30 min to extract warm water-soluble compounds and then cooled to room temperature. Proline in the water extract was measured as described by Bates et al., (1973) with minor modifications. 250 μl of the extract was reacted with 1 ml acid ninhydrin and 1 ml glacial acetic acid. The mixture was placed in water bath for 1 h at 100°C, and the reaction was ceased in an ice bath. 4 ml Toluene was added to the reaction mixture and its optical density was measured at 520 nm. The amount of proline was determined from a standard curve.
H2O2 Content
Hydrogen peroxide content was determined by homogenizing plant material (100 mg FW) in an ice bath with 3% TCA. The homogenate was centrifuged at 12000 rpm for 15 min and supernatant was taken. One hundred (100) mM KH2PO4 and 1M KI were added to the supernatant. Optical density was measured at 390 nm by spectrophotometer. H2O2 was quantified based on a standard curve.
Sugar Content
Total sugar was determined by method described by Duboius et al., (1956). Approximately 100 mg of leaf tissue was homogenized in 1 ml of distilled water and 1 ml of 5% phenol was added to sample. Sample tubes were shaken for 10 min after adding 5 ml concentrated H2SO4. Absorbance was measured at 490 nm through spectrophotometer. The amount of sugar was determined from standard curve constructed with different concentrations of D-glucose.
Protein Extraction and Quantification
Protein was extracted by grinding ~100 mg lyophilized plant material in pre-cooled mortar and pestle. The slurry was homogenized with buffer containing 100 mM Tris HCl (pH 6.8), 1 % SDS and 0.1 % ß merceptoethanol, and centrifuged at 15,000 rpm for 10 minutes at 4oC. The supernatant was collected, and protein was quantified through the method described by Bradford (1976) using bovine serum albumin as standard.
Statistical Analysis
Statistical analysis for the CRD was performed using GenState discovery (version 3.0). LSD was applied to discriminate between treatment means.
Results and Discussion
Seedling FW and DW
A significant decrease was observed in the fresh weight of wheat seedlings under water stressed condition as compared to the control (Figure 1A). In WW conditions, maximum FW of 1.553 ± 0.187 g. seedling-1 was obtained in the genotype PR-102 followed by PR-97 (1.322 ± 0.153 g. seedling-1) and minimum FW of 0.847 ± 0.119 g. seedling-1 was obtained in Saleem-2000. In the WS conditions, on the other hand maximum FW of 0.837 ± 0.078 g. seedling-1 was obtained in Pirsabak-2004 followed by Pirsabak-2008 (0.810 ± 0.044 g. seedling-1) and minimum FW of 0.467 ± 0.067 g. seedling-1 was obtained in PR-100. Drought stress conditions significantly decrease the dry mass of wheat genotypes (Figure 1B). In well water (WW) conditions maximum DW of 0.363 ± 0.015 g. seedling-1 was obtained in the genotype PR-97, followed by Saleem-2000 (0.303 ± 0.032 g. seedling-1) and minimum DW of 0.183 ± 0.021 g. seedling-1 was noted in PR-90. In the water stress (WS) condition, on the other hand, maximum DW of 0.243 ± 0.010 g. seedling-1 was obtained in Pirsabak-2008, followed by PR-97 with a DW of 0.241 ± 0.042 g. seedling-1 and minimum DW of 0.068 ± 0.007 g. seedling-1 was noted in PR-90. Drought and water stress or deficiency among the different environmental stresses is the most important and visible stress which direct effect the plant growth and development. Our current results showed that drought significantly reduced seedling fresh and dray weight (Figure 1B). Similar results were found in the previous findings that drought stress significantly decreased the shoot fresh weight, dry weights, stomatal conductance and maximum photosynthetic capacity (Alireza et al. 2017). Furthermore, drought stress has direct effect on both above and below the ground growth tissues along the photosynthesis activities while lastly on dry matter accumulation (Lu et al. 2015; Khalili et al. 2016). Moreover, for the food security, breeders are bound to develop new tolerant or resistant varieties of wheat to decrease the food security risk. The ability of a plant to absorb water and rapid growth are also affected by drought stress and this affect proceed further to a series of other metabolic activities such as osmotic stress, reduced leaf water content, oxidative damage and stomatal closure etc (Wang et al. 2008).
Drought Tolerance Index
Because significant differences were noted in the FW and DW of the wheat genotypes under both well watered (WW) and water stress (WS) conditions, the Drought Tolerance Index (DTI), was calculated from the DW to determine the drought tolerance potential of each genotype (Figure 1C). Statistically significant differences were noted in the DTI of each genotype. The DTI ranged between 0.22 and 0.97. DTI values showed that the genotype Pirsabak-2004 (DTI of 0.22) was the most tolerant to WS conditions, followed by Pirsabak-2008 (DTI of 0.28) and Pirsabak-2005 (DTI of 0.40). In contrast, the genotype PR-90 was the most susceptible genotype to WS conditions (DTI of 0.97).
 |
Figure 1: Seedling Fresh Weight (A), Dry Weight (B), and Drought Tolerance Index (C) of the Wheat Genotypes Under Well Water (WW) (Blue Bars) and Water Stress (WS) (Indigo Bars) Conditions. |
Relative Water Content (RWC)
There was a distinct decline in the relative water content of the leaves of wheat seedlings when exposed to drought stress with contrast to control (Figure 2A). In WW condition maximum RWC of 87.18 ± 3.00 % was obtained in the genotype PR-97 followed by Nowshera-96 (86.89 ± 3.15 %) and minimum RWC of 80.47 ± 3.42 % was noted in Fakhr-e-Sarhad. After exposure to the WS conditions, maximum RWC of 73.22 ± 3.49 % was obtained in Pirsabak-2004, followed by Pirsabak-2008 (67.78 ± 1.92 %) and minimum RWC of 31.05 ± 2.39 % under WS conditions was noted in PR-90. Minimum reduction in the RWC and maximum water loss was noted in PR-98. Similarly, there was a strong positive correlation (r = 0.796) between the DW and RWC (Table 2). Abbasi et al., (2003) also reported high positive correlation (r = 0.89) between fresh biomass yield and RWC. Our current results are very similar to the previous findings of Alireza et al. (2017), where they indicated that RWC can be used as an important secondary trait for selecting wheat genotypes that could maintain better performance under drought conditions. In the current experiment, drought stress decreased RWC in the leaves of wheat seedlings. Relative water content is one of the important physiological conditions to check the tissue grade and cell hydration which is compulsory for normal physiological and biochemical attributes and growth in plant (Silva et al. 2007). In different experiments, the scientists have observed that the preservation of a high RWC during drought is indicative of drought resistance (Colom and Vazzana 2003; Ozkur et al. 2009). Low decrease in RWC was observed in Aegilops than Triticum accessions after the severe treatment of drought stress and recommended that the using of RWC should be indicator for drought response while it is suitable physiological trait for screening drought-tolerant genotypes (Pampino et al. 2006).
Table 2: Pooled Genotypes Correlation Coefficient (r) of the DW with the Different Parameters in WW and WS Conditions in Wheat Seedlings
| WW | WS | |
| Fresh Weight | 0.494 | 0.654* |
| Relative Water Content | 0.229 | 0.796** |
| Leaf Area | 0.472 | 0.390 |
| H2O2 Content | 0.044 | -0.824** |
| Membrane Stability | 0.402 | 0.812** |
| Chlorophyll | -0.061 | 0.134 |
| Proline | -0.629* | 0.385 |
| Sugar | 0.148 | 0.217 |
| Protein | -0.516 | -0.415 |
Correlation coefficient significant at * p = 0.05 and **p = 0.01 levels
Leaf Area
Drought stress significantly alters the leaf area of wheat genotypes (Figure 2B). In WW condition maximum leaf area was obtained in the genotype Pirsabak-2008 (9.56 ± 0.99 cm2) followed by PR-100 (9.15 ± 0.50 cm2) and minimum leaf area (4.13 ± 0.17 cm2) was noted in Nowshera-96. In the WS condition, on the other hand, maximum leaf area (8.53 ± 0.91 cm2) was obtained in Pirsabak-2008, followed by Pirsabak-2004 (7.08 ± 0.61 cm2) and the minimum leaf area (3.01 ± 0.48 cm2) was noted in the genotype Saleem-2000. The data regarding leaf area of the different wheat genotype in WW and WS conditions showed statistically significant differences in the leaf area of the genotypes under both WW and WS condition (Figure 2B). The average leaf area of all the genotypes under WW conditions decreased significantly after exposure to WS conditions. In WW condition maximum leaf area was obtained in the genotype Pirsabak-2008 and minimum leaf area was noticed in Nowshera-96. In the WS condition, on the other hand, maximum leaf area was noticed in Pirsabak-2008 and minimum leaf area, on the other hand, was noted in the genotype Saleem-2000. The genotypes also suffered variable decrease in leaf area because of WS conditions. Compared with the leaf area under WW conditions, minimum reduction in leaf area was noted in Pirsabak-2004 and maximum decrease was noted in Saleem-2000. During this experiment, a strong positive correlation was noted between the DW and LA under both WW and WS conditions (r = 0.472 and 0.390, respectively). Reduction in leaf area under drought stress was also documented by Heinigre (2000) in his research. It was reported earlier that the leaf thickness, shoot fresh weight and stem diameter were severely affected by harsh drought stress (17% and 17.9%) as compared to mild (3 and 5 %) and moderate stress (11% and 13.6%) (Huang et al. 2013).
Chlorophyll Content
A significant decrease in the chlorophyll content of wheat genotypes under drought stress was detected (Figure 2C). In WW condition maximum chlorophyll content was obtained in the genotype Fakhr-e-Sarhad (714.112 ± 30.981 µg.g-1 FW) followed by PR-98 (704.880 ± 4.329 µg.g-1 FW) and minimum chlorophyll content (602.607 ± 32.058 µg.g-1 FW) was noted in PR 100. On the other hand, in the WS condition, maximum chlorophyll content (471.990 ± 13.474 µg.g-1 FW) was obtained in Saleem-2000, followed by PR-98 (378.273 ± 16.850 µg.g-1 FW) and the minimum chlorophyll content (236.940 ± 19.021µg.g-1 FW) was noted in the genotype Fakhr-e-Sarhad. A rapid decrease in the chlorophyll content has been noted in wheat after exposure to drought stress (Ommen et al. 1999). During this experiment, the average chlorophyll content of all the wheat genotypes decreased when exposed to WS conditions, however, statistically significant differences were noted in the chlorophyll content of different wheat genotypes under both WW and WS conditions (Figure 2C). In WW condition maximum chlorophyll content was obtained in the genotype Fakhr-e-Sarhad followed by PR-98. In contrast, minimum chlorophyll content was noted in PR 100. In the WS condition, on the other hand, maximum chlorophyll content was obtained in Saleem-2000, followed by PR-98.
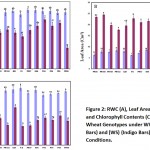 |
Figure 2: RWC (A), Leaf Area (B), and Chlorophyll Contents (C) of the Wheat Genotypes under WW (Blue Bars) and (WS) (Indigo Bars) Conditions. |
The minimum chlorophyll content, on the other hand, was noted in the genotype Fakhr-e-Sarhad. Compared with the chlorophyll content under WW conditions the minimum reduction of chlorophyll content was noted in the genotype Saleem-2000, while on the other hand, maximum reduction was noted in Fakhr-e-Sarhad. Stability of chlorophyll content is associated with tolerance under various abiotic stresses (Mohammadi et al. 2009). Positive, but insignificant, correlation (r = 0.134) was noted between the DW and chlorophyll content under WS conditions (Table 2). In current study there are conflicting reports on the correlation between chlorophyll content and plant performance under drought stress conditions. In agreement with current results, Painawadee et al., (2009) reported positive but statistically insignificant correlation (r = 0.10) between the biomass production and chlorophyll content under drought stress. However, Keyvan (2010) reported a strong correlation (r = 0.843) between grain yield and chlorophyll content. Moreover, a thylakoid membrane is used for the protection of chlorophyll while it might be possible that loss in chlorophyll will be due to increase in temperature or drought-induced lipid peroxidation of chloroplast membranes and electrolytic leakage from thylakoid membranes (Pradhan et al. 2012; Tian et al. 2013). In crops, leaf senescence occurs by drought stress and ultimately decreases the chlorophyll content (Yang et al. 2001). Our results are very similar to the previous findings of Alireza et al. (2017), where they stated that drought stress reduced the relative chlorophyll content in different studied species. Parallel to our findings, in an experiment conducted by Ergen and Budak (2009), closure of stomata as small dehydration using transpiration is one of the first responses to water deficit. Econopouly et al. (2013) also reported a high level of genetic variability and drought tolerance in Ae. cylindrica accessions in response to water deficit stress.
Membrane Stability Index (%)
Membrane stability was marked decrease under water stressed conditions (Figure 3A). Under WW conditions the highest membrane stability index was recorded for Pirsabak-2004 (93.58 ± 2.55 %) followed by Fakhr-e-Sarhad (93.04 ± 1.33 %) and minimum membrane stability index of 80.11 ± 1.94 % was noted in PR-98. In the WS condition, on the other hand maximum membrane stability index of 81.92 ± 3.43 % was obtained in Pirsabak-2008 followed by Pirsabak-2004 (81.33 ± 4.64 %) and minimum membrane stability index of 42.45 ± 8.70 % was noted in PR-98. The MSI of different wheat genotypes shows statistically significant differences under both WW and WS conditions (Fig. 3A). The average membrane stability of all the genotypes decreased when exposed to WS condition. Similarly, there was a highly significant positive correlation (r = 0.812) between the DW and MSI (Table 2). Under WW conditions the highest membrane stability was recorded in Pirsabak-2004 followed by Fakhr-e-Sarhad. Conversely, minimum membrane stability was noted in PR-98. In the WS condition, on the other hand maximum membrane stability was obtained in Pirsabak-2008 followed by Pirsabak-2004. Similarly, minimum membrane stability was noted in PR-98. It can be inferred from the data that Pirsabak-2008 has suffered the least damage to the cellular membranes, as evident from a decrease in MSI under WS compared to WW conditions and PR-98 has suffered the maximum damage. A strong correlation between cell membrane stability with growth and field performance of wheat seedlings has been previously reported (Bajji et al., 2001), which resemble our results. Similar to our results, Hairat and Khurana, (2015) and Alireza et al. (2017), reported that different species have different affect to drought stress as some are less affected than others while depends on the their thylakoid membrane stability under drought stress.
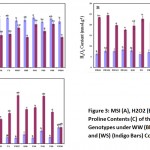 |
Figure 3: MSI (A), H2O2 (B), and Proline Contents (C) of the Wheat Genotypes under WW (Blue Bars) and (WS) (Indigo Bars) Condition. |
H2O2 Content
There was a striking increased seen in the H2O2 content of wheat genotypes under drought stress (Figure 3B). In WW condition maximum H2O2 content of 8.48 ± 0.14 nmol. g-1 FW was obtained in the genotype Nowshera-96 followed by PR-102 (8.34 ± 0.44 nmol. g-1 FW) and minimum H2O2 content of 6.01 ± 0.07 nmol. g-1 FW was noted in Pirsabak-2005. In contrast to WW, in the WS condition maximum H2O2 content of 24.44 ± 0.26 nmol. g-1 FW was obtained in PR-100 followed by PR-90 (24.14 ± 0.24 nmol. g-1 FW). Similarly, minimum H2O2 content of 14.44 ± 0.88 nmol. g-1 FW was noted in Pirsabak-2004. Hydrogen peroxide is one of the most important and mobile ROS, which is also involved in cell signaling Mittler, Zilinskas (1994). Water limitation resulted in an increased accumulation of H2O2 Celina (2005). Statistically significant differences were noted in the H2O2 content of different wheat genotypes under both WW and WS conditions (Fig. 3B). The average H2O2 content of all the genotypes increased after exposure to WS condition. In WW condition maximum H2O2 content was obtained in the genotype Nowshera-96 followed by PR-102. Conversely, minimum H2O2 content was noted in Pirsabak-2005. In the WS condition, on the other hand maximum H2O2 content was obtained in PR-100 followed by PR-90. Similarly, minimum H2O2 content was noted in Pirsabak-2004. Variable increase in the H2O2 content was noted among the wheat genotypes after exposure to WS conditions. Minimum increase in H2O2 content was noted in Pirsabak-2004, followed by Pirsabak-2008. The maximum increase in H2O2 content as a consequence of exposure to WS conditions, on the other hand, was noted in the wheat genotypes PR-97. Furthermore, a highly negative correlation (r = -0.824) was found between the DW and H2O2 content (Table 2). Thus, it can be used as a reliable secondary trait for identification of drought tolerant wheat genotypes. Celina, (2005) also reported the accumulation of H2O2 in wheat leaves after exposure to water stressed conditions.
Proline Content
A significant increase in proline content in the leaves of wheat seedling was detected under drought stressed conditions (Figure 3C). In WW condition maximum Proline content (63.35 ± 4.56 FW nmol. g-1 FW) was obtained in the genotype PR-90 followed by Pirsabak-2005 (49.10 ± 3.93 nmol. g-1 FW), whereas, the minimum was recorded for PR-97 (21.22 ± 1.87 nmol. g-1 FW). Alternatively, in WS condition, maximum Proline content of 186.52 ± 7.63 nmol. g-1 FW was obtained in PR 101 followed by PR-97 (185.56 ± 5.24 nmol. g-1 FW) and minimum Proline content of 63.48 ± 6.46 nmol. g-1 FW was noted in PR-90. The Proline content of different wheat genotypes shows statistically significant differences under both WW and WS conditions (Figure 3C). Under drought stress conditions the average Proline content of all the genotypes increase. In WW condition maximum Proline content was obtained in the genotype PR-90 followed by Pirsabak-2005. Whereas, the minimum Proline content was noted in PR-97. In the WS condition, alternatively maximum Proline content was obtained in PR 101 followed by PR-97. On the other hand, minimum Proline content was noted in PR-90. The maximum increase in Proline content because of exposure to WS conditions was noted in the wheat genotypes PR-97 followed by PR-101, while minimum increase in Proline content was noticed in PR-90. We found a positive, but statistically non-significant, correlation (r = 0.385) was found between the proline content and DW under WS conditions. However, there was a negative correlation between the proline content and DW when the seedlings were adequately irrigated (Table 2). Thus, not all genotypes with high proline content have accumulated higher dry biomass. Zarei et al., (2007) have found a significant correlation between the proline content with yield under stress conditions but not with the potential yield. In previous findings different researchers observed a maximum increase in proline contents with time of stress application in two wheat genotypes up to maximum level (Aneela et al. 2017; Nayyar, 2003).
Sugar Content
Figure 4A shows a distinct increase in sugar level of wheat genotypes under drought stress conditions as compared to the control. In WW condition maximum sugar content of 11.16 ± 1.40 nmol. g-1 FW was obtained in the genotype Pirsabak 2008 followed by Pirsabak-2005 (10.48 ± 1.26 nmol. g-1 FW). Conversely, minimum sugar content of 6.90 ± 0.45 nmol. g-1FW was noted in PR-90. In the WS condition, on the other hand, maximum sugar content of 19.66 ± 0.96 nmol. g-1 FW was obtained in Fakhr-e-Sarhad followed by Saleem 2000 (19.19 ± 1.46 nmol. g-1 FW) and minimum sugar content of 13.61 ± 1.39 nmol. g-1 FW was noted in PR-101. A 2-fold increase in sugar content was obtained after the exposure of wheat genotypes to WS conditions (Figure 4A). Significant differences were present in the sugar content in the genotypes under both WW and WS conditions. In WW condition maximum sugar content was obtained in Pirsabak-2008 followed by Pirsabak-2005 and minimum sugar content was noticed in PR-90. In the WS condition, on the other hand, maximum sugar content was found in Fakhr-e-Sarhad followed by Saleem-2000 and minimum Sugar content was noted in PR-101. A positive but non-significant correlation was noted between the DW and sugar content under both WW and WS conditions (r = 0.148 and 0.217, respectively). The genotypes capacity to enhance sugar production in response to drought stress was also different. Compared with the sugar content under WW conditions the minimum increase was noted in the genotype PR-101 and maximum increase was noted in PR-90. Hare et al. (1995) found a rapid increase in sugar contents in the leaves and roots of drought stressed plants. We observe in literature that soluble sugar accumulations in leaf of different wheat cultivars are different because genetic structure in the wheat clarifies its tolerance to water stress. Therefore, the maximum amount of soluble compounds was observed in drought tolerant wheat cultivars than sensitive cultivars (Nayyar and Walia, 2003; Aneela et al. 2017).
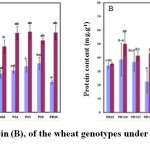 |
Figure 4: Sugar (A), Protein (B), of the wheat genotypes under WW (blue bars) and (WS) (indigo bars) conditions. |
Protein Content
Compared with the control, a marked increase occurred in the protein concentration of wheat genotypes when exposed to drought stress (Figure 4B). In WW condition, maximum protein content of 42.86 ± 4.16 mg. g-1 FW was obtained in the genotype Nowshera-96 followed by PR-100 (38.62 ± 11.88 mg. g-1FW) and minimum Protein content of 22.38 ± 8.07 mg. g-1FW was noted in PR-102. In the WS condition, on the other hand maximum Protein content of 62.05 ± 4.85 mg. g-1FW was noted in Nowshera-96 followed by PR-100 (49.99 ± 7.79 mg. g-1FW). Whereas, a minimum Protein content of (28.87 ± 2.57 mg. g-1FW) was recorded for Pirsabak-2008. Statistically significant differences were noted in the protein content of different wheat genotypes under both WW and WS conditions (Figure 4B). The average protein content of all the genotypes increased under WS condition. In WW condition maximum Protein content was obtained in the genotype Nowshera-96 followed by PR-100. Conversely, minimum Protein content was noted in PR-102. In the WS condition, on the other hand maximum Protein content was noted in Nowshera-96 followed by PR-100. On the other hand, a minimum Protein content was recorded for Pirsabak-2008. Compared with the protein content under WW conditions a minor decrease was noted in the genotypes Fakhr-e-Sarhad, Pirasbak-2004 and Pirsabak-2005, respectively. All the other wheat genotypes, on the other hands, showed an increase in protein content. Though statistically not significant, a negative correlation was found between the protein content and DW under both WW (r = -516) and WS (r = -415) conditions (Table 2). Our results are in the line with the previous findings of Aneela et al. (2017), where they reported that variations in protein contents were observed in all tested genotypes under both control and drought stressed condition. Furthermore, under drought stress condition, protein contents were recorded in greater amount as compared to normal (Aneela et al. 2017). For instance, maximum protein contents were recorded in AARI-11, FSD-08 and PAKISTAN-13 under drought as compared to normal (Aneela et al. 2017).
Conclusion
Our obtained results on the basis of different biochemical and related characteristics associated with drought adaptation were assessed of wheat genotypes in control environmental condition. Thus after getting the above results specially drought tolerance index, it was concluded that among all the studied genotypes that Pirsabak-2004 was the most drought tolerant genotype. However, Pirsabak-2008 has suffered minimum reduction in fresh weight (28%) and cellular membranes stability (10.89%). Maximum increase in proline (7.75 fold) and sugar content (163.51 %) was noted in PR-97 and PR-90, respectively. Therefore we recommend wheat genotype ‘Pirsabak-2004’ for further field trials to finalize its anti-drought habitats for the field growing condition.
References
- Abbasi F., Feyen J., Roth RL., Sheedy M., Genuchten MTV. 2003. Water flow and solute transport in furrow-irrigated fields.Irrigation Science, 22(2): 57-65.
- Alireza PA., Jafar A., Ali AM., Alireza E., Mohammad M., Kadambot HMS. 2017. Physiological responses to drought stress in wild relatives of wheat: implications for wheat improvement. Acta Physiologia Plantarum, 39: 106.
- Aneela U., Syed AM., Amjad M. 2017. Hormonal Seed Priming Improves Wheat (Triticum aestivum L.) Field Performance Under Drought And Non-Stress Conditions. Pakistan Journal of Botany, 49(4): 1239-1253.
- Anjum SA., Ashraf U., Zohiab A., Tanveer M., Naeem M., Ali I., Tabassum T., Nazir U. 2017. Growth and developmental responses of crop plants under drought stress. Zemdirbyste-Agriculture, 104(3): 267–276.
- Arnon DI. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris.Plant physiology, 24(1): 1.
- Bajji M., Lutts S., Kinet JM. 2000. Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in callus cultures issued from durum wheat (Triticum durum) cultivars differing in drought resistance.Journal of Plant Physiology, 156(1): 75-83.
- Bajji M., Lutts S., Kinet JM. 2001. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions.Plant Science, 160(4): 669-681.
- Bates LS., Waldren RP., Teare ID. 1973. Rapid determination of free proline for water-stress studies.Plant and soil, 39(1): 205-207.
- Bolanos J., Edmeades GO. 1996. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize.Field Crops Research, 48(1): 65-80.
- Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical biochemistry, 72(1-2): 248-254.
- Celina M., Gillen KT., Assink RA. 2005. Accelerated aging and lifetime prediction: review of non-Arrhenius behaviour due to two competing processes.Polymer Degradation and Stability, 90(3): 395-404.
- Chen TH., Murata N. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes.Current opinion in plant biology, 5(3): 250-257.
- Colom MR., Vazzana C. 2003. Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping love grass plants. Environmental and Experimental Botany, 49:135–144.
- Delauney AJ., Verma DPS. 1993. Proline biosynthesis and osmoregulation in plants.The plant journals, 4(2): 215-223.
- DuBois M., Gilles KA., Hamilton JK., Rebers PAT., Smith F. 1956. Colorimetric method for determination of sugars and related substances.Analytical chemistry, 28: 350-356.
- Econopouly B., Mckay J., Westra P., Reid S., Helm A., Byrne P. 2013. Phenotypic diversity of Aegilops cylindrica (jointed goatgrass) accessions from the western United States under irrigated and dryland conditions. Agriculture, Ecosystem and Environment, 164:244–251.
- Ergen NZ., Budak H. 2009. Sequencing over 13000 expressed sequence tags from six subtractive cDNA libraries of wild and modern wheats following slow drought stress. Plant Cell Environment, 32:220–236.
- Falconer D. S.1989. Introduction to quantitative genetics. Longman Scientific and Technical, New York (3rd).
- Fischer RA., Maurer R. 1978. Drought resistance in spring wheat cultivars. I. Grain yield responses.Crop and Pasture Science, 29(5): 897-912.
- Hairat S., Khurana P. 2015. Evaluation of Aegilops tauschii and Aegilops speltoides for acquired thermotolerance: implications in wheat breeding programmes. Plant Physiology and Biochemistry, 95:65–74.
- Hare PD., Cress WA. 1997. Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regulators, 21: 79–102.
- Heinigre R.W. 2000. Irrigation and Drought management. Crop Science Department.
- Huang C., Zhao S., Wang L., Anjum S. A., Chen M., Zhou H., Zou C. 2013. Alteration in chlorophyll fluorescence, lipid peroxidation and antioxidant enzymes activities in hybrid ramie (Boehmeria nivea L.) under drought stress. Australian Journal of Crop Science, 7 (5): 594–601.
- Jaleel CA., Manivannan P., Sankar B., Kishorekumar A., Gopi R., Somasundaram R., Panneerselvam R. 2007. Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation.Colloids and surfaces B: Biointerfaces, 60(2): 201-206.
- Kemp CD. 1960. Methods of estimating the leaf area of grasses from linear measurements.Annals of Botany, 24(4): 491-499.
- Keyvan S. 2010. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars.Journal of Animal and Plant Science, 8(3): 1051-1060.
- Khalili M., Pour-Aboughadareh A., Naghavi MR. 2016. Assessment of drought tolerance in barley: integrated selection criterion and drought tolerance indices. Environmental and Experimental Biology, 14:33-41.
- Kishor PK., Hong Z., Miao GH., Hu CAA., Verma DPS. 1995. Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants.Plant Physiology, 108(4): 1387-1394.
- Lu HB., Qiao YM., Gong XC., Li HQ., Zhang Q., Zhao ZH., Meng LL. 2015. Influence of drought stress on the photosynthetic
characteristics and dry matter accumulation of hybrid millet.
Photosynthetica, 53:306-311. - Mittler R., Zilinskas BA. 1994. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought.The Plant Journal, 5(3): 397-405.
- Mohammadi MO., Karimizadeh RA., Naghavi MR. 2009. Selection of bread wheat genotypes against heat and drought tolerance based on chlorophyll content and stem reserves.Journal of Agriculture and Social Sciences, 5(5): 119-122.
- Nayyar M., Walia DP. 2003. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biologia Plantarum,46(2): 275-279.
- Ommen OE., Donnelly A., Vanhoutvin S., Oijen MV., Manderscheid R. 1999. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 concentrations and other environmental stresses within the ‘ESPACE-wheat’project.European Journal of Agronomy, 10(3): 197-203.
- Ozkur O., Ozdemir F., Bor M., Turkan I. 2009. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovate Desf. to drought. Environmental and Experimental Botany, 66:487-492.
- Painawadee M., Jogloy S., Kesmala T., Akkasaeng C., Patanothai A. 2009. Heritability and correlation of drought resistance traits and agronomic traits in peanut (Arachis hypogaea).Asian Journal of Plant Sciences, 8(5): 325.
- Pampino P., Pataleo S., Gerardi C., Mita G., Perrotta C. 2006. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environment, 29:2143-2152.
- Pradhan G., Prasad V., Fritz AK., Kirkhan M., Gill B. 2012. Response of Aegilops species to drought stress during reproductive stage of development. Functional Plant Biology, 39:51-59.
- Rhodes D., Samaras Y. 1994. Genetic control of osmoregulation in plants.Cellular and molecular physiology of cell volume regulation, 347-361.
- Silva M., Jifon J., Silva JAG., Sharma V. 2007. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology, 19:193-201.
- Tian S., Mao X., Zhang H., Chen S., Zhai C., Yang S., Jing R. 2013. Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat. Journal of Experimental Botany, 64:2063–2080.
- Waggoner PE. 1993. Preparing for climate change. In: Inter. Crop Sci. Society of America, Madison, WI, USA, Chapter 30, pp. 239-245.
- Wang R., Chen S., Zhou X., Shen X., Deng L., Zhu H., Shao J., Shi Y., Dai S., Fritz E., Huttermann A., Polle A. 2008. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiology, 28:947-957.
- Wang W., Vinocur B., Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance.Planta, 218(1): 1-14.
- Yancey P.H. 1994. Compatible and counteracting solutes.Cellular and molecular physiology of cell volume regulation, 81-109.
- Yang J., Zhang J., Wang Z., Zhu Q., Liu L. 2001. Water deficit–induced senescence and its relationship to the remobilization of prestored carbon in wheat during grain filling. Agronomy Journal, l93:196–206.
- Zareri L., Farshadfar E., Haghparast R., Rajabi R., Mohammadi M. 2007. Evaluation of some indirect traits and indices to identify drought tolerance in bread wheat. Asian Journal of Plant Sciences. 6: 1204-1210.

This work is licensed under a Creative Commons Attribution 4.0 International License.





