How to Cite | Publication History | PlumX Article Matrix
Thatsanee Luangharn1,2,3,4  , Peter E. Mortimer1,4*
, Peter E. Mortimer1,4* , Samantha C. Karunarathna1,3,4
, Samantha C. Karunarathna1,3,4  , Kevin D. Hyde3,5
, Kevin D. Hyde3,5 , Jianchu Xu1,3,4
, Jianchu Xu1,3,4
1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China
2University of Chinese Academy of Sciences, Beijing 100049, China
3East and Central Asia Regional Office, World Agroforestry Centre (ICRAF), Kunming 650201, Yunnan, China
4Centre for Mountain Futures, Kunming Institute of Botany, Kunming 650201, Yunnan, China
5Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand
Corresponding Author E-mail : peter@mail.kib.ac.cn
DOI : http://dx.doi.org/10.13005/bbra/2806
ABSTRACT: Ganoderma mushrooms have been used in traditional medicines for centuries and as such are highly sought after, especially in Asia. The present study is the first report of the successful cultivation of G. leucocontextum, G. resinaceum, and G. gibbosum collected from the wild, in Yunnan Province, China. One mature fruiting body of the laccate G. leucocontextum, one mature fruiting body of the laccate G. resinaceum, and seven non-laccate G. gibbosum fruiting bodies were collected and isolated into culture. These strains were cultivated using both soil casing layer and non-casing layer methods. The highest yield and biological efficiency (BE) of G. leucocontextum was obtained when using the soil casing layer method (60.43% BE, with 253.82 g/Kg-1 of the total yield) with the non-casing layer method (13.60% BE, with 58.18 g/Kg-1 of the total yield), respectively. Only one cycle of production (26.94% BE and 7.02 g/Kg-1) was obtained for G. resinaceum KUMCC19-0001 when the soil casing layer method was applied, while a high yield of 109.26% BE, with a total yield of 27.75 g/Kg-1, was obtained when the non-casing layer method was used. A BE of 73.80% and total yield of 284.15 g/Kg-1 were obtained for the G. gibbosum KUMCC17-0005 when it was cultivated with a soil casing layer, while a BE of 40.26% and a total yield of 172.08 g/Kg-1 was obtained when the non-casing layer method was used. Based on this comprehensive study, this result will be helpful for the commercial cultivation for laccate G. leucocontextum, G. resinaceum, and non-laccate G. gibbosum.
KEYWORDS: Medicinal Mushroom; Morphological Characteristics; Mushroom Cultivation; Soil Casing Method; White Rot
Download this article as:| Copy the following to cite this article: Luangharn T, Mortimer P. E, Karunarathna S. C, Hyde K. D, Xu J. Domestication of Ganoderma Leucocontextum, G. Resinaceum, and G. Gibbosum Collected from Yunnan Province, China. Biosci Biotech Res Asia 2020;17(1). |
| Copy the following to cite this URL: Luangharn T, Mortimer P. E, Karunarathna S. C, Hyde K. D, Xu J. Domestication of Ganoderma Leucocontextum, G. Resinaceum, and G. Gibbosum Collected from Yunnan Province, China. Biosci Biotech Res Asia 2020;17(1). Available from: https://bit.ly/2VH3Fpj |
Introduction
Ganoderma is one of the most important medicinal mushroom genera and has been widely used in traditional Chinese medicine systems for over 2000 years (Paterson 2006). Members of the genus Ganoderma are renowned medicinal mushrooms and subsequently species from this group are highly sought after and of great economic value, especially in Asia (Dai et al., 2009; Hapuarachchi et al., 2018b, 2019a). Ganoderma was typified by G. lucidum (W. Curt.:Fr.) Karst, which belongs to the order Polyporales (Justo et al., 2017; He et al., 2019); however, Cui et al. (2019) stated that Ganoderma should not be included in Polyporales because their double-walled basidiospores are quite different from other members of the Polyporales. The genus Ganoderma is characterized by its unique laccate and non-laccate basidiocarps, double-walled basidiospores, and interwall pillars (Moncalvo and Ryvarden, 1997; Hapuarachchi et al., 2019a). Ganoderma is a cosmopolitan genus, distributed worldwide in temperate and tropical areas (Cao and Yuan, 2013; Hapuarachchi et al., 2019a, b).
Ganoderma is rich in several biologically active compounds: more than 400 bioactive compounds have been found in various Ganoderma species, including fatty acids, polysaccharides, steroids, and triterpenoids, providing nutrition and containing medicinal properties (Wasser and Weis, 1999; Gao et al., 2003; De Silva et al., 2012a, b; Li et al., 2013; Richter et al., 2015). These valuable natural compounds are used to treat a variety of pathological diseases (Cheng et al., 2010; Teng et al., 2011; De Silva et al., 2013; Richter et al., 2015). Commercial Ganoderma products are available in many forms such as dried fruiting bodies, spore capsules, dietary supplements, and cosmetic products (Shiao 2003; Hapuarachchi et al., 2018a, 2019a, b).
Laccate Ganoderma leucocontextum was discovered on the Tibetan Plateau. It is commonly called the “white Ganoderma” referring to its white context (Li et al., 2015). Its fruiting bodies contain novel triterpenes and meroterpenes, which are used to treat nerve system diseases (Wang et al., 2015; Chen et al., 2018; De Silva et al., 2012a, b). Ganoderma resinaceum contains high numbers of triterpenoids (Chen et al., 2019), with strong inhibitory effects against α-glucosidase, and is used to treat hepatoprotective activity (Chen et al., 2017; Chen et al., 2018). Ganoderma gibbosum also contains triterpenoids, which are used in clinical treatment for their hypertension and cholesterol-reducing properties (Pu et al., 2017). Therefore, triterpenoids have been regarded as an important indicator in the quality evaluation of Ganoderma species and related products (Guo et al., 2013; Hennicke et al., 2016). Ganoderma species also show significant cytotoxic activities and contain the following properties: immunostimulatory, anticancer, antiviral, anti-inflammatory, and antioxidant (treating liver, lung, kidney, spleen, and stomach functions), allowing for its use in the clinical treatment of various types of diseases (Niu et al., 2007; De Silva et al., 2012a, b; Chen et al., 2017). However, a number of Ganoderma species remain poorly studied: for example, there are few reports on the chemistry and bioactivity of non-laccate G. gibbosum, and the only known pharmacologically active compounds derived from G. gibbosum are a few forms of lanostane triterpenes, which were found to possess immunoregulatory and anti-inflammatory activities (Pu et al., 2017).
Most Ganoderma cultivation is carried out in China, with local raw materials such sawdust or wood chips used as substrates (Stamets 2000; Jo et al., 2010; Roy et al., 2015; Tan et al., 2015; Ćilerdžić et al., 2016; Luangharn et al., 2017). Non-casing layer methods are usually applied to Ganoderma cultivation (Zhou et al., 2012; Tan et al., 2015; Liu et al., 2017), while soil casing methods are rarely used (Nicholas and Ogame, 2006). Although G. leucocontextum, G. resinaceum, and G. gibbosum have been domesticated, the use and effectiveness of a soil casing layer has not been previously reported on. Thus, the objectives of this study were to determine most effective methods of cultivation for G. leucocontextum, G. resinaceum, and G. gibbosum, for which most scientists report sawdust as the optimal substrate, and to introduce a new protocol in Ganoderma cultivation for inducing the production of fruiting bodies via soil casing and non-casing layer methods in Kunming, Yunnan Province, China.
Materials and Methods
Mushroom Collection and Isolation
One G. leucocontextum (KUMCC17-0007) strain, one G. resinaceum (KUMCC19-0001) strain, and seven non-laccate G. gibbosum strains were included in this study. The G. gibbosum strains consisted of five mature fruiting bodies (KUMCC17-0004, KUMCC17-0005, KUMCC17-0013, KUMCC18-0007, KUMCC19-0002) and two young fruiting bodies (KUMCC17-0009 and KUMCC19-0003), collected and isolated in the temperate climate of Yunnan Province, China (Figure 1). Detailed site information regarding location, climate, average monthly temperature during the rainy season, and host trees species of the study sites are provided in Table 1. Pure cultures of the nine Ganoderma strains were isolated by the method described by Stamets (2000). The voucher numbers of the cultures were obtained by depositing the cultures in the Kunming Institute of Botany Culture Collection, China (KUMCC).
Table 1: Detailed site information, including location, substrate, host tree species, and average monthly temperatures during the rainy season collection period of this study.
| Fungal species | Fungal
strain |
Location | Substrate,
host species |
Monthly temperature |
| Ganoderma leucocontextum | KUMCC
17-0007 |
Baoshan, Yunnan | Decay stump of Pinus sp. | 18 °C |
| G. resinaceum | KUMCC
19-0001 |
Kunming Institute of Botany, Kunming | Living tree of Albizia mollis | 20 °C |
| G. gibbosum | KUMCC
17-0004 |
Kunming Botanical Garden, Kunming | Decay of Chamaecyparis pisifera | 19 °C |
| G. gibbosum | KUMCC
17-0005 |
Kunming Botanical Garden, Kunming | Living tree of Albizia mollis | 19 °C |
| G. gibbosum | KUMCC
17-0009 |
Kunming Botanical Garden, Kunming | Living tree of Albizia mollis | 18 °C |
| G. gibbosum | KUMCC
17-0013 |
Kunming Botanical Garden, Kunming | Decay wood of unknown tree | 20 °C |
| G. gibbosum | KUMCC
18-0007 |
Jinning, Yunnan | Stump of unknown tree | 22 °C |
| G. gibbosum | KUMCC19-0002 | Kunming Botanical Garden, Kunming | Decay wood of unknown tree | 20 °C |
| G. gibbosum | KUMCC
19-0003 |
Baoshan, Yunnan | Living tree of Albizia sp. | 18 °C |
 |
Figure 1: Morphological characteristics of the nine strains of Ganoderma |
Grain Media for Inoculum Production
Ganoderma spawn was prepared using wheat (Triticum aestivum L.) as the main spawn medium, following the procedure described in Luangharn et al. (2017). Broken wheat grains were avoided as a potential cause of contamination (Narh et al., 2011). Wheat grains were washed and soaked for 18 hours. Next, 200 grams of wheat grain were filled into spawn bottles and autoclaved at 121 °C for 30 min. After being left to cool down, three pieces of active mycelia from agar media were cut 2–3 cm in diameter and inoculated into the spawn media bottles. The spawn media bottles were incubated at 25 ± 1 °C. 24–30 days after inoculation, and once the white mycelia fully covered the wheat grains, they were then considered to be viable mother spawn.
Bag Preparation for Fruiting Body Production
Alnus cremastogyne sawdust was obtained locally and used as the main substrate. Our basic formula for growing Ganoderma substrate was determined on the basis of dry weight (Smith et al., 2002). The substrate was composed of sawdust (80%), wheat bran (17%), calcium carbonate (CaCO3) (1%), magnesium sulfate (MgSO4) (1%), and sucrose (1%). All organic and inorganic additives were mixed well. The pH was stabilized by CaCO3 and MgSO4 at pH 7–8 (Fang et al., 2002), and levels below optimal pH values were adjusted with CaCO3. Water was gradually added until the moisture content reached 70–75%, or until the substrate clumped when squeezed by hand. Eight hundred grams of sawdust substrate were filled into the thermo-stable polypropylene bags (6.50 × 12.50 inch). Holes (~5 cm) were punched in the top of the substrate bags, and a plastic ring was used to seal the tops of the growing bags. The substrate bags were sterilized in 121 °C for 40 min. After sterilization, the substrate bags were left to cool for 18 hrs.
Approximately 5–8 grams of active mother spawn were placed in the substrate bags under aseptic conditions, after which the bags were sealed and incubated at 28 ± 2 °C for 26–30 days. All substrate bags were incubated at a relative humidity between 65–75% and maintained under dark conditions to induce mushroom mycelial formation. After the mycelia completely colonized the substrate in the bags, all bags were moved to the growing rooms to apply the two different cultivation treatments: the soil casing and non-casing methods. The soil casing method was performed in a clean area, with sufficient ventilation and adjustable humidity. A dark sheet was used to cover the base of the growing area. Next, approximately 5–8 cm of clay soil was placed as a on top of the black sheet, acting as a ground layer. The soil was sourced from a nearby village (Xiaoshao Village, Ciba Town, Panlong District, Kunming). The fully colonized mushroom bags were cut at top and bottom, placed on the soil bed, and finally covered with a casing layer of the same clay-based soil. For the non-casing method, the fully colonized growing bags were kept in a clean room with good ventilation. Both bags were grown at 25–28 °C, and water was sprayed daily to maintain the relative humidity at 75–85% during primordia initiation, while 70–80% humidity was maintained during the formation of mature fruiting bodies until the fruiting bodies fully developed. Mycelia growth, primordia initiation, fruiting development, and fructification periods were recorded regularly. All experiments were carried out with ten replicates.
Harvesting and Yield Data
The number of primordia, young primordia, and mature fruiting bodies per bag were recorded during the total harvest period of 180–185 days. Mature fruiting bodies (indicated by caps becoming completely red and the white margin disappearing) were manually harvested (Royse 1996), counted, and weighed. The biological efficiency (BE) and total yields (g/Kg-1) were determined by following the protocols described by Royse (2010) and Peksen and Yakupoglu (2009).
Data Collection and Statistical Analysis
Data analysis was carried out using the SPSS statistical program (Softonic International SA, Barcelona, Spain) with ten replicates. Data for initiation of primordia, young primordia production, mature fruiting body production, and time period for the development of young to mature fruiting bodies were collected. All the data were compared to obtain a mean separation using Tukey’s test (p < 0.05), followed by post-hoc tests that were expressed in a one-way ANOVA.
Results
Mushroom Cultures and Spawn Production
Colonization of Grow Bags
After 14 days of incubation, Ganoderma leucocontextum, G. resinaceum, and G. gibbosum were fully colonized with white mycelia on a PDA medium under dark conditions. Ganoderma leucocontextum, G. resinaceum, and G. gibbosum mycelia were produced on wheat grain media after being incubated for 3–4 days. White mycelia of G. leucocontextum and G. resinaceum were seen on wheat grain media after being incubated at 25 ± 1 °C for 3–4 days, and the grains were fully colonized after 22 days of incubation. After only 3 days we observed colonization of the wheat grain media from the cultures derived from the mature G. gibbosum, white massive mycelia were seen after 2–4 days of further incubation, and the grains were fully covered after 18 days of incubation at 25 ± 1 °C. The cultures derived from the young G. gibbosum spawn took 5 days before we observed grain colonization, and grains were fully covered after 26 days of incubation at 25 ± 1 °C.
Ganoderma Leucocontextum Production
Cultivation Using a Soil Casing Layer
Ganoderma leucocontextum (KUMCC17-0007) was successfully cultivated with the soil casing and non-casing layer methods, obtaining higher total yields when cultivated with the casing layer method. Results of the fruiting cycles of G. leucocontextum grown with the soil casing layer are shown in Table 2, 3, and Figure 2. Three fruiting cycles were obtained when the casing layer method was applied. In the first cycle, initial primordia were seen on day 26, developing into young fruiting bodies on day 36, and harvested as mature fruiting bodies on day 58. The second cycle began 18 days after the first cycle was harvested, and its mature fruiting bodies were harvested after 35 days. The third cycle was initiated much later than the first two cycles: primordia were seen on day 25, and mature fruiting bodies were harvested after 41 days. A single fruiting body per bag was produced with both soil casing and non-casing methods. Fruit body sizes in the first fruiting cycle were larger than in other cycles, and generally this mushroom showed strongly laccate fruiting bodies. Morphological characteristics of the mature fruiting bodies resulting from the casing layer method were stipitate and annual, applanate, and dimidiate pilei shape, with plump pilei (Figure 2).
Table 2: Results of the total numbers of initial primordial formed, primordial, and mature fruiting bodies of Ganoderma leucocontextum, G. resinaceum, and G. gibbosum.
| Fungal species | Fungal strains | No. of initial primordial formed | No. of primordia | No. of mature fruiting bodies | |||
| SC | NC | SC | NC | SC | NC | ||
| Ganoderma leucocontextum | KUMCC
17-0007 |
102 | 31 | 98 | 24 | 61 | 14 |
| G. resinaceum | KUMCC
19-0001 |
22 | 57 | 18 | 50 | 9 | 35 |
| G. gibbosum | KUMCC
17-0004 |
36 | 22 | 33 | 20 | 33 | 20 |
| G. gibbosum | KUMCC
17-0005 |
39 | 32 | 39 | 31 | 35 | 30 |
| G. gibbosum | KUMCC
17-0009 |
24 | 0 | 22 | 0 | 20 | 0 |
| G. gibbosum | KUMCC
17-0013 |
36 | 33 | 36 | 33 | 34 | 32 |
| G. gibbosum | KUMCC
18-0007 |
48 | 23 | 43 | 23 | 40 | 22 |
| G. gibbosum | KUMCC
19-0002 |
39 | 35 | 37 | 34 | 37 | 33 |
| G. gibbosum | KUMCC
19-0003 |
20 | 0 | 20 | 0 | 20 | 0 |
Notes: SC = Soil casing; NC = Non-casing
Table 3: Results of the fruiting cycles of Ganoderma leucocontextum, G. resinaceum, and G. gibbosum cultivated by both soil casing and non-casing layer methods. Values with the same column followed by the same letter are not significantly different (p < 0.05) by Tukey’s test.
| Fungal species | Fungal strains | Average wet weight of
fruiting bodies (g) |
Biological Efficiency (BE) (%) | Total yield
(g/Kg-1) |
|||
| SC | NC | SC | NC | SC | NC | ||
| Ganoderma leucocontextum | KUMCC
17-0007 |
203.06 ± 0.17b | 46.54 ± 0.11e | 60.43 | 13.60 | 253.82 | 58.18 |
| G. resinaceum | KUMCC
19-0001 |
21.55 ± 0.8g | 87.41 ± 0.45d | 26.94 | 109.26 | 7.02 | 27.75 |
| G. gibbosum | KUMCC
17-0004 |
147.05 ± 0.93d | 119.26 ± 0.64c | 42.24 | 33.15 | 183.81 | 149.07 |
| G. gibbosum | KUMCC
17-0005 |
227.32 ± 0.71a | 137.67 ± 0.22ab | 73.80 | 40.26 | 284.15 | 172.08 |
| G. gibbosum | KUMCC
17-0009 |
88.70 ± 0.85e | 0f | 26.72 | 0 | 110.88 | 0 |
| G. gibbosum | KUMCC
17-0013 |
182.96 ± 0.15c | 125.10 ± 0.11bc | 61.20 | 39.09 | 228.70 | 156.37 |
| G. gibbosum | KUMCC
18-0007 |
154.54 ± 0.10d | 116.76 ± 0.11c | 45.98 | 30.39 | 193.17 | 145.95 |
| G. gibbosum | KUMCC
19-0002 |
183.84 ± 0.13c | 141.81 ± 0.41a | 60.66 | 44.45 | 229.80 | 177.26 |
| G. gibbosum | KUMCC
19-0003 |
73.02 ± 0.42f | 0f | 20.92 | 0 | 91.28 | 0 |
Notes: SC = Soil casing; NC = Non-casing
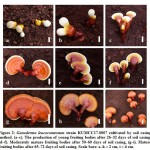 |
Figure 2: Ganoderma leucocontextum strain KUMCC17-0007 cultivated by soil casing method. |
Cultivation Without a Casing Layer
The non-casing layer method resulted in two fruiting cycles (Figure 3). The highest yield was obtained from the first fruiting cycle. The initial primordia appeared at the top of the grow bags after 18 days of incubation at 25 ± 1 °C; the mushroom primordia developed into young fruiting bodies within 34 days; and the mature fruiting bodies were harvested after 60 days. The second cycle was initiated 24 days after the first harvest, and mature fruiting bodies were harvested after 54 days. Only one out of four bags produced a fruiting body, and six bags out of the total used in the experiment produced primordia. This mushroom strain required 180 days to complete three cycles via the casing layer method, while it took 138 days to complete two cycles with the non-casing method. Morphological characteristics of the mature fruiting bodies resulting from the non-casing layer method formed a long stipe with non-pilei when young that became pilei when mature, with some wrinkled pilei (Figure 3).
 |
Figure 3: Ganoderma leucocontextum strain KUMCC17-0007 cultivated by non-casing method. |
Ganoderma Resinaceum Production
Cultivation Using a Soil Casing Layer
Laccate Ganoderma resinaceum (KUMCC19-0001) was cultivated successfully, with one fruiting cycle obtained using the soil casing method, and two fruiting cycles using the non-casing method (Table 2, 3). G. resinaceum had a lower yield when cultivated using the casing layer method. On day 36 after the soil casing was applied, initial primordia were observed. The primordia developed to the young stage within 26 days, and mature fruiting bodies were harvested on day 30. Although 1–4 initial primordia were observed on the surface, only 1–3 mature fruiting bodies per bag developed and were collected. Morphological developments of the fruiting bodies exposed to the soil casing layer method are shown in Figure 4. Base on the morphological characteristics of G. resinaceum, when the soil casing was used, G. resinaceum were generally composed of white and strongly laccate fruiting bodies, whereas with non-casing treatment, the fruiting bodies were dull and pale when young, weakly laccate when mature, and strongly laccate when old.
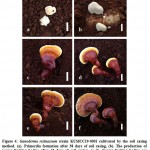 |
Figure 4: Ganoderma resinaceum strain KUMCC19-0001 cultivated by the soil casing method. |
Cultivation without a Casing Layer
The morphological developments of its fruiting bodies produced in the non-casing layer treatment are shown in Figure 5. Primordia formation was seen 22 days after incubation at 25 ± 1 °C. Young fruiting bodies were seen on day 33, and mature fruiting bodies were harvested on day 54. The primordia of the second cycle were observed 24 days after the first cycle was harvested, and mature fruiting bodies of the second cycle were harvested after 41 days. However, no primordia were produced after the second fruiting cycle. We obtained a single mature fruiting body per growing bag. Primordia were not observed in one of the growing bags, and one of the harvested fruiting bodies was abnormal. Morphological characteristics of the mature fruiting bodies resulting from the non-casing layer method were weakly laccate when young and strongly laccate when old.
 |
Figure 5: Ganoderma resinaceum strain KUMCC19-0001 cultivated without a casing layer. |
Ganoderma Gibbosum Production
Cultivation Using a Soil Casing Layer
Three fruiting cycles of G. gibbosum with well-developed fruiting bodies were obtained with the casing layer method. Five strains of G. gibbosum gave similar results with the casing layer method. Results of the fruiting cycles are shown in Table 2, 3. G. gibbosum KUMCC17-0005 was the most successful strain of this species, giving the highest total yield after the first cycle, followed successively by the strains KUMCC19-0002, KUMCC17-0013, KUMCC18-0007, and KUMCC17-0004 (Table 3). The initial primordia of these five G. gibbosum strains formed on the entire soil surface 22–24 days after the soil casing was applied. The production of the first cycle was harvested after 33–35 days. 25–27 days after the first cycle was harvested, the initial primordia of the second cycle were seen, and mature fruiting bodies were harvested after 46–48 days. The primordia of the third cycle were seen 25–28 days after the fruiting bodies of the second cycle were harvested, and mature fruiting bodies were again harvested after 40–42 days. However, in the fourth cycle, all G. gibbosum strains produced initial primordia with the soil casing layer method, but fruiting body development was not observed. The morphological development of the fruiting bodies of these five G. gibbosum strains are shown in Figures 6–7, 9–11. The two younger G. gibbosum strains (KUMCC17-0009 and KUMCC19-0003) were also cultivated successfully using the soil casing layer method (Figure 12–13). However, only two fruiting cycles were obtained from these two strains (Table 3). The morphological development of the two younger G. gibbosum fruiting bodies under the soil casing method are shown in Figures 13–14. The initial primordia of these two strains of G. gibbosum formed over the entire soil surface 36 days after the soil casing was applied. Production from the first cycle was harvested after 42 days. The initial primordia of the second cycle were seen 40 days after the fruiting bodies of the first cycle were harvested, and mature fruiting bodies were harvested after 56 days. Small primordia were seen in the third cycle, but the development of fruiting bodies was not seen.
 |
Figure 6: Ganoderma gibbosum strain KUMCC17-0004 cultivated using a soil casing layer. |
 |
Figure 7: Ganoderma gibbosum strain KUMCC17-0005 cultivated using a soil casing layer. |
 |
Figure 8: Ganoderma gibbosum strain KUMCC17-0005 cultivated using a soil casing layer. |
 |
Figure 9: Ganoderma gibbosum strain KUMCC17-0013 cultivated using a soil casing layer. |
 |
Figure 10: Ganoderma gibbosum strain KUMCC18-0007 cultivated using a soil casing layer. |
 |
Figure 11: Ganoderma gibbosum strain KUMCC19-0002 cultivated using a soil casing layer. |
 |
Figure 12: Ganoderma gibbosum strain KUMCC17-0009 cultivated using a casing layer. |
 |
Figure 13: Ganoderma gibbosum strain KUMCC19-0003 cultivated using a casing layer. |
Cultivation without a Casing Layer
Using the non-casing cultivation method, the following total yields were obtained for the listed strains: KUMCC19-0002 (177.26 g/Kg-1), KUMCC17-0005 (172.08 g/Kg-1), KUMCC17-0013 (156.37 g/Kg-1), KUMCC17-0004 (149.07 g/Kg-1), and KUMCC18-0007 (145.95 g/Kg-1) (Table 3). The formation of primordia initiation was seen after 18–22 days of incubation at 25 ± 1 °C; fruiting bodies were harvested after 23–26 days. After the first harvest the primordia of the second cycle were observed after 20–24 days, and their mature fruiting bodies were harvested after 36–40 days. Initial primordia of the third cycle were seen 35–42 days after the second cycle was harvested, and mature fruiting bodies were harvested after 48–50 days. The morphological developments of the Ganoderma gibbosum fruiting bodies under the non-casing method are shown in Figures 8, 14. The five strains of G. gibbosum showed non-laccate pilei when immature and a slightly dull or pale surface when developing to full maturity and into old age. They usually grew as single fruiting bodies, although a few formed chains of fruiting bodies (Figure 10d). A single fruiting body produced 1–3 pinheads (Figures 6a, 9a, 13a, c), and each substrate bag produced 1–3 single fruiting bodies.
 |
Figure 14: Ganoderma gibbosum strain KUMCC19-0002 cultivated without a casing layer. |
Discussion and Conclusion
The soil casing layer method has been investigated for some mushroom species (Zhed et al., 2012; Thongklang et al., 2014; Subbiah and Balan, 2015; Martos et al., 2017; Thongbai et al., 2017), but very few studies have reported on its application for Ganoderma species (Chen 2012; Hapuarachchi et al. 2018a). This study documents on the first successful cultivation of laccate and non-laccate Ganoderma by using both soil casing layer and non-casing layer methods.
Using the soil casing layer method in Ganoderma cultivation is the most suitable way to induce the production of fruiting bodies, since the soil casing layer helps maintain the moisture content in the growing substrate, an important factor also noticed by Cho et al., (2008). Colauto et al. (2010) stated that moisture content is a factor of great importance to promoting mushroom formation, and the soil casing layer traps additional water that can be accessed by Ganoderma spawn. However, selecting the appropriate soil, whether it be clay, loam, or sand, is dependent on the specific needs of the mushroom species (Amin et al., 2010; Martos et al., 2017). In this study, we selected clay soil, which is considered a suitable substrate material with a reasonable water-holding capacity that also retains good air flow to facilitate gaseous exchanges in the mushroom-growing substrate (Smerdon 1983).
Our new casing layer method had the higher number of growth cycles, highest yield within a short period of time when compared to the non-casing method for Ganoderma species. Cultivation using the soil casing method led to a higher number of growth cycles and yields (Table 3) by which we determined that these three Ganoderma species reached higher production levels than the non-casing method used for G. lucidum (Erkel 2009; Roy et al., 2015), and G. neojaponicum (Jo et al., 2010). Previous studies reported that total mushroom yield was obtained from one or two cycles in a harvest period of 90 days (Peksen and Yakupoglu, 2009), while our Ganoderma strains required approximately 180–185 days to complete all cycles and reach their harvest period. In addition, morphological characteristics found in the strains G. leucocontextum and G. resinaceum on which the casing layer method was used were strongly laccate when compared to the non-casing method. G. gibbosum resulted in a darker-colored and larger-sized pilei when compared to the non-casing method. In the first of the three cycles of G. gibbosum, large pilei dark in color were seen, whereas in the third cycle, smaller, lighter-colored pilei were observed.
We recommend isolating the original mature fruiting bodies to achieve a high yield of production, rather than isolating the originals from young fruiting bodies. For example, under the soil casing method, three cycles of G. gibbosum production were achieved when the originals were isolated from mature fruiting bodies, whereas only two cycles were achieved when the originals were isolated from young G. gibbosum fruiting bodies. Under the non-casing layer method, all G. gibbosum strains produced initial primordia, whereas using the non-casing layer method they did not produce initial primordia. Thus, this study also showed that strain type affects total yield and quality, i.e., strains originating from mature fruiting bodies had better yields than strains originating from young fruiting bodies, which has also been noted in other studies (Sakamoto 2018). Strains originating from young fruiting bodies (KUMCC17-0009 and KUMCC19-0003) had lower yields, longer incubation times, and required more time to produce mature pileus than the other five strains that originated from mature fruiting bodies. Thus, we agree with Sakamoto (2018) that in order to obtain a good yield, the original mushroom strain should be isolated from the mature fruiting body.
The conditions of our mushroom-growing are very similar to those of Zhou et al. (2012), whose study mentioned the temperature range for optimal Ganoderma mycelia growth to be between 15–35 °C. Jo et al. (2009), by contrast, stated it to be 25–27 °C (Jo et al., 2009). According to Cao and Yuan (2013), a temperature range of 20–25 °C is suitable for primordia formation, while a temperature range of 24–28 °C is suitable for fruiting body development. We agree with Cao and Yuan (2013) that ideal spawn-running temperature ranges between 28–30 °C. Our results suggest that a temperature range of 18–20 °C or lower affects the production of fruiting bodies, leading to slower development and ultimately stopping growth altogether. Chen et al. (2017) mentioned that temperatures higher than 30 °C negatively affect the growth and development of Ganoderma. In our study, we maintained the optimum relative humidity at 70–75% to promote the formation of mushroom mycelia; 80–85% during the formation of primordia initiation; and 70–80% during the formation of mature fruiting bodies. Our results resemble Peksen and Yakupoglu (2009), who have stated that the moisture content for promoting G. lucidum mycelia growth should be above 70%. Habijanic and Berovic (2000) showed that a moisture content less than 55% is not appropriate for mycelia growth, while Luangharn et al. (2017) also reported that humidity below 40% can cause slow mycelia growth in non-laccate G. australe.
Our study also revealed that wild laccate Ganoderma cultivation via the casing method has a higher yield than the non-casing method. Results from this study should prove valuable for the commercial production of G. leucocontextum, G. resinaceum, and non-laccate G. gibbosum. It should be noted that Ganoderma species cause disease and even death of trees (Hapuarachchi et al. 2019b). Therefore, growers need to be careful when disposing of spend Ganoderma compost.
Acknowledgements
We appreciate the kind support given by the University of Chinese Academy of Sciences, Beijing 100049, China; the Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China; and the Key Laboratory for Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China. Yinfeng and Huangfei are acknowledged for their invaluable assistance. The authors also thank Austin G. Smith and William A. Julian for their contributions to the English editing.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
This research was funded by the National Sciences Foundation, China, NSFC-TRF, Grand No. 41761144055, the National Sciences Foundation, China (NSFC), Grant No. 41771063, and the Chinese Academy of Sciences (CAS), Grant No. 2017CASSEABRIZD003, CAS President’s International Fellowship Initiative (PIFI) for funding his postdoctoral research (No. 2018PC0006) and the National Science Foundation of China (NSFC) for funding this work under the project code 31750110478, 2019 high-end foreign expert introduction plan (granted by the Ministry of Science and Technology of the People’s Republic of China, Grant Number G20190139006).
References
- Amin R, Khair A, Alam N and Lee T. S. Effect of different substrates and casing materials on the growth and yield of Calocybe indica. Mycobiology., 2010; 38(2): 97–101.
CrossRef - Cao Y and Yuan H. S. Ganoderma mutabile sp. nov. from southwestern China based on morphological and molecular data. Mycological Progress., 2013; 12: 121–126.
CrossRef - Chen A. W. Natural-log cultivation of the medicinal mushroom Ganoderma lucidum (Reishi). Mushroom grower’s newsletter., 2012; 3(9): 2–6.
- Chen X, Chen L, Li S and Zhao J. Meroterpenoids from the fruiting bodies of higher fungus Ganoderma resinaceum. Phytochemistry Letters., 2017; 22: 214–218.
CrossRef - Chen L, Chen X, Wang S, Bian Y, Zhao J and Li S. Analysis of triterpenoids in Ganoderma resinaceum using liquid chromatography coupled with electrospray ionization quadrupole – time – of – flight mass spectrometry. Int J Mass Spectrom., 2019; 436: 42–51.
CrossRef - Chen X. Q, Zhao J, Chen, L. X, Wang S. F, Wang Y and Li S. P. Lanostane triterpenes from the mushroom Ganoderma resinaceum and their inhibitory activities against α-glucosidase, Phytochemistry., 2018; 149: 103–115.
CrossRef - Cheng C. R, Yue Q. X, Wu Z. Y, Song X. Y, Tao S. J, Wu X. H, Xu P. P, Liu X, Guan S. H and Guo D. A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry., 2010; 71; 1579–1585.
CrossRef - Cho Y. S, Weon H. Y, Joh J. H, Lim J. H, Kim K. Y, Son E. S, Lee C. S and Cho B. G. Effect of casing layer on growth promotion of the edible mushroom Pleurotus ostreatus. Microbiology., 2008; 36(1): 40–44.
CrossRef - Ćilerdžić J, Stajić M and Vukojević J. Degradation of wheat straw and oak sawdust by Ganoderma applanatum. Int Biodeter Biodegr., 2016; 114: 39–44.
CrossRef - Colauto N. B, Silveira A. R, Eira A. F and Linde G. A. Production flush of Agaricus blazei on Brazilian casing layers. Braz J Microbiol., 2011; 42: 616–623.
CrossRef - Cui B. K, Li H. J, Ji X, Zhou J. L, Song J, Si J, Yang Z. L and Dai Y. C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers., 2019; 1–256.
CrossRef - Dai Y. C, Yang Z. L, Cui B. K, Yu C. J and Zhou L. W. Species diversity and utilization of medicinal mushrooms and fungi in China. Int J Med Mushrooms., 2009; 11; 287–302.
CrossRef - De Silva D. D, Rapior S, Fons F, Bahkali, A. H and Hyde K. D. Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Divers., 2012a; 55: 1–35.
CrossRef - De Silva D. D, Rapior S, Hyde K. D and Bahkali A. H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers., 2012b; 56: 1–29.
CrossRef - De Silva D. D, Rapior S, Sudarman E, Stadler M, Xu J, Alias S. A and Hyde K. D. Bioactive metabolites from macrofungi: ethnopharmacology, biological activities and chemistry. Fungal Divers., 2013; 62: 1–40.
CrossRef - Erkel E. I. The effect of different substrate mediums on yield of Ganoderma lucidum (Fr.) Karst. JFAE., 2009; 7; 841–844.
- Fang Q. H and Zhong J. J. Effect of initial pH on production of ganoderic acid and polysaccharide by submerged fermentation of Ganoderma lucidum. Process Biochem., 2002; 37(7): 769–774.
CrossRef - Hapuarachchi K. K, Elkhateeb W. A., Karunarathna S. C, Cheng C. R, Bandara A. R, Kakumyan P, Hyde K. D, Daba G. M and Wen T. C. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere., 2008; 9(5): 1025–1052.
CrossRef - Gao Y, Zhou S, Huang M and Xu A. Antibacterial and antiviral value of the genus Ganoderma P. Karst. Species (Aphyllophoromycetidae): a review. Int J Med Mushrooms., 2003; 5(3): 235–246.
CrossRef - Guo X. Y, Liu D, Ye M, Han J, Deng S, Ma X. C, Zhao Y, Zhang B, Shen X and Che Q. M. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal., 2013; 75: 64–73.
CrossRef - Habijanic J and Berovic M. The relevance of solid-state substrate moisturing on Ganoderma lucidum biomass cultivation. Food Sci. Biotechnol., 2000; 38: 225–228.
- Hapuarachchi K. K, Elkhateeb W. A, Karunarathna S. C, Cheng C. R., Bandara A. R, Kakumyan P, Hyde K. D, Daba G. M and Wen T. C. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere., 2018a; 9: 1025–1052.
CrossRef - Hapuarachchi K. K, Karunarathna S. C, Raspé O, De Silva K. H. W. L, Thawthong A, Wu XL, Kakumyan P, Hyde K. D and Wen T. C. High diversity of Ganoderma and Amauroderma (Ganodermataceae, Polyporales) in Hainan Island, China. Mycosphere., 2018b; 9: 931–982.
CrossRef - Hapuarachchi K. K, Karunarathna S. C, McKenzie E. H. C, Wu X. L, Kakumyan P, Hyde K. D and Wen T. C. High phenotypic plasticity of Ganoderma sinense (Ganodermataceae, Polyporales) in China. AJOM., 2019a; 2: 1–47.
CrossRef - Hapuarachchi K. K, Karunarathna S. C, Phengsintham P, Yang H. D, Kakumyan P, Hyde K. D and Wen T. C. Ganodermataceae (Polyporales): Diversity in Greater Mekong Subregion countries (China, Laos, Myanmar, Thailand and Vietnam). Mycosphere., 2019b; 10: 221–309.
CrossRef - He M. Q, Zhao R. L, Hyde K. D, Begerow D, Kemler M, Yurkov A, McKenzie O, Kakishima M, Sánchez-Ramírez, Vellinga E. C, Halling R, Papp V, Zmitrovich I. V, Buyck B, Ertz D, Wijayawardene N. N, Cui B. K, Schoutteten N, Liu X. Z, Li T. H, Yao Y. J, Zhu X. Y, Liu A. Q, Li G. J, Zhang M. Z, Ling Z. L, Cao B, Antonıín V, Boekhout T, da Silva B. D. B, De Crop E, Decock C, Dima B, Dutta A. K, Fell J. W, Gem J, Ghobad-Nejhad M, Giachini A. J, Gibertoni T. B, Gorjón S. P, Haelewaters D, He S. H, Hodkinson B. P, Horak E, Hoshino T, Justo A, Lim Y. W, Menolli Jr N, Mešié A, Moncalvo J. M, Mueller G. M, Nagy L. G, Nilsson R. H, Noordeloos M, Nuytinck J, Orihara T, Ratchadawan C, Rajchenberg M, Silva-Filho A. G. S, Sulzbacher M. A, Tkalčec Z, Valenzuela R, Verbeken A, Vizzini A, Wartchow F, Wei T. Z, Wei M, Zhao C. L and Kirk P. M. Notes, outline and divergence times of Basidiomycota. Fungal Divers., 2019; 99: 105–367.
CrossRef - Hennicke F, Cheikh-Ali Z, Liebisch T, Maciá-Vicente J. G, Bode H. B and Piepenbring M. Distinguishing commercially grown Ganoderma lucidum from Ganoderma lingzhi from Europe and East Asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry., 2016; 127: 29–37.
CrossRef - Hiroo S. Method for cultivating Ganoderma amboinense. 2008; http://www.sumobrain.com/patents/jp/Method-cultivating-ganodermaamboinense/
- Jo E. Y, Cheon J. L and Ahn J. H. Effect of food waste compost on the antler-type fruiting body yield of Ganoderma lucidum. Mycobiology., 2013; 41: 42–46.
CrossRef - Jo W. S., Cho Y. J., Cho D. H., Park S. D., Yoo Y. B and Seok S. J. Culture conditions for the mycelial growth of Ganoderma applanatum. Mycobiology., 2009; 37(2): 94–102.
CrossRef - Jo W. S, Park H. N, Park S. H, Jung H. Y and Yoo Y. B. Fruit-body production of Ganoderma neojaponicum by sawdust cultivation. Korean J Mycol., 2010; 38(2): 199–201.
CrossRef - Justo A, Miettinen O, Floudaas D, Ortiz-Santana B, Sjökvist E, Lindner D, Nakasone K, Niemela D, Nakasone K, Niemelö T, Larsson K. H, Ryvarden L and Hibbett D. S. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol., 2017; 121(9): 798–894.
CrossRef - Li T. H, Hu H. P, Deng W. Q, Wu S. H, Wang D. M and Tsering T. T. Ganoderma leucocontextum, a new member of the G. lucidum complex from southwestern China. Mycoscience., 2015; 56: 81–85.
CrossRef - Liu S. R, Ke B. R, Zhang W. R, Liu X. R and Wu X. P. Breeding of new Ganoderma lucidum strains simultaneously rich in polysaccharides and triterpenes by mating basidiospore-derived monokaryons of two commercial cultivars. Sci Hortic., 2017; 216: 58–65.
CrossRef - Luangharn T, Karunarathna S. C, Khan S, Xu J. C, Mortimer P. E and Hyde K. D. Antibacterial activity, optimal culture conditions and cultivation of the medicinal Ganoderma australe, new to Thailand. Mycosphere., 2017; 8(8): 1108–1123.
CrossRef - Martos E. T, Zied D. C, Junqueira P. D. G, Rinker D. L, Silva R. D, Toledo R. C. C and Diasa E. S. Casing layer and effect of primordia induction in the production of Agaricus subrufescens mushroom. ANRES., 2017; 51(4): 231–234.
CrossRef - Moncalvo J. M and Ryvarden L. A nomenclatural study of the Ganodermataceae Donk. Fungi Flola., 1997; 11: 1–114.
- Narh D. L, Obodai M, Baka D and Dzomeku M. The efficacy of sorghum and millet grains in spawn production and carpophores formation of Pleurotus ostreatus (Jacq. Ex. Fr) Kummer. Int Food Res J., 2011; 18: 1143–1148.
- Nicholas L. G and Ogame K. Psilocybin mushroom handbook: easy indoor & outdoor cultivation. Canada, Quick American, 2006.
- Niu X. M, Li S. H, Xiao W. L, Sun H. D and Che C. T. Two new lanostanoids from Ganoderma resinaceum. J Asian Nat Prod Res., 2007; 9(7): 659–664.
CrossRef - Paterson R. R. M. Ganoderma – A therapeutic fungal biofactory. Phytochemistry., 2006; 67: 1985–2001.
CrossRef - Peksen A and Yakupoglu G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World J Microb Biot., 2009; 25: 611–618.
CrossRef - Pu D. B, Zheng X, Gao J. B, Zhang X. J, Qi Y, Li X. S, Wang Y. M, Li X. N, Li X. L, Wan C. P and Xiao W. L. Highly oxygenated lanostane-type triterpenoids and their bioactivity from the fruiting body of Ganoderma gibbosum. Fitoterapia, 2007; 119: 1–7.
CrossRef - Richter C, Wittstein K, Kirk P. M and Stadler M. An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fungal Divers., 2015; 71: 1–15.
CrossRef - Royse D. J. Specialty mushrooms. Arlington, VA, ASHS Press, 1996.
- Royse D. J. Effects of fragmentation, supplementation and the addition of phase II compost to 2nd break compost on mushroom (Agaricus bisporus) yield. Bioresour Technol., 2010; 101: 188–192.
CrossRef - Roy S, Jahan M. A. A, Das K. K, Munshi S. K and Noor R. Artificial cultivation of Ganoderma lucidum (Reishi Medicinal Mushroom) using different sawdust as substrates. AJBIO., 2015; 3(5): 178–182.
CrossRef - Sakamoto Y. Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol Rev., 2018; 32: 236–248.
CrossRef - Shiao M. S. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chemical Rec., 2003; 3(3): 172–180.
CrossRef - Smith J. E, Rowan N. J and Sullivan R. Medicinal mushroom: A rapid developing area of biotechnology for cancer therapy and other bioactivities. Biotechnol Lett., 2002; 24: 1839–1845.
CrossRef - Smerdon M. Thoughts on casing. Mushroom J., 1983; 124: 193–194.
- Stamets P. Growing gourmet and medicinal mushrooms. Berkeley, CA, Ten Speed Press, 2000.
- Subbiah K. A and Balan V. A comprehensive review of tropical milky white mushroom (Calocybe indica P&C). Mycobiology., 2015; 43(3): 184–194.
CrossRef - Tan W. C, Kuppusamy U. R, Phan C. W, Tan Y. S, Raman J, Anuar A. M and Sabaratnam V. Ganoderma neo-japonicum Imazeki revisited: domestication study and antioxidant properties of its basidiocarps and mycelia. Sci Rep., 2015; 5: 1–10.
CrossRef - Teng B. S, Wang C. D, Yang H. J, Wu J. S, Zhang D, Zheng M, Fan Z. H, Pan D and Zhou P. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J Agr Food Chem., 2011; 59: 6492–6500.
CrossRef - Thongbai B, Wittstein K, Richter C, Miller S. L, Hyde K. D, Thongklang N, Klomklung N, Chukeatirote E and Stadler M. Successful cultivation of a valuable wild strain of Lepista sordida from Thailand. Mycol Prog., 2017; 16(4): 311–323.
CrossRef - Thongklang N, Sysouphanthong P, Callac P and Hyde K. D. First cultivation of Agaricus flocculosipes and a novel Thai strain of A. subrufescens. Mycosphere., 2014; 5(6): 814–820.
CrossRef - Wang K, Bao L, Xiong W, Ma K, Han J, Wang W, Yin W and Liu H. (2015). Lanostane triterpenes from the Tibetan medicinal mushroom Ganoderma leucocontextum and their inhibitory Effects on HMG-CoA reductase and α‑Glucosidase. J Nat Prod., 78; 1977−1989.
CrossRef - Wasser S. P and Weis A. L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspective (review). Int J Med Mushrooms., 1999; 1: 31–62.
CrossRef - Zhed D. C, Pardo-Giménez A, de Almeida Minhonia M. T, Villas-Boas R. L, Alvarez-Orti M and Pardo-Gonzálezd J. E. Characterization, feasibility and optimization of Agaricus subrufescens growth based on chemical elements on casing layer. Saudi J Biol Sci., 2012; 19(3): 343–347.
CrossRef - Zhou X. W, Su K. Q and Zhang Y. M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl Microbiol Biotechnol., 2012; 93: 941–963.
CrossRefe

This work is licensed under a Creative Commons Attribution 4.0 International License.






