How to Cite | Publication History | PlumX Article Matrix
Dhaval V Parmar1 , Valentina V Umrania2
, Valentina V Umrania2 , Madhulika A Mistry3
, Madhulika A Mistry3 , John J Georrge4
, John J Georrge4
![]() , Nutan P Vishwakarma5
, Nutan P Vishwakarma5 and R. Z. Sayyed6
and R. Z. Sayyed6![]()
1Department of Microbiology, M. V. M. Science and Home Science College, Rajkot, Gujarat, India.
2Department of Microbiology, PDU Government Medical College, Rajkot, Gujarat, India.
3Department of Bioinformatics, Christ College, Rajkot, India.
4Department of Biotechnology, Shree M. and N. Virani Science College, Rajkot, Gujarat, India.
5Department of Microbiology, PSGVPM'S Arts, Science and Commerce College, Shahada, 425409 Maharashtra, India.
Corresponding Author E-mail : sayyedrz@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2833
ABSTRACT: Urinary tract infections are a major infection burden globally and antimicrobial resistance can lead to treatment failures as well as upsurge cost of healthcare. The study was aimed to know the common pathogens responsible for urinary tract infection, their antibiotic resistance pattern, and bundle care effectiveness. 2352 urine samples were studied from the year 2013 to 2017. Urine culture and antibiotic sensitivity testing were carried out. Care bundle compliance for catheter-associated urinary tract infections (CAUTI) was observed during the starting year (2013) and end year (2017). 46.3% of samples found positive for the presence of significant bacteriuria. Escherichia coli was the commonest isolate followed by Pseudomonas aeruginosa and Klebsiella pneumoniae. All antibiotics found more or less resistant against various organisms, most trusted antibiotics against UTI, Nitrofurantoin found 20.8% (CI = 18.0-23.8) resistant overall. Out of all E. coli isolated,86.0% were possible extended-spectrum beta-lactamase (ESBL) producers, 18.3% were carbapenem-resistant, 20.7% were amikacin resistant and 0.7% were colistin-resistant.The number of CAUTIand CAUTI rates per 1000 catheter days decreased where, p = 0.0482 and p = 0.0783 respectively.CAUTI bundle shows no significant difference;p = 0.8475.Non-bias compliance surveys and continuous monitoring with quality care can limit nosocomial infections.
KEYWORDS: Antibiotic Resistance; Bundle Care Approach; Urinary Isolates; Urinary Tract Infection
Download this article as:| Copy the following to cite this article: Parmar D. V, Umrania V. V, Mistry M. A, Georrge J. J, Vishwakarma N. P, Sayyed R. Z. Antibacterial Resistance Trend in Urinary Tract Infections and Their Control at a Tertiary Care Hospital in the Saurashtra Region of Gujarat, India. Biosci Biotech Res Asia 2020;17(2). |
| Copy the following to cite this URL: Parmar D. V, Umrania V. V, Mistry M. A, Georrge J. J, Vishwakarma N. P, Sayyed R. Z. Antibacterial Resistance Trend in Urinary Tract Infections and Their Control at a Tertiary Care Hospital in the Saurashtra Region of Gujarat, India. Biosci Biotech Res Asia 2020;17(2). Available from: https://bit.ly/2N6kIxf |
Introduction
Urinary tract infections (UTIs) are very common and diagnosed with the help of urine culture technique.1,2 It is always advisable to correlate clinical symptoms with the results of the test. Organisms like Escherichia coli which are the most frequently isolated organism in uncomplicated and complicated UTIs. However, it creates ambiguity towards choosing correct empirical treatment.3 Antimicrobial resistance in nosocomial UTIs, especially catheter-associated urinary tract infections poses grave concerns for antimicrobial effectiveness in treating.4,5 It is necessary to measure and compare the antimicrobial resistance in hospitals regularly because the effects of antimicrobial resistance are mainly felt in healthcare facilities.6
Urinary catheters are used in critical patients, especially those who are unable to move from their bed or unable to empty the bladder naturally due to some clinical condition. The catheter remains attached for a long period, which leads to catheter-associated urinary tract infection (CAUTI) because of the catheter act as a reservoir for multidrug-resistant organisms and responsible for hospital-acquired infections. Such infections are preventable by implementing a bundle of care.7,8 CAUTI bundle care is an evidence-based guideline to assess the need, proper handling, and earliest removal of catheters to alleviate the risk in the patient.9
The study aimed to summarize the most common pathogens of UTIs and their antimicrobial susceptibility patterns, so this may be helpful while preparing the local empirical treatment regimens. The study also aims to evaluate the effectiveness of CAUTI bundle care as it checks whether it succors to reduce CAUTI by minimizing the number of days of catheterization or not.
Materials and Methods
Urine Culture and Antibiotic Sensitivity
Urine samples received from the inpatient department as well as the outpatient department were processed as per the standard operating procedure followed by the hospital.10 Samples were streaked on sheep blood agar and MacConkey’s agar with a calibrated nichrome wire loop and incubated for 24 h at 35oC. After 24 h, if any microbial growth found, it was carried for identification procedure. If no growth observed, then re-incubated and observed for microbial growth after a total of 48 h from the first incubation. Identification of organism and antibiogram was carried out by using automated system MicroScanautoSCAN (Siemens, Germany; now takeover by Beckman Coulter, U.S.A.).
Bundle Care Compliance
Infection control program surveillance regarding urinary tract infections was carried out with the help of a trained infection control nurse. Bundle care assessment and data about that were collected from the daily registers. Starting year (2013) and end year (2017) survey was taken in the account for CAUTI bundle care compliance to observe the difference.
Mostly two events were observed in bundle care: 1) Insertion of the catheter, and 2) Maintenance of catheter. Insertion care included the following points: hand hygiene must be performed before starting the insertion procedure, gloves must be worn before handling the catheter, the catheter must be secured in a comfortable position. Maintenance care included following points: catheter care or perineal care must be carried out in each shift, bladder wash must be given as per the treating doctor’s recommendations, urobag must be emptied when it gets 2/3rd full, or 8 h and also before transporting the patient, all junctions and connections in the tubing must be kept closed or not, urobag musts kept below the level of the urinary bladder, drainage bag, and tubing must not touch the floor, before collection of the urine sample, collection site must be disinfected with 70% alcohol swab, the patient must be educated regarding the care of catheter.
Data Analysis
Software WHONET-2019 (developed by WHO Collaborating Centre for the surveillance of antimicrobial resistance), EpiInfo (version 7.2.3.1), and SPSS were used for antibiotic resistance trends and statistical analysis, respectively.
Results
Urinary Isolates and Their Antibiogram
In this study, 2352 urine samples were tested for culture and sensitivity, out of which 46.3% of urine samples showed significant bacteriuria (colony-forming unit > 100000 per mL). Distribution according to gender shows 3.6% more significant bacteriuria in the case of females than male, statistically, fisher exact one-tailed p-value is < 0.05 which states that the rise is significant (Table 1).
Table 1: Gender wise distribution of occurrence of UTI
| Gender | Total samples | Total positive | % positivity |
| Male | 1224 | 545 | 44.5 |
| Female | 1128 | 543 | 48.1 |
| Total | 2352 | 1088 | 46.3 |
A total of 1088 isolates were recovered from the urine sample. Out of total isolates, 57 (5.2%) were Gram-positive organisms, 61 (5.6%) were Candida sp. and 970 (89.2%) were Gram-negative organisms. Escherichia coli (541, 49.7%) was isolated most frequently followed by Ps. aeruginosa (193, 17.7%), Klebsiella pneumoniae (190, 17.5%), Candida albicans (32, 2.9%), Enterococcus faecalis (26, 2.4%), Candida tropicalis (25, 2.3%), Enterococcus faecium(24, 2.2%). The current study had excluded Candida sp. as well as organisms that were less frequently isolated (<2%) in this study. Out of all E. coli (54, 100%) isolated, 86.0% were possible extended-spectrum beta-lactamase (ESBL) producers, 18.3% were carbapenem-resistant, 20.7% were amikacin resistant and 0.7% were colistin-resistant.
Out of all Ps. aeruginosa (193, 100%) isolated, 1.6% resistant to colistin, 76.7% to tobramycin, 81.9% to ciprofloxacin, 74.6% to cefepime, 64.8% to piperacillin/tazobactam, 68.1% to meropenem, and 65.7% to aztreonam. Out of all K. pneumoniae total (190, 100%) isolated, 88.4% were possible ESBL producers, 64.7% were carbapenem-resistant, 55.8% were amikacin resistant and 1.6% were colistin-resistant.Out of all E. faecalis (26, 100%), 7.7% were resistant to penicillin whereas, in the case of E. faecium (24, 100%), 8.3% were resistant to linezolid. Overall resistance pattern of Gram-negative and Gram-positive isolates with their 95% R% confidence interval (C.I.) (Figures 1 and 2).
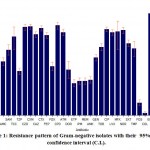 |
Figure 1: Resistance pattern of Gram-negative isolates with their 95% R% confidence interval (C.I.). |
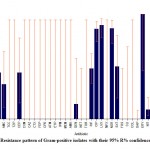 |
Figure 2: Resistance pattern of Gram-positive isolates with their 95% R% confidence interval (C.I.). |
Bundle Care Compliance
Catheterised patients during the starting and end year survey were 4686 and 3859 respectively. An unpaired t-test shows a significant difference in the number of catheterized patients in starting and in end-year conditions; t=5.821, p =0.0001. Catheter-associated urinary tract infections were 37 and 19 for starting and end year respectively. An unpaired t-test shows a significant reduction in CAUTI patients in end year as compared to the starting year where t=2.092, p = 0.0482. Urinary catheter days were 18744 and 16107 for starting and end year respectively. An unpaired t-test shows a significant reduction in the urinary catheter days in the end year as compared to the starting year, where t=3.890, p = 0.0008. CAUTI rate per 1000 catheter days were 2.0 and 1.1 for starting and end year respectively. An unpaired t-test shows no significant difference where t=1.846, p = 0.0783. CAUTI bundle care compliance was 92.6% and 93.0% for starting and end year respectively. CAUTI bundle compliance shows no significant rise in end-year which was demonstrated by an unpaired t-test where t=0.1946, p = 0.8475.
Emphasis on the reduction of usage of higher antibiotics like colistin, nitrofurantoin, and vancomycin showed a reduction in resistance during the end line. Table 2 shows the difference between the starting year and end year survey upon such antibiotics.
Table 2: Antibiotics which shows decline in resistance
| Antibiotic | Baseline R% (R%95% C.I.) | End line R% (R%95% C.I.) |
| Gram negative isolates | ||
| Colistin | 6.0 (3.4-10.1) | 0.0 (0.0-3.2) |
| Tigecycline | 13.1 (9.1-18.4) | 0.0 (0.3-6.3) |
| Nitrofurantoin | 24.3 (18.4-31.2) | 20.7 (14.0-29.4) |
| Gram positive isolates | ||
| Vancomycin | 0.0 (0-43.9) | 0.0 (0-37.1) |
| Teicoplanin | 0.0 (0-48.3) | (0-34.5) |
| Daptomycin | 0 (0-48.3) | 0 (0-34.5) |
Discussion
This study proffers the details about the bacterial and fungal isolates responsible for UTIs as well as also gives antibiotic resistance patterns for bacterial isolates. It also proffers the cognizance about the importance of the selection of antibiotics in the UTI treatment. Effectiveness of the infection control program with the aspect of bundle care compliance can be well understood.
The current study shows the highest culture positivity i.e. 46.3%; whereas Pondei et al11 shows 37.38%and Aboderin et al12shows 35.8%. This study complies with Patel et al.13 and Demir et al14 study that shows the prevalence of UTI is more in females than males. E. coli remains the most common organism which was upheld by other studies too,13,15-22 whereas Aboderin et al12 found Klebsiella spp. as a major isolate. The distribution of bacteria is different in different parts of the world and studying the influencing factors that cause this infection in unassociated geographical regions, indicates their dissipation.23
The present study shows that E. coli was less resistant to nitrofurantoin, amoxicillin/clavulanic acid, tetracycline, gentamicin except for ciprofloxacin as compare to Aboderin et al study12. This shows the importance of local analysis of antibiotics. E. coli shows resistance to ampicillin, ampicillin/sulbactam, cefuroxime, levofloxacin, meropenem, nitrofurantoin, tobramycin, cefazolin in Patel et al study13 which is quite lower than the current study except for Nitrofurantoin. Similar results are seen in K. pneumoniae and Ps. aeruginosa except for nitrofurantoin for K. pneumoniae, where resistant pattern remains similar. Antibiotics advised under empirical treatment by a government body are also showing a certain level of resistance in different studies24. Misuse of antibiotics leads to increasing resistance which becomes a matter of attention. General Practitioners should consider about the microbiological profile and the antibiotic sensitivity pattern during management to avoid misuse of antibiotics.25
It has been estimated that due to symptomatic urinary tract infections, 7 million people take treatment at emergency units and 100,000 people seek for hospitalizations yearly. UTI has become the most frequent hospital-acquired infection, and responsible for as many as 35% of hospital-acquired infections. It is the second most common cause responsible for bacteremia in hospitalized patients.20 In the present study, it was found that CAUTI decreased in end year (p = 0.0482) with CAUTI rate per 1000 catheter days (p = 0.0783) which suggests though the number of infections significantly decreased infection rate change remains statistically insignificant. In Taiwan, the CAUTI rate decreased by 22.7% after the successful implementation of UTI bundle care26. However, a study conducted in the US shows no change in catheter-days in the end year as compared to the starting year (p = 0.90)27. Similarly, Agodi et al.28 show 4.2 CAUTI rate per 100 catheter days (the year 2006-2007) to 3.7 CAUTI rates per 100 catheter days (the year 2010-2011) with relative risk 1.13 and CI = 0.71-1.78 which was statistically significant.
CAUTI bundle care compliance was 92.6% and 93.0% for starting and end year respectively (p =0.8475) which suggests that difference was statistically insignificant. Upon implementation of CAUTI bundle care in the hospital, a decline in nosocomial infection was observed in many of the studies. Effective bundle implementation requires the dedication of nursing staff and with continuous monitoring.29-32
Conclusion
Urinary tract infections are the most common and some timeslife-threatening infections. Catheter-associated UTIs are also emerging and most of them are hospital-acquired. It was observed that antibiotic resistance has been reported for all known antibiotics and to confine prevention of infection is the convenient way. Effective implementation of bundled care approach can alleviate the burden of CA-UTI in the hospitals and implementation of antibiotic policy can save higher antibiotics as an option for an emergency. The in-vivo and in-vitro difference in antibiotic resistance should be studied. Continuous monitoring of resistance patterns and monitoring of the infection control program is inevitable.
Conflict of Interest
All authors declare that no conflicts of interest exist.
Funding Source
This research neither had any funding source nor received any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors acknowledge the authorities of the participating colleges for providing all the necessary facilities required for the present research.
Reference
- Gajdács M., Urbán E. Resistance Trends and Epidemiology of Citrobacter–Enterobacter–Serratiain Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-Year Survey. Medicina 2019;55(6):285
- Gajdács M., Ábrók M., Lázár, A., Burián, K. Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina2019;55(7):356
- Foxman B. The epidemiology of urinary tract infection. Rev. Urol. 2010;7(12):653-60.
- Fasugba, O., Mitchell, B. G., Mnatzaganian, G., Das, A., Collignon, P., and Gardner, A. Five-Year Antimicrobial Resistance Patterns of Urinary Escherichia coli at an Australian Tertiary Hospital: Time Series Analyses of Prevalence Data. PloS one.2016;11(10):e0164306.
- Bjerklund Johansen T.E., Cek M., Naber K., Stratchounski L., Svendsen M.V., Tenke P. Prevalence of Hospital-Acquired Urinary Tract Infections in Urology Departments. Urol. 2007;51(4):1100-12.
- Coxeter P., Looke D., Hoffmann T., Lowe J., Del Mar C. The antibiotic crisis: charting Australia’s path towards least resistance. N. Z. J. Public Health. 2013;37(5):403-4.
- Edwardson S., Cairns C. Nosocomial infections in the ICU. Intens. Care2019;20(1):14-8.
- Pallett A., Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. Antimicrob. Chemother.2010;65(3):iii25-iii33.
- Andreessen L., Wilde M.H., Herendeen P. Preventing Catheter-Associated Urinary Tract Infections in Acute Care: The Bundle Approach. Nurs. Care Qual.2012;27(3):209-17.
- Baron E.J., Finegold S.M., Bailey W.R., Scott E.G., Baron E.J. Bailey and Scott’s diagnostic microbiology. St. Louis; Baltimore: C.V. Mosby; 1990.
- Pondei K., Oladapo O., Kunle-Olowu O.E. Anti-microbial susceptibility pattern of micro-organisms associated with urinary tract infections in a tertiary health institution in the Niger Delta Region of Nigeria. J. Microbiol. Res.2012;6(23):4976-82.
- Aboderin O.A., Abdu A.R., Odetoyin B.W., Lamikanra A. Antimicrobial Resistance in Escherichia coli Strains From Urinary Tract Infections. Natl. Med. Assoc.2009;101(12):1268-73.
- Patel S., Taviad P.P., Sinha M., Javadekar T.B., Chaudhari V.P. Urinary tract infections (UTI) among patients at GG hospital and medical college, Jamnagar, Natl J. Commu. Med. 2012;3(1):138-41.
- Demir M., Kazanasmaz H. Uropathogens and Antibiotic Resistance in the Community and Hospital-Induced Urinary Tract Infected Children. of Glob. Antimicrob. Re.2020;20:68-73
- Tajbakhsh E., Tajbakhsh S., Khamesipour F. Isolation and Molecular Detection of Gram-Negative Bacteria Causing Urinary Tract Infection in Patients Referred to Shahrekord Hospitals of Iran. Red. Crescent Med. J. 2015;17(5):e24779.
- Cordoba, G., Holm, A., Hansen, F., Hammerum A.H., Bjerrum L.Prevalence of antimicrobial-resistant Escherichia colifrom patients with suspected urinary tract infection in primary care, Denmark. BMC Infect. Dis. 2017;17(1):
- Price T.K., Dune T., Hilt E.E., Thomas-White K.J., Kliethermes S., Brincat C.,…and Schreckenberger, P. C. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. Clin.Microbiol.2016;54(5):1216-22
- Sánchez-García J.M., Sorlózano-Puerto A., Navarro-Marí J.M., Gutiérrez Fernández J. Evolution of the antibiotic-resistance of microorganisms causing urinary tract infections: A 4-year epidemiological surveillance study in a hospital population. Clin. Esp. 2019;219(3):116-23.
- Wang Y., Li H., Chen B. Pathogen distribution and drug resistance of nephrology patients with urinary tract infections. Saudi Pharm. J. 2016;24(3):337-40.
- Wagenlehner F.M.E., Naber K.G. Emergence of antibiotic resistance and prudent use of antibiotic therapy in nosocomially acquired urinary tract infections. J.Antimicrob. Agents 2004;23:24-29.
- Lu P.L., Liu Y.C., Toh H.S., Lee Y.L., Liu Y.M., Ho C.M., … Hsueh P.R. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). J. Antimicrob. Agents 2012;40:S37-S43.
- Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of Resistance in Gram-Negative Urinary Isolates Using Existing and Novel Indicators of Clinical Relevance: A 10-Year Data Analysis. Life 2020;10(2):16.
- Benachinmardi, K., Padmavathy, M., Malini, J., and Navaneeth, B. (2015). Microbiological profile and antibiogram of uropathogens in the pediatric age group. J. Health Allied Sci. 2015;4(1):61-64.
- Venkatesh S., Chauhan L., Gadpayle A., Jain T., Wattal C., Aneja S., …Jain S. National Treatment Guidelines for Antimicrobial use in Infectious Diseases2016; pp 18-19.
- Hummers-Pradier E., Ohse A.M., Koch M., Heizmann W.R., Kochen M.M. Urinary tract infection in men. Int J Clin Pharmacol Ther. 2004;42(7):360-66.
- Lai C.C., Lee C.M., Chiang H.T., Hung C.T., Chen Y.C., Su L.H.,…Hsueh P.R. Implementation of a national bundle care program to reduce catheter-associated urinary tract infection in high-risk units of hospitals in Taiwan. Microbiol. Immunol. Infect. 2017;50(4):464-70.
- Saint S., Greene M.T., Krein S.L., Rogers M.A.M., Ratz D., Fowler K.E.,…Fakih M.G. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. Engl. J. Med.2016;374(22):2111-9.
- Agodi A., Auxilia F., Barchitta M., Brusaferro S., D’Alessandro D., Grillo O.C.,…GISIO-SITI. Trends, risk factors, and outcomes of healthcare-associated infections within the Italian network SPIN-UTI. J. Hosp. Infect. 2013;84(1):52-8.
- Marra A.R., Sampaio Camargo T.Z., Gonçalves P., Sogayar A.M., Moura D.F. Jr., Guastelli LR,…Edmond M.B. Preventing catheter-associated urinary tract infection in the zero-tolerance era. J. Infect Control 2011;39(10):817-22.
- Leblebicioglu H., Ersoz G., Rosenthal V.D., Nevzat-Yalcin A., Akan Ö.A., Sirmatel F.,…Bacakoglu F. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). J. Infect Control2013;41(10):885-91.
- Tatham M., Macfarlane G., MacRae M., Tully V., Craig K. Development and Implementation of a Catheter-Associated Urinary Tract Infection (CAUTI) ‘Toolkit’. BMJ Open Qual.2015;4(1):u205441.w3668.
- Sampathkumar P., Barth J.W., Johnson M., Marosek N., Johnson M., Worden W.,…Thompson R. Mayo Clinic Reduces Catheter-Associated Urinary Tract Infections Through a Bundled 6-C Approach. Comm. J. Qual. Saf. 2016;42(6):254-261.
Abbreviations
AMK-Amikacin, SAM-Ampicillin sulbactam, AMC-Amoxycillin/clavulanic acid, AMP-Ampicillin, ATM-Aztreonam, CZO-Cefazolin, FEP-Cefepime, CSL-Cefoperazone/Sulbactam, CTX-Cefotaxime, FOX-Cefoxitin, CPD-Cefpodoxime, CAZ-Ceftazidime, CMX-Cefuroxime, CEP-Cephalothin, CHL-Chloramphenicol, CIP-Ciprofloxacin, CLR-Clarithromycin, CLI-Clindamycin, COL-Colistin, DAP-Daptomycin, DOR-Doripenem, ETP-Ertapenem, ERY-Erythromycin, FOS-Fosfomycin, FUS-Fusidic acid, GEN-Gentamycin, IPM-Imipenem, LVX-Levofloxacin, LNZ-Linezolid, MEM-Meropenem, MEZ-Mezlocillin, MFX-Moxifloxacin, NET-Netilmicin, NIT-Nitrofurantoin, NOR-Norfloxacin, OXA-Oxacillin, PEN-Penicillin, TZP-Piperacillin/tazobactam, PIP-Piperacillin, RIF-Rifampicin, TEC-Teicoplanin, TCY-Tetracycline, TCC-Ticarcillin/clavulanic acid, TGC-Tigecycline, TOB-Tobramycin, STX-Trimethoprim/Sulfamethoxazole, TMP-Trimethoprim, VAN-Vancomycin

This work is licensed under a Creative Commons Attribution 4.0 International License.





