How to Cite | Publication History | PlumX Article Matrix
Hassan I. El-Sayyad1*![]() , Osama A. Abbas2 and Zahraa A. Greash2
, Osama A. Abbas2 and Zahraa A. Greash2
1Zoology Department, Faculty of Science, Mansoura Univ., Egypt.
2Zoology Department, Faculty of Science, Port Said Univ., Egypt.
Corresponding Author E-mail : elsayyad@mans.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2826
ABSTRACT: Consumption of processed high cholesterol diet is associated with the development of metabolic diseases. The present work evaluated the role of soaked barley on decreasing the high cholesterol level that impairing the structure and function of the gastrocnemius muscle of mother rats and their offspring. Sixty virgin female albino rats and twenty healthy males (100g body weight) were used in the experimental work. Mating was carried out and pregnancy was determined. Pregnant were arranged into 4 groups (n=15); control (C), barley supplemented group (B) (20%), hypercholesterolemic-group (H) (3%) and hypercholesterolemic & malted barley group (H+B). The third (H) and fourth groups (HB) were consumed hypercholesterolemic diet for 4 months prior to conception and through gestation and lactation period. The fourth group supplemented malted barley containing a hypercholesterolemic diet during gestation and lactation period. At 21 days post-partum, the mother rats and their offspring were anesthetized with sodium phenobarbitone (9.1 mg/kg) and sacrificed. Blood was collected from heart and their sera were separated. Also, the gastrocnemius of both mother and their offspring were removed. Histological, transmission electron microscopy and biochemical investigations were carried out. The present findings showed that the high cholesterol diet possessed abnormal expression of protein bands (SDS-PAGE), associated with poor expression of lactic dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G6PD) isoenzymes coincides with damage of the gastrocnemius muscle fibers. The elevated sera cholesterol level induces a decrease of the muscle contents of glutathione-s-transferase and superoxide dismutase. These were followed by increased the lipid peroxidation malondialdhyde (MDA) and apoptic marker caspase-3. On the other side, dietary supplementation of barley plus a hypercholesterolemic diet increased the muscle contents of the assayed antioxidant enzymes and decreased MDA, caspase 3 and LDH isoenzyme expression. This led to improvement of the muscle structure. The authors concluded that malted barley contained flavonoids and phytonutrients with a potent antioxidant activity which reduced the oxidative stress resulted from ingestion of hypercholesterolemic diet. This led to improvement of the myoblast structures in mother rats and their offspring.
KEYWORDS: Barley, Cholesterol Diet, Gastrocnemius, Light And Transmission Electron Microscope ; Offspring, Oxidative Stress, Pregnant
Download this article as:| Copy the following to cite this article: El-Sayyad H. I, Abbas O. A, Greash Z. A. Malted Barley Improved the Structure and Function of Gastrocnemius Muscle of Hypercholesterolemic Mother Rats and their Offspring. Biosci Biotech Res Asia 2020;17(2). |
| Copy the following to cite this URL: El-Sayyad H. I, Abbas O. A, Greash Z. A. Malted Barley Improved the Structure and Function of Gastrocnemius Muscle of Hypercholesterolemic Mother Rats and their Offspring. Biosci Biotech Res Asia 2020;17(2). Available from: https://bit.ly/30l2mjL |
Introduction
The skeletal muscles make up about 55% of human body mass and play important roles in contraction and relaxation. They are the main site for glucose utilization, oxidation of fatty acids and metabolism of proteins (Kuo and Ehrlich, 2015).
Skeletal muscles are also made up of muscle fibers population (I, IIA, IIX and IIB). They are regulated by their myosin heavy chains, which control the contraction and relaxation (Hoh, 2011). This involved the conversion of the chemical energy through hydrolysis of ATP into contraction of the cytoskeletal apparatus (Schiaffino and Reggiani, 2011; Pataky et al., 2019). Two points are important of the muscle. First of it is the higher inclusion of cholesterol in their sarcolemma which facilitate the membrane fluidity (Barrientos et al., 2017). The second its high content of mitochondria required for ATP production.The muscle fibers were highly susceptible to ROS-induced damage of their fibers (Zhang et al., 2017).
Myocytes are highly susceptible to the obesity associated inflammation and generation of ROS through the reduction of superoxide dismutase, catalase and glutathione peroxidase (Moylan and Reid, 2007). Consumption of fat diet was found to decrease the actin filaments (F-actin) necessary to control glucose transporter 4 (GLUT4) and predicting early insulin resistance in the skeletal muscle (Grice et al., 2019). Oxidative stress associated with obesity is significantly involved in establishing insulin resistance in both adipocytes and myocytes (Gozalez-Franquesa and Patti, 2017). High fat diet significantly decreased type I fiber In lumbar muscles, while increased type II. Such phenomena can indicate skeletal muscle reaction to overload of dietary lipid to regulate metabolic homeostasis (Hua et al., 2017).
Soaked barley foods are a prebiotic that increased the production luminal butyrate. It provided the enzymes (α-amylase, β-amylase) required for various forms of sugar, such as monosaccharide glucose, disaccharide maltose , disaccharide maltotriose, and maltodextrines (Hanai et al., 2004; Kranz et al., 2015). Germinating barley food attenuated inflammation by reducing sera levels of tumor necrosis factor-α and IL-6 and 8 of ulcerative colitis (Faghfoori et al., 2011) modulating gut microbiota (Zhong et al.2015), and anti-hyperglycaemia (Ramakrishna et al., 2017).
Little of work is available about the phytomedicinal importance of fermented barley against the ingestion of hypercholesterolemic diet which induces skeletal muscle damage. The present work focused on assessing the histopathological, ultrastructural and biochemical changes of high cholesterol diet on gastrocnemius muscle of mother rats and their offspring as well as the effect of ingested soaked barley grain.
Material and Methods
Ethical Consideration
This research was carried out according to the National Institute of Health, s guide to the use of the laboratory animals and supported by the Mansoura University,s Egyptian Committee for Animal Care and Bioethics.
Preparation of a High Cholesterol Diet and Assessment of Hyperlipidemia
It was done by ingestion of 3% cholesterol diet containing 10% animal fat, 2% cholic acid and 1% thiouracil in addition to the normal diet for 4 months prior to conception and throughout pregnancy and lactation period (Enkhmaa et al., 2005). For critical assessment of hyperlipidemia, under sterile condition, the blood is aspirated from the ocular artery by capillary tube, centrifuged at 2000 rpm and sera were removed. Total Cholesterol (Deeg and Ziegenhorn, 1983) and LDL (Friedewald et al., 1972) were estimated.
Diets Containing Soaked Barley
The standard diet is composed of 20% malted barley. The hypercholesterolemic groups were fed on diet containing 20% barley prior to conception and during the gestation and lactation period.
Experimental Animal
Sixty virgin female and twenty adult male albino rats (Rattus norvegicus) weighing approximately 100gm body weight, obtained from Helwan Breading Farm, Ministry of Health, Egypt, have been used for experimental investigation. It has been provided free access to food and water ad libitum. They kept in good ventilation with 12 hour light/dark cycle. Virgin females were allowed for mating with fertile male (3 female/1male) for overnight. In the next morning, visualizing sperm in the vaginal smear determine the onset of gestation. The pregnant rats were arranged into 4 groups (n=15) including; control (C), barley supplemented group (B), hypercholesterolemic-group (H) and hypercholesterolemic & soaked barley group (H+B. Mothers of the studied groups were anaesthetized by subcutaneous injection of sodium phenobarbitone (9.1 mg/kg) at 3 weeks post-partum and sacrificed. Blood was collected from the heart of both mothers and their offspring, allowed to coagulate, and sera were separated and kept in refrigerator. At the same time, their gastrocnemius were dissected and investigated as follows:
Histological Investigation
The specimens were fixed in 10 % phosphate buffered formalin (pH 7.4), dehydrated in the highest degree of ethyl alcohol, cleared in toluene and mounted in melted paraplast 58-62ºC. Five µm histological sections were cut, stained with hematoxylin & eosin and examined under a bright field light microscope.
Transmission Electron Microscopy (TEM)
Extra- specimens were fixed in 2.5% in 0.1 M phosphate buffer (pH 7.4), followed by 1 % osmium tetraoxide. They were then dehydrated in ascending ethyl alcohol, cleared with acetone and embeded in epoxy resin. Ultrathin sections were cut on a LKB Ultratome IV (LKB Instruments, Bromma, Sweden) with a diamond knife and mounted on grids, stained with uranyl acetate and lead citrate, and viewed under a Joel 100CX electron transmission microscope (Musashino 3-chome, Akishima, Tokyo 196-8558, Japan).
Biochemical Assessments of Sera Lipid Profiles
Sera levels of total cholesterol (TC) (Deeg and Ziegenhorn, 1983), Triglycerides (TG) (Fossati and Prencipe, 1982) and high density lipoproteins (HDL) (Grove, 1979) were assayed. Low density lipoproteins (LDL) was calculated from the total concentrations of cholesterol (TC), HDL-cholesterol and triglycerides according to Friedewald et al (1972). The glucose was determined by blood glucometers one touch ultra (Life Scan Milipitas, CA, USA).
Assessment of Sera Antioxidants Glutathione-S-Transferase and Superoxide Dismutase Activities
Glutathione S-transferase (GST) is determined through the conjugation of 1- chloro- 2,4- dinitrobenzene with reduced glutathione followed by measuring the absorbance at 340 nm ( Prins and loose, 1969). Also, determination of superoxide dismutase (SOD) depends on the reduction of nitroblue tetrazolium (NBT) by superoxide radicals to the blue colored formazan and assayed calorimetrically at 560 nm (Nishikimi et al. (1972),
Determination of Serum MDA Content
It is evaluated on the basis of red colour development according to Ohkawa et al. (1979), and calculated at 532 nm for specifying the degree of peroxidation and expressed as nmol/ mg protein.
Determination of Serum Caspase-3
It is determined calorimetrically by using a Stressgen kit (catalog No. 907-013). The cleavage of the peptide can be quantitated spectrophotometrically at a wavelength of 405nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the color reaction.
Determination of Muscle Actin Beta and Keratin 18
Actin beta (ACTb, catalogue no. SEB340Mi) and keratin 18 (KRT18, catalogue no. SEB231Ra) were assayed by using Enzyme-linked Immunosorbent Assay Kit of Cloud-Clone Corp.
Assessment of Muscle Isoenzymes Electrophoresis
Fresh samples of gastrocnemius muscle of the studied groups were homogenized and analyzed by SDS-PAGE (Laemeli, 1970).The protein content was separated and determined (Lowry et al., 1951). Electrophoresis was done at 4°C in polyacrylamide gel and protein bands were visualized by staining with coomassie blue R-250 (60 mg/L) in an acidic medium (Andrew, 1970). The assayed enzymes are:
Glucose-6-Phosphate Dehydrogenase (G6PD)
The electrophoretic buffer contained 5mM tris, 80 mM aspartate and 20 µM NADP+ at (pH 7.4) and visualized the enzyme activity at 30°C in a media composed of in 20 mL, 1.2 mM tris-phosphate buffer (pH 8.5), 25% (v/v) glycerol, 30 µM glucose-6-phosphate, 4 mM NADP+, 6mg p-nitroblue tetrazolium and 0.5mg phenazine methosulfate (Gaal et al., 1980).
Lactic Dehydrogenase Isoenzymes
After electrophoresis, the gels were incubated into media containing 18.4 mL H2O, 4mL 1M tris, 12mL tetrazolium-blue, 4 mL phenazine methosulphate, 4mL Na-lactate and 1.3 mL NAD to develop color reaction for 20 min. Investigating the gel, the appearance or absence of a certain isoenzymatic band of the isoenzymes were recorded (to Lehnert and Berlet,1979).
Statistical Analysis
Statistics were calculated with SPSS for windows version 15.0 (SPSS Inc., Chicago, IL, USA). The means value obtained in the different groups were compared by one-way post-hoc analysis of variance (ANOVA) test. All results were expressed as mean±standard error (SE) and significance were defined at p <0.05 and highly significant at p<0.01.
Results
Light and Ultra-Structural Observations
Gastrocnemius of Mother Rats
At light microscopic level, mother rats ingested a high cholesterol diet showed a considerable widening of the interstitial spaces between the muscle fibers. The muscle fibers possessed focal necrosis (Fig. 1A2). On the other side, barley supplementation in diet containing a high fat diet showed restoration of the muscle fibers. Its histological picture seemed to be of nearly normal characteristic feature (Fig.1A3). Regard the control (Fig.1 A) and barley fed groups (Fig.1 A1)
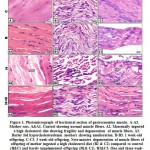 |
Figure 1: Photomicrograph of horizontal section of gastrocnemius muscle. |
At transmission electron microscopy, compared to the control (Fig.3 A), the myofibrils were disorganized and lacked normally oriented muscle bands in mother fed on a high cholesterol diet. The nuclei of the myocytes become pyknotic. They were enclosed by electron dense heterochromatin. Many necrotic foci were diffusely scattered in the muscle fibers. Lipid deposits were appeared in between the myofibers. Damaging of the mitochondria was become evident (Fig. 3 A1 and A2).
In mother rat ingested barley and a hypercholesterolemic diet, the muscle fibers restored their regularly orientated muscle bands. Glycogen granules and mitochondria were detected in between muscle fibrils (Fig. 3A-A3).
Gastrocnemius of Offspring
At light microscopy level, offspring of mother ingested a high cholesterol diet showed abnormal structure of muscle fibers characterized by expansion of the endomysium and increased perimysial connective tissue. The thickening of muscle fibers varied markedly between each other. Some of the muscle fibers appeared damaging and fragmented (Fig.1B2 & C2). In cross sections, the muscle fibers become widely separated and possessed dense infiltration of leukocytes. In 1 week -old, there was a remarked indistinct cross striation associated with the presence of necrotic areas. Dense inflammation associated with necrotic foci and lipid deposits were observed in 3-week-old offspring (Fig. 2 B1, B2). Regard the normal structural of control and barley fed group (Figs. 1 B and C, 2 A1 and A2).
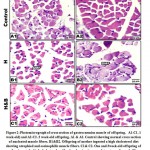 |
Figure 2: Photomicrograph of cross section of gastrocnemius muscle of offspring. |
In offspring of mother’s ingested barley and a high cholesterol diet, the histological picture of the gastrocnemius appeared comparatively improved (Figs. 1 B3, C3 and 2 C1, C2).
At transmission electron microscopy, the muscle of offspring of hypercholesterolemic mother showed a detected disorganization of muscle fibers with corrugated sarcolemma. The myocyte nuclei become pyknotic and enclosed by electron dense chromatin. Their nucleolar envelope become convoluted (Fig.3 B1, B2 and C1, C2) compared to the normal ordinary myocytes of H, A, I and Z bands in control (Fig. 3 B and C).
 |
Figure 3: Transmission electron micrograph of gastrocnemius muscle. |
On the other hand, offspring of mother ingested barley containing a high cholesterol diet preserved most of the elementary structure of the muscle associated with nearly almost the normal muscle bands of the muscle fibers. The distribution of nuclear chromatin appeared to be of normal characteristic structure. The intefibillar structure showed normal mitochondrial distribution with characteristic internal cristae (Figs. 3 B3 and C3).
Biochemical Observations of Mothers
Compared to both of the control and barley fed mother rats, sera of mothers ingested a high cholesterol diet showed significant decrease of the activities of both glutathione s-transferase and superoxide dismutase with a concomitant significantly increase of MDA and casp3. However, there was a detected decrease of MDA and casp 3 and increase to almost normal value of the assayed antioxidant enzymes in muscle of hypercholesterolemic mothers ingested barley (Figs. 4 and 5).
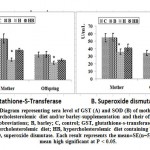 |
Figure 4: Diagram representing sera level of GST (A) and SOD (B) |
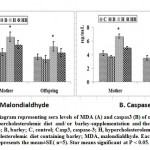 |
Figure 5: Diagram representing sera levels of MDA (A) and caspas3 (B) |
In experimental mother rats fed on a high cholesterol diet, there was a detected significant decrease of the sera total protein and B-actin s with a concomitant increase of keratin. However, ingestion of barley and a high cholesterol diet, showed a detected improvement of the assayed parameters but their levels were still less than the control values (Figs. 6& 7).
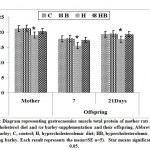 |
Figure 6: Diagram representing gastrocnemius muscle total protein of mother rats |
 |
Figure 7: Diagram representing gastrocnemius muscle |
Biochemical Observations of Offspring
Offspring of mothers ingested a high cholesterol diet possessed a significant depletion of the activities of both the sera GST and SOD (Fig.4 A and B). The decrease of the antioxidant enzymes were associated with increased sera levels of MDA and Casp-3. The mentioned dramatic alterations were ameliorated in those of mothers ingested barley plus a high cholesterol diet but were still not matched with the control values (Fig. 5 A and B).
In one and three week-old offspring of mothers ingested a high cholesterol diet, the muscle contents of total protein and B-actin were markedly decreased coincides with a marked increase of keratin. However, in those of mothers ingested barley plus a high cholesterol diet, the muscle contents of the assayed actin and protein were significantly improved but still differed from the control values (Figs. 6 & 7 A and B).
Sodium Dodecylsulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Sodium dodecylsulfate–polyacrylamide gel electrophoresis of gastrocnemius muscle of mother rat ingested a high cholesterol diet and their offspring showed a decreased expression of the protein bands compared to the control and barley fed group (Fig. 8).
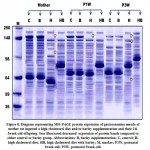 |
Figure 8: Diagram representing SDS-PAGE protein expression of gastrocnemius muscle |
Lactic Dehydrogenase Isoenzymes Electrophoresis (LDH)
Following differential LDH isoenzymes of gastrocnemius muscle of mother, LDH expressed five isoenzyme fractions. In mother ingested a high cholesterol diet, the second and third isoenzyme fractions become denser, while the fractions IV and V appeared faintly compared to the other groups. On the other side, 1week-old offspring showed increased densities of the isoenzyme fractions II, III, IV and V compared to the other groups. The densities of the isoenzyme fractions were comparatively decreased in 3week-old offspring, compared to that of the control and barley fed groups. On offspring of mother ingested a high cholesterol diet containing barley, the LDH isoenzymes of the gastrocnemius muscle were improved (Fig. 9).
 |
Figure 9: Diagram representing LDH and G6-PHD isoenzyme electrophoresis |
Glucose-6-Phosphatase Dehydrogenase Isoenzyme Electrophoresis (G6PD)
In mother G6-PHD, three isoenzyme fractions were identified in gastrocnemius muscle. Mother ingested a high cholesterol diet showed faint expression of the isoenzyme fractions I, II & III comparing with either control or barley group. In mother ingested a high cholesterol diet containing barley, there was a detected newly isoenzyme fraction IV and faint expression of fraction III.
In 1week-old offspring G6-PDH, four isoenzyme fractions are observed. Offspring of mother rats ingested on a high cholesterol diet showed a weak expression of the isoenzyme fractions III and IV compared to either the control or barley groups. In offspring of mother ingested a high cholesterol diet containing barley, there was a detected decreased expression of the isoenzyme fraction III and newly expression of the isoenzyme fraction V. In 3week-old offspring G6-PDH, four isoenzyme fractions were observed. Offspring of mothers ingested on a high cholesterol diet showed decreased expression of the isoenzyme fractions compared to the control or barley group. In those maternally fed on a high cholesterol diet containing barley, decreased expression of the isoenzyme fractions were observed compared to the other groups (Fig. 9).
Discussion
The present study showed that mothers ingested a high cholesterol diet was abnormally disorganized the gastrocnemius muscle clarified by expanding of the interstitial space, focal necrosis and disorganized muscle bands. The affected myocytes had compacted nuclear chromatin materials that displayed sign of pyknosis. Leukocytic infiltration was detected in the necrotic zone. Ultrastructurally, lipid deposits were observed in between the muscle fibers. The alterations in mother rats reflected the abnormal structural organization of gastrocnemius muscle in their offspring. The muscle fibers varied in thickness, expanding of the endomysium, increased perimysial connective tissue, splitting and damaged myofibrils as well as dense collection of inflammatory cells especially in necrotic patches.
The present results are consistent with authors who reported increased accumulation of lipid in the soleus and tibialis anterior muscles associated damage of muscle fibers (Collino et al., 2014) and atrophy of the tibialis anterior of mice ingested a high fat diet (Abrigo et al., 2016). Also, mitochondrial damage and increased intracellular oxidative stress were reported in skeletal muscle in obese diabetic patients (Dos Santos et al., 2018).
Rats fed on either 45 percent or 60 percent fat diet for 3 weeks had injured gastrocnemius assessed by acidophilic staining and deposition of lipid droplets in between muscle fibers (Ickin et al., 2015). Extra-myocellular deposition of lipid coincides with decrease of type 1 fiber and increase of type 2 in lumbar muscle (Hua et al., 2017).
The observed increase of sera maternal levels of LDL, and cholesterol associated depletion of the antioxidant enzyme defense of glutathione s-transferase and superoxide dismutase as well as increased lipid peroxidation assessed by malondialdhyde reflected the increased oxidative stress and muscle damage. This was represented by increased expression of sera levels of caspase 3. The depletion of the antioxidant enzymes are known to increase the production of reactive oxygen species superoxide, post– myocyte damage resulting from leakage electron from the mitochondrial transport chain (Kim et al., 2008).
Also, the diseased gastrocnemius muscle of mother rats ingested a high cholesterol diet and their offspring was explained decreased protein synthesis and missing of protein bands following SDS-PAGE analysis, associated increased expression of LDH isoenzyme fractions predicting the muscle damage. At the same time, the G6-PD isoenzymes expression was decreased. Glucose-6-phosphate dehydrogenase is known to be involved in the myosin light chain phosphorylation via pentose phosphate pathway which is a major source of NADPH, which regulates various enzymatic (Gupta et al., 2011). This can explain the muscle damage.
In addition, the gastrocnemius muscle of mother rats ingested a high cholesterol diet and their offspring showed a significant decrease of total protein and B-actin concomitant with increased keratin content. This finding is consistent with that of Lóry et al. (2019) who reported depletion of protein in muscle of obese rat due to reduction of the aminopeptidase A activity and angiotensin-converting enzyme in the skeletal muscle leading to dysfunction of myosin heavy chain.
Also, the high cholesterol diet was found to decrease the protein muscle content and β-actin correlates with increased muscle keratin. β-actin is known to be localized in stress fibers of circular bundles. This may be resulted from decreased phosphorylation (Sun et al., 2019) and associated depletion of cortical filamentous actin of glucose transporter 4 (Grice et al., 2019). Reduction of protein and β-actin explained the muscle dysfunction.
Keratin is also the intermediate protein filament required for transducing contractile force (Muried et al., 2020). Overexpression of keratin is associated with the development of muscular atrophy Toivola et al., 2008).
On the other side, barley supplementation to mother rat ingested a high cholesterol diet restored the striation of muscle fibers associated with increased the assayed antioxidant enzymes and decreased the MDA marker of oxidative stress and caspase 3 that assessed cell death. This improvement of muscular structure reflected the increase of G6PD and protenin and β-catenin content.
Barley contain varieties of nutrients such as β-glucan which decrease blood cholesterol (Aludatt et al., 2012) in animals and human (Wilson et al., 2004; AbuMweis et al., 2010). Also, it is rich in antioxidant and phenolic components (Wilson et al., 2004; AbuMweis et al.,2010), which increased the high-density lipoprotein (HDL) (Shimizu et al.,2019), that scavenge free radicals in cell membranes, preventing damage to their cellular lipids and proteins or DNA (Lobo et al.,2010)
The authors concluded that supplementation with barley has therapeutic potential against muscular dystrophy caused by hypercholesterolemia. The dietary soaked barley restored almost the antioxidant enzymes and reduced oxidative stress. The gastrocnemius muscle restored almost the normal structural pattern and protein and actin content for performing its function in both mother rats and their offspring.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Abrigo J, Rivera JC, Aravena J et al. High Fat Diet-induced skeletal muscle wasting is decreased by mesenchymal stem stem cells administration: Implications on oxidative stress, Ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev. 2016; 2016 (9047821), 13 pages.
- AbuMweis SS, Jew S, Ames NP. β-glucan from barley and its lipid-lowering capacity: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 2010; 64(12):1472-1480.
- Aludatt MH, Abu-Zaiton A, Alrababah M et al. Anti-oxidant, Anti-diabetic, and anti-hypertensive eEffects of extracted phenolics and hydrolyzed peptides from barley protein eractions. Int J. Food Prop. 2012; 15(4): 781-795.
- Andrews AT. Electrophoresis: theory, techniques, biochemical and clinical applications, clarendon press, Oxford.1986
- Andrich DE, Melbouci L, Ou Y et al. A Short-term high-fat diet alters glutathione levels and IL-6 gene expression in oxidative skeletal muscles of young rats. Front Physiol. 2019; 10: 372.
- Barrientos G,Sánchez-Aguilera P, Jaimovich E et al. Membrane cholesterol in skeletal muscle: A novel player in excitation-contraction coupling and insulin resistance. J Diabetes Res. 2017; 2017: 3941898.
- Bartłomiej S, Justyna RK, Ewa N. Bioactive compounds in cereal grains – occurrence, structure, technological significance and nutritional benefits – a review. Food Sci Technol Int. 2012; 18(6): 559-568.
- Collino M, Mastrocola R, Nigro D et al. Variability in myosteatosis and insulin resistance induced by high- fat diet in mouse skeletal muscles. Biomed Res Int. 2014; 2014:569623.
- Deeg R, Ziegenhorn J. Kinetic enzymic method for automated determination of total cholesterol in serum. 1983; 29: 1798–1802.
- Dos Santos JM, de Oliveira DS, Moreli ML et al. The role of mitochondrial DNA damage at skeletal muscle oxidative stress on the development of type 2 diabetes. Mol Cell Biochem. 2018;449(1-2):251-255.
- Enkhmaa B, Shiwaku K, Anuurad E et al. Prevalence of the metabolic syndrome using the Third Report of the National Cholesterol Educational Program Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults (ATP III) and the modified ATP III definitions for Japanese and Mongolians. Clin Chim Acta. 2005; 352(1-2):105-113.
- Faghfoori Z, Navai L, Shakerhosseini R et al. Effects of an oral supplementation of germinated barley foodstuff on serum tumor necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem.2011; 48(Pt 3):233-7.
- Fossati P, Prencipe L . Serum triglycerides determined colorimetrically with an enzyme that proceduces hydrogen peroxide. Chem. 1982; 28: 2077–2080.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of low density lipoprotein cholesterol in plasma without use preparative ultracentrifuge. Chem. 1972; 18: 499–502.
- Gaal O, Medgyesi GA, Vereczkey L. Electrophoresis in the separation of biological macromolecules O. Gaal, G. A. Medgyesi, L. Vereczkey John Wiley & Sons (1980) Price-$57.75. J Immunopharmacol.. 1980; 2 (4): 573-574.
- Gamel T, Abdel-Aal ESM. Phenolic acids and antioxidant properties of barley wholegrain and pearling fractions. Food Sci. 2012; 21(2):118-131.
- Gonzalez-Franquesa A, Patti ME. Insulin resistance and mitochondrial dysfunction. Adv Exp Med Biol. 2017; 982:465–520.
- Grice BA, Barton KJ, Covert JD et al. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. Am J Physiol Endocrinol Metab. 2019;317(2):E362-E373.
- Grove TH. Effect of reagent PH on determination of the high-density lipoprotein cholesterol by precipitation with sodium phototungestate-magnesium. Chem. 1979; 25: 560–564.
- Gupte RS, Ata H, Rawat D et al. Glucose-6-phosphate dehydrogenase is a regulator of vascular smooth muscle contraction. Antioxid Redox Signal. 2011 ;14(4):543-558.
- Hanai H, Kanauchi O, Mitsuyama K et al. Germinated barley food-stuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13(5):643-647.
- Hoh JF. Muscle fiber types and function. Curr Opin Rheumatol. 1992; 4(6):801–808.
- Hua N, Takahashi H, Yee GM et al. Influence of muscle fiber type composition on early fat accumulation under high-fat diet challenge. PLoS One. 2017;12(8): e0182430.
- Ickin Gulen M, Guven Bagla A et al. Histopathological changes in rat pancreas and skeletal muscle associated with high fat diet induced insulin resistance. Biotech Histochem. 2015; 90(7):495-505.
- Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008; 102: 401–414.
- Kranz B, Koch M, Schapfl M et al. Investigation of the germination of barley and wheat grains with a design of experiments for the production of hydrolases. Food Technol Biotechnol. 2015; 53(2):127–135.
- Kuo IY, Ehrlich BE. Signaling in muscle contraction. Cold Spring Harb Perspect Biol. 2015 ;7(2):a006023
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970; 227:680-685.
- Lehnert T, Berlet HH. Selective inactivation of lactate dehydrogenase of rat tissues by sodium deoxycholate. Biochem J.1979; 177:813–8.
- Lobo V, Patil A, Phatak A et al. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010; 4(8): 118-126.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1): 265-275.
- Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35(4):411–429.
- Muriel JM, O’Neill A, Kerr JP et al. Keratin 18 is an integral part of the intermediate filament network in murine skeletal muscle. Am J Physiol Cell Physiol. 2020;318(1):C215–C224.
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46(2), 849-854.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2): 351-358.
- Pataky MW, Yu CS, Nie Y et al. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. Am J Physiol Endocrinol Metab. 2019; 316(5): E695–E706.
- Pinho RA, Sepa-Kishi DM, Bikopoulos G et al. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med. 2017;110:381–389.
- Prins HK, Loose JA . Glutathione “chapter 4” in biochemical methods in red cell genetics. Edited by J.J. Yunis. Academic press, N.Y.D. London. 1969, 126-129.
- Ramakrishna R, Sarkar D, Manduri A et al. Shetty K. Improving phenolic bioactive-linked anti-hyperglycemic functions of dark germinated barley sprouts (Hordeum vulgare L.) using seed elicitation strategy. J Food Sci Technol. 2017; 54(11):3666–3678.
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011; 91(4):1447–1531.
- Shimizu C, Wakita Y, Kihara M et al. Association of lifelong intake of barley diet with healthy aging: Changes in physical and cognitive functions and intestinal microbiome in senescence-accelerated mouse-prone 8 (SAMP8). Nutrients 2019; 11, 1770.
- Sun YN, Huang JQ, Chen ZZ et al. Amyotrophy induced by a high-fat diet is closely related to inflammation and protein degradation determined by quantitative phosphoproteomic analysis in skeletal muscle of C57BL/6 J Mice. J Nutr. 2020; 150(2):294-302.
- Toivola DM, Nakamichi I, Strnad P et al. Keratin overexpression levels correlate with the content of spontaneous pancreatic injury. Am J Pathol. 2008; 172(4):882-892.
- Wilson TA, Nicolosi RJ, Delaney B et al. Reduced and high molecular weight barley beta-glucans decrease plasma total and non-HDL-cholesterol in hypercholesterolemic Syrian golden hamsters. J Nutr. 2004; 134(10): 2617-2622.
- Zhang L, Zhou Y, Wu W et al. Skeletal muscle-specific overexpression of PGC-1α induces fiber-type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int J Biol Sci. 2017;13(9):1152–1162.
- Zheng XK, Zhang L, Wang WW et al. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol. 2011;137(1):662–668.
- Zhong Y, Nyman M, Fåk F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res. 2015; 59(10):2066–2076.

This work is licensed under a Creative Commons Attribution 4.0 International License.






