How to Cite | Publication History | PlumX Article Matrix
Ali Hazazi1*, Waleed Alomaim2*, Mohammed Almubarak1, Fawaz Albloui1, Omer Alsaweed1, Waleed Tamimi3, Ali. A. Rabaan4, Fahad Aldakheel5, AbdulKarim S. Bin Shaye6 and Faisal Alseraye1*
1Department of Pathology and Laboratory Medicine, Security Forces Hospital Program, P.O. Box 3643, Riyadh 11481, Saudi Arabia
2King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
3King Abdulaziz Medical City, King Saud Bin Abdulaziz University for Health Sciences, King Abdullah Research Centre, Riyadh, Saudi Arabia
4Molecular Diagnostic Laboratory, Johns Hopkins Aramco Healthcare, Dhahran, Saudi Arabia.
5King Saud University, College of Applied Medical Sciences, Riyadh, Saudi Arabia
6Prince Sattam Bin Abdulaziz University, College of Applied Medical Sciences, Riyadh, Saudi Arabia
DOI : http://dx.doi.org/10.13005/bbra/2851
ABSTRACT: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a potentially lethal pathogen recently found to be responsible for the pandemic coronavirus disease 2019 (COVID-19). At present PCR testing remains the standard method of diagnosing COVID-19 patients. Recently, testing for SARS-CoV-2 immunoglobulin was identified as a promising method of diagnosing COVID-19 and assessing an individual’s exposure to the virus. In the current study, four different techniques—CLIA, ELISA, ECLIA, and rapid testing—were used to assess the IgG antibody response in 20 patients following COVID-19 exposure. The data obtained using the CLIA and ELISA techniques illustrated that 90 percent of COVID-19 patients produced the SARS-COV-2 IgG antibody. Processing samples using the ECLIA method showed that these antibodies were present in 80 percent of all patients; however, the rapid testing technique showed that only 70 percent of patients were able to generate an immune response. The CLIA and ELISA techniques seemed to be more sensitive in terms of detecting SARS-COV-2 IgG, as they revealed that a high percentage of COVID-19 patients developed the IgG antibody. Conducting further research on the ongoing pandemic COVID-19, particularly studying antibody testing, will be valuable for diagnosing and monitoring patients.
KEYWORDS: Anti-SARS-CoV-2; COVID-19; Serological Testing
Download this article as:| Copy the following to cite this article: Hazazi A, Alomaim W, Almubarak M, Albloui F, Alsaweed O, Tamimi W, Rabaan A. A, Aldakheel F, Shaye A. S. B, Alseraye F. Comparison of Various Diagnostic Techniques Used to Identify the Presence of Anti-SARS-CoV-2 Immunoglobulin G. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Hazazi A, Alomaim W, Almubarak M, Albloui F, Alsaweed O, Tamimi W, Rabaan A. A, Aldakheel F, Shaye A. S. B, Alseraye F. Comparison of Various Diagnostic Techniques Used to Identify the Presence of Anti-SARS-CoV-2 Immunoglobulin G. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/2SUQHU1 |
Background
In 2019 a new strain of the coronavirus disease, now dubbed COVID-19, was first transmitted to humans. COVID-19 can lead to serious and even life-threatening infectious respiratory disease. This disease is principally triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has been found to be highly transmissible between humans [1-3].
COVID-19 shares many symptoms of the common flu, but it has extrapulmonary manifestations, including pneumonia and acute respiratory distress syndrome. Other effects of COVID-19 infections include acute kidney injury and detrimental impacts on other organ systems, particularly the neurologic, hepatic, cardiac, endocrine, and gastrointestinal systems; it may also cause thromboembolisms [4,5].
Accurately diagnosing SARS-CoV-2 is essential so that patients with the virus can be quickly quarantined to stop the spread of the disease, thus saving people’s lives. Polymerase chain reaction (PCR) testing remains the standard approach used by healthcare workers to directly detect viral RNA in suspected COVID-19 cases in the early stage of contagion [6]. The human antibody response is an altered technique that is often used to assess a patient’s viral exposure and detect the formed immunity to COVID-19 infection. Any antibodies to COVID-19 developed following infection may be detectable in a patient’s blood within a few days. Such antibodies may provide some protection to the human body, but at present there is no clear evidence of how long this formed immunity lasts or the possibility of reinfection [7,8].
Antibody testing may identify certain forms of antibodies linked to COVID-19 infections, including binding antibodies and neutralizing antibodies. Binding antibodies are developed in any response to COVID-19, while neutralizing antibodies are effective in blocking the virus. Although COVID-19 antibody testing is still not fully understood, it could help significantly in identifying people who may possibly be protected from reinfection with the virus [9].
In this study we present the overall experience of a serological test for COVID-19 patients by comparing the various diagnostic techniques used to identify the presence of SARS-CoV-2 Immunoglobulin G, which will benefit the healthcare system.
Materials and Method
Serum was collected from COVID-19 patients of various ages and genders being treated at the Security Forces Hospital in Riyadh, Saudi Arabia, whose results were confirmed by PCR. A total of 20 serum samples were examined for SARS-COV-2 IgG antibody following a minimum of 3 days from the confirmed PCR result. The tested samples were drawn from 10 male and 10 female patients in total; the mean age for both genders was 36.85 years. Four different techniques were applied to determine the presence of SARS-COV-2 antibodies: Chemiluminescence immunoassay (CLIA), enzyme-linked immunosorbent assay (ELISA), electro-chemiluminescence immunoassay (ECLIA), and rapid testing.
CLIA was used to identify specific IgG antibodies to SARS-CoV-2 (COVID-19) in the human serum samples. This technique comprises a “flash” chemiluminescence technology with paramagnetic microparticle solid phase. The patient, controls, and calibrators were measured by a photomultiplier as relative light units. The anti-SARS-COV-2 ELISA (IgG) uses the plate-based assay technique ELISA to detect and quantify peptides, proteins, and antibodies. In this method an antigen must be immobilized on a solid surface and then complexed with an antibody linked to an enzyme. The patient, controls, and calibrator are then measured by photometric based on color intensity. ECLIA testing is based on a ruthenium complex and tripropylamine. The chemiluminescence reaction used to detect the reaction complex is initiated by applying a voltage to the sample solution. Rapid testing is a one-step method of determining SARS-CoV-2 IgG: A lateral flow immunochromatographic assay is used to detect the presence of IgG specific to SARS-CoV-2 virus in human serum. The reaction forms an antigen-antibody-antibody-gold particle complex and the results are interpreted using the color band at the T-line area.
Results
In the current study examining the 20 selected patients for SARS-CoV-2 IgG antibodies using four different methods produced different outcomes depending on the technique used (See Table 1). The CLIA test indicated that 18 of the 20 patients were producing SARS-CoV-2 IgG; the other two patients tested negative, one of whom had contracted the virus more than 14 days previously and the other only 3 days before. The ELISA test produced results identical to those of the CLIA method, and the same patients were found to be positive and negative for antibodies, respectively. The ECLIA technique revealed the presence of SARS-CoV-2 IgG in the samples of 16 out of 20 patients. Of the four patients who tested negative, two of them were the same patients who tested negative using the CLIA and ELISA tests; of the two remaining patients, one had contracted the virus 14 days previously and the other more than 21 days previously. Finally, rapid testing showed clearly inconsistent results in terms of determining SARS-CoV-2 IgG in the examined patients. A total of six patients tested negative for SARS-CoV-2 IgG: The two patients who showed no detectable antibodies in any of the methods used, the two patients who were also found to be negative using the ECLIA method, and two more patients who tested positive using the other methods. Correspondingly, analysis of the data obtained using the CLIA technique revealed SARS-CoV-2 IgG in 90 percent of all of the examined patients, which was confirmed using the ELISA technique. The ECLIA method revealed an immune response in 80 percent of the examined patients, while rapid testing detected antibodies in only 70 percent of the patients (Fig.1).
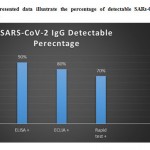 |
Figure 1: The presented data illustrate the percentage of detectable SARs-CoV-2 IgG in four different methods. |
Table 1: The presented data illustrate the determination of SARs-CoV-2 IgG using different techniques. Age and gender is presented.
| Patient NO# | Sex | Age (years) | Period of Serum Collection (days) | CLIA
|
ELISA | ECLIA | RAPID TEST |
| PATIENT 1 | F | 38 | 17 | + | + | + | + |
| PATIENT 2 | M | 39 | 11 | + | + | + | + |
| PATIENT 3 | F | 44 | 29 | + | + | + | + |
| PATIENT 4 | F | 37 | 13 | – | – | – | – |
| PATIENT 5 | M | 19 | 14 | + | + | – | – |
| PATIENT 6 | F | 43 | 25 | + | + | + | + |
| PATIENT 7 | F | 31 | 23 | + | + | + | + |
| PATIENT 8 | M | 33 | 27 | + | + | + | + |
| PATIENT 9 | F | 50 | 30 | + | + | + | + |
| PATIENT 10 | F | 35 | 36 | + | + | + | + |
| PATIENT 11 | F | 38 | 34 | + | + | + | + |
| PATIENT 12 | F | 33 | 36 | + | + | + | + |
| PATIENT 13 | M | 40 | 25 | + | + | + | + |
| PATIENT 14 | M | 34 | 24 | + | + | + | + |
| PATIENT 15 | M | 36 | 24 | + | + | + | + |
| PATIENT 16 | M | 33 | 22 | + | + | – | – |
| PATIENT 17 | F | 35 | 15 | + | + | + | – |
| PATIENT 18 | M | 45 | 20 | + | + | + | – |
| PATIENT 19 | F | 29 | 24 | + | + | + | + |
| PATIENT 20 | M | 45 | 3 | – | – | – | – |
Discussion
The antibodies known as immunoglobulins are produced by lymphocytes and act as a protective proteins to counteract the expression of a foreign body (antigen), such as a pathogen. Testing for serological antibodies is often used diagnoses and to determine whether a person has been exposed to a particular virus [6,9]. Antibody tests for COVID-19 could play a key role in diagnosing SARS-CoV-2 infections, but this issue is still not clear and further research is unquestionably needed. In the current study, four methods (CLIA, ELISA, ECLIA, and rapid testing) were applied to investigate the immune response following exposure to SARS-CoV-2; however, the results were inconsistent. The CLIA and ELISA techniques detected SARS-CoV-2 antibodies in all excluding two of the examined serum specimens, indicating that the immune systems of 18 patients had produced antibodies to the SAR-CoV-2 antigen and that these individuals could be immune to reinfection. Both the CLIA and ELISA techniques revealed that those patients tested only 3 days after exposure to the virus were negative for SARS-CoV-2 IgG, which seems to be normal given that the human body requires time to generate antibodies when first infected. Neither the CLIA nor the ELISA method detected SARS-CoV-2 IgG in the patients who had been exposed 13 days prior to testing, which could indicate the importance of the timing of the tests. The production of IgG begins within 21 days of the onset of symptoms, which could be another reason for the negative immune response results. Comparing ECLIA technique to the CLIA and ELISA methods indicated that fewer number patients had generated IgG; only 16 patients tested positive, while the other four tested negative. Two of these four had also tested negative using the CLIA and ELISA methods, as discussed above, and the other two had been exposed to the virus 14 days and 22 days, respectively, prior to testing. The reason that no immunoglobulin was detected in these patients using this method could be the sensitivity of the technique. Using rapid testing to investigate SARS-CoV-2 IgG in the selected patients revealed obvious inconsistencies with the other three methods. In this case, six of the 20 patients were found to be negative for IgG. In addition to the two patients found to be negative using CLIA and ELISA tests and the further two patients identified using the ECLIA test, the additional two patients had been exposed more than 14 days before testing. Rapid testing was limited in terms of detecting IgG antibodies compared to the other three techniques, which is a disadvantage and could indicate that this technique is not ideal for assessing the presence of SARS-CoV-2 IgG in the human body. More importantly, various factors can affect testing for antibodies, including the amount of antibodies present in the sample, the time since exposure to the virus, and the sensitivity of the technique used [8].
Concluding Remarks
Antibody testing is valuable when performed appropriately with sensitive techniques. Accurate serological testing methods are needed to determine the immune response to SARS-COV2 infection and subsequent, aiding in diagnosis. More importantly, it is essential that further studies be conducted to elucidate the remaining mysteries of COVID-19 antibody testing.
Acknowledgements
We acknowledge our colleagues in nursing department in security forces hospital for sustenance and Blood sample Collection from COVID19 patients.
Conflict of Interest
The authors declare there are no conflict of interest
Funding Sources
No funding to declare
References
- Fauci, A.S., H.C. Lane and R.R. Redfield, Covid-19 – Navigating the Uncharted. N Engl J Med, 2020. 382(13): p. 1268-1269.
- Bai, Y., L. Yao, T. Wei, F. Tian, D.Y. Jin, L. Chen and M. Wang, Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA, 2020. 323(14): p. 1406-1407.
- Kucharski, A.J., T.W. Russell, C. Diamond, Y. Liu, J. Edmunds, S. Funk, R.M. Eggo and C.-w.g. Centre for Mathematical Modelling of Infectious Diseases, Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis, 2020. 20(5): p. 553-558.
- Gupta, A., M.V. Madhavan, K. Sehgal, N. Nair, S. Mahajan, T.S. Sehrawat, B. Bikdeli, N. Ahluwalia, J.C. Ausiello, E.Y. Wan, et al., Extrapulmonary manifestations of COVID-19. Nat Med, 2020. 26(7): p. 1017-1032.
- Rothan, H.A. and S.N. Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun, 2020. 109: p. 102433.
- Xiang, F., X. Wang, X. He, Z. Peng, B. Yang, J. Zhang, Q. Zhou, H. Ye, Y. Ma, H. Li, et al., Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clin Infect Dis, 2020.
- Lu, H., C.W. Stratton and Y.W. Tang, An evolving approach to the laboratory assessment of COVID-19. J Med Virol, 2020.
- Jacofsky, D., E.M. Jacofsky and M. Jacofsky, Understanding Antibody Testing for COVID-19. J Arthroplasty, 2020. 35(7S): p. S74-S81.
- Deshpande, G.R., G.N. Sapkal, B.N. Tilekar, P.D. Yadav, Y. Gurav, S. Gaikwad, H. Kaushal, K.S. Deshpande, O. Kaduskar, P. Sarkale, et al., Neutralizing antibody responses to SARS-CoV-2 in COVID-19 patients. Indian J Med Res, 2020.

This work is licensed under a Creative Commons Attribution 4.0 International License.





