How to Cite | Publication History | PlumX Article Matrix
Conversion of Lignocellulosic Sugarcane Bagasse Waste into Bioethanol Using Indigenous Yeast Strain
K .Vijayaraghavan* and G. M. Akshaya
Department of Biotechnology Hindustan Institute of Technology and Science Hindustan University No 1 Rajiv Gandhi Salai (OMR), Padur, Chennai 603103, Tamil Nadu, India.
Corresponding Author E-mail: kvijay@hindustanuniv.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2859
ABSTRACT: The article explores a novel way towards effective utilization of solid waste generated from sugarcane bagasse towards maximum energy extraction. Adopting acid treatment coupled with steam explosion deconstructed the hemicelluloses and lignocellulosic material maximizing the fermentable sugar production. The pretreatment of bagasse was optimized with respect to sulphuric acid dosage and steam explosion period. Sugarcane bagasse being source of second generation feedstock proved its capability in bioethanol production based on the effectiveness of pretreatment process. The fermentation of bagasse was carried out at an initial pH 4.5 using indigenous yeast strain isolated from the bagasse. The results showed that pretreated bagasses produced a fermentable sugar of 74±3 g/l. The bioethanol production was found to be 34±2 g/l during a fermentation period of 36 h. Steam exploded acid bagasse led to the efficient breakage of hemicelluloses and lignocellulosic matrix, thus paving the way for scaling-up towards bioethanol production at industrial level.
KEYWORDS: Acid Hydrolysis; Bagasse; Bioethanol; Lignocellulosic Wastes; Saccharomyces Species; Stream Explosion; Sugarcane Crop Residue; Yeast Strain
Download this article as:| Copy the following to cite this article: Vijayaraghavan K, Akshaya G. M. Conversion of Lignocellulosic Sugarcane Bagasse Waste into Bioethanol Using Indigenous Yeast Strain. Biosci Biotech Res Asia 2020;17(3). |
| Copy the following to cite this URL: Vijayaraghavan K, Akshaya G. M. Conversion of Lignocellulosic Sugarcane Bagasse Waste into Bioethanol Using Indigenous Yeast Strain. Biosci Biotech Res Asia 2020;17(3). Available from: https://bit.ly/313lXVf |
Introduction
The second generation energy derived from waste biomass serves as a bio-based economy towards a carbon neutral1,2. Renewable energy seems to be a potential candidature in meeting the energy deficit due to decline in fossil fuel deposit and to curb environmental pollution by reducing by 30-85% of greenhouse gas emissions. Recycling of lignocellulosic biomass residue towards biofuel production has garnered greater attraction as it offers solid waste treatment coupled with energy production. More explicitly bioethanol serves as a petrol additive that can be produced from various cellulosic biomass reserves like food crops, herbaceous and woody plants, agricultural and forestry residues. Though bioethanol was initially produced using food crop like corn, wheat and sugar beet, it was later shifted towards residual lignocellulosic substrate. Among the members of highest cultivator of sugarcane, India has a place for itself along with Brazil and China. The sugarcane lignocellulosic residue (bagasse) obtained after extraction of sugarcane juice, served as the raw material for bioethanol production 3-7.
On an average for every ton of sugarcane processed in the extraction of cane juice leaves 23-25% as residue which accounts for 60-80% carbohydrate. The bagasse residues can be composted or their burnt ashes finds use in agriculture for soil amendment else as a fuel in boilers. In the above stated applications the energy available in residue are not utilized to the maximum extent. Thus fermentation of bagasse yields bioethanol along with the digested residue as agricultural resource 8.
Pretreatment of lignocelluloses seems to be an important step for the release of fermentable sugars from the lignocellulosic matrix. Different pretreatment methods incorporating physical, biological, chemical, and physicochemical were analyzed inorder to rupture the lignocellulosic matrix9. Various schemes have been devised for pre-treatment processing which includes acidic, alkali, steam explosion, microwave, wet oxidation and a combination of the above said process. Pretreatment methods are devised to loosen the lignocelluloses matrix, reduce inhibitory products and to minimize carbohydrate degradation. Cellulose, hemicelluloses and lignin are the fraction present in sugarcane bagasse. Cellulose being a linear β(1→4)-linked D-glucose units generates crystalline regions and consequently increases resistance to the hydrolytic process. Hemicelluloses and lignin encloses the cellulose matrix thus resisting the act due to enzymatic and chemical process10.
Hence it is essential to hydrolyze the cellulose and hemicelluloses inorder to obtain fermentable sugars and decrease the recalcitrant nature. The acid used during the hydrolysis of lignocellulosic biomass served as a catalyst in breaking down the heterocyclic ether bonds between sugar monomers in the polymeric chain. High concentrated acids causes corrosion and necessitate addition chemicals for pH neutralization. The pre-treated and hydrolysed substrates can undergo fermentation using microbial species including bacteria, fungi, yeast and enzymes in bioethanol produced11,12. Earlier studies had shown that hydrolysis of pretreated bagasse using acid was found to be economic on comparison with enzymatic hydrolysis13.
Concentration of acid-catalysed pretreatment technologies dictates the release of chemicals from biomass, which includes furans, phenols, organic acids and cinnamic acid. These products exhibits inhibitory effect on enzymatic hydrolysis and fermentation, depending on their concentration in biomass hydrolysates. The various products formed during acid pretreatment process were found to be correlated to the reaction condition and catalyst concentration14,15. Sugarcane bagasse contains arabinoxylans as the main hemicellulose component, but this fraction is normally lost after exposure to acid pretreatments such as diluted acid hydrolysis for cellulosic ethanol production. However, when acid pretreatments such as steam explosion are carried out at lower intensity such as in the absence of an added catalyst, the pretreated materials retain as much as 50% of their original hemicellulose content. The efficiency of pretreatment depends on the glucose and xylose released16.
Steam explosion is carried out by exposing the biomass to high-pressure saturated steam for required time interval, followed by sudden release of pressure. During this operation structure and composition of the lignocellulosic material is changed. The hydrolysis process converted hemicelluloses into monomeric and oligomeric sugars, with the release of acetic and organic acids17.
The objectives of the present investigation were to optimize the operating parameters based on pH, temperature and reaction time on bioethanol production. The effectiveness of acid treatment, steam explosion and their combined effect were also investigated on the sugarcane bagasse in-order to break the lignocellulosic matrix to produce fermentable sugar. The potential of indigenous yeast strain belonging to Sccharomyces species isolated from the sugarcane bagasse was tested for its fermentable capacity towards bioethanol production.
Material and Methods
Analytical Methods
The cellulose, hemicelluloses, lignocelluloses and ash content of the sugarcane bagasse was measured as per NREL LAP Method18. Total reducing sugar content was measured by dinitrosalicylic acid (DNS) method19. The bioethanol yield was estimated using dichromate by spectrophotometric method20. The characteristics of sugarcane bagasse are as shown in Table 1.
Table 1: Composition of sugarcane bagasse
| Parameter | Characteristics (%) |
| Cellulose | 39±0.7 |
| Hemicellulose | 20±0.9 |
| Lignin | 13±0.4 |
| Reducing sugar | 7.4±0.3 |
| Ash | 1.8±0.2 |
Isolation of Yeast Cultures
Bagasse samples were collected from sugarcane juice crushing unit. The yeast was isolated using malt agar media from the bagasse samples. The isolated yeast was investigated morphologically through microscope and tested for its fermentation capability using glucose as substrate towards bioethanol production.
Pretreatment of Sugarcane Bagasse
Bagasse lignocelluloses material was collected freshly from the sugarcane juice crushing unit. Physical treatment of sugarcane bagasse was carried out by shredding to a length of 300 mm. The effect of pretreatment on the release of reducing sugar was investigated using 250 g of shredded bagasse with various methods like acid hydrolysis, steam explosion and combined effect of steam exploded acid hydrolysis. The acid hydrolysis was carried out using a final volume of 150 ml with varying dosage of concentrated sulphuric acid 3, 5, 10 and 15 % for 12, 24, 36 and 48 h respectively. The steam explosion of sugarcane bagasse was carried out at 121oC at 15 psi for varying duration viz: 10, 20, 30, 40 and 50 min. Steam explosion of acid treated sugarcane bagasse was carried out using the optimized value of acid hydrolysis and steam explosion.
Fermentation Media for Ethanol Production
250 gm of bagasse was acid treated using 150 ml of 10% sulphuric acid for a hydrolysis period of 36 h. This was followed by steam explosion at 121oC and 15 psi for 40 min. Analysis of the filtrate showed a reducing sugar concentration of 7.4 ± 0.3 g/l. The batch anaerobic fermentation was carried in 1 litre fermentor using the filtrate obtained from pretreatment process. The initial pH of the broth was adjusted to pH 4.5 using phosphate buffer and the fermentation period was carried out for 48 h using 5% yeast culture obtained from overnight culture broth.
Results and Discussion
The preliminary experiments were conducted using the isolated yeast from the bagasse on ethanol production using glucose as substrate. The fermentation broth was adjusted in the ratio C:N:P of 100:2.5:1. The initial experiments were conducted using 10 g/l of glucose, 0.25 g/l urea and 0.1 g/l potassium dihydrogen phosphate. During the fermentation period samples were drawn at regular interval viz 24, 36 and 48 h, which resulted in a bioethanol concentration of 28, 37 and 42 g/L respectively.
Physical treatment of sugarcane bagasse was carried out by shredding to a length of 300 mm. Decrease in bagasse size lead to increase in surface area which helps to reduce the cellulose crystallinity and polymerization. Size reduction helps to hydrolyse the cellulose and hemicelluloses. Earlier studies showed that delignification resulted in increased production of ethanol21.
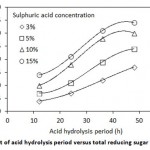 |
Figure 1: Effect of acid hydrolysis period versus total reducing sugar concentration |
Figure 1 shows the effect of pretreatment when of bagasse was hydrolyzed with sulphuric acid, steam exploded and steam explosion of acid hydrolysed bagasse. The results showed that using acid treatment at varying dosage viz 3, 5, 10 and 15 %, the reducing sugar concentrations at 36 h were found to be 12, 19, 28 and 31 g/L respectively. In the pretreatment stage the sugarcane bagasse gets digested to yield fermentable sugar. During the acid treatment, hydrolysis takes place due to the penetration of acid in lignin. The release of sugar molecule occurs due to the break of cellulose and hemicelluloses polymers. Acidic hydrolysis at lower temperatures below 90°C are less effective in hydrolysing hemicelluloses compared to higher temperature (180°C). Hydrolysis at higher temperature results in the formation of furfural and 5-hydroxymethyl-furfural as the hemicelluloses decomposes22. Earlier researcher had used a dilute acid at a concentration of 1.8-10% at 100– 120 °C for 40–120 min23,24.
In the case of steam explosion at 121oC at 15 psi for varying duration viz: 10, 20, 30, 40 and 50 min, the corresponding value of reducing sugar were found to be 7, 16, 31, 34 and 37 g/L respectively. Earlier researcher had studied steam explosion at 0.6–4.8 MPa at 160–260°C for 15 min25,26.
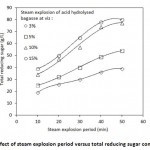 |
Figure 2: Effect of steam explosion period versus total reducing sugar concentration |
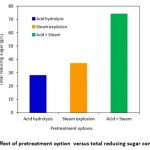 |
Figure 3: Effect of pretreatment option versus total reducing sugar concentration |
The combined of effect of steam explosion of acid hydrolysed bagasse at an optimized acid dosage of 10% sulphuric acid and 40 minutes steam explosion resulted in reducing sugar of 34, 47, 57, 74 and 77 g/L respectively as shown in Figure 2. Earlier researcher had stated that H2SO4, CO2 and SO2 when served as a catalyst in the recovery of hemicelluloses sugar accompanied with minimal inhibitory products. In the pretreatment stage the sugarcane bagasse gets digested to yield fermentable sugar27. Steam explosion technique helps to deconstruction the hemicellulose and lignocellulosic matrix due the acid hydrolysis coupled with temperature and pressure28. Figure 3 shows the effect of different pretreatment techniques in realising the reducing sugar. The results showed that on acid hydrolysis, steam explosion and steam explosion of acid treated bagasse resulted in a reducing sugar yield of 28, 37 and 74 respectively.
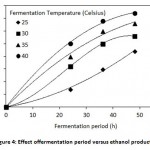 |
Figure 4: Effect offermentation period versus ethanol production |
Bioethanol production from sugarcane bagasse was carried out using steam exploded acid hydrolysed bagasse. The batch fermentation bagasse was at different fermentation temperatures viz: 25, 30, 35 and 40°C under intermittent agitated state. The experiments were carried out for 48 h, and samples were drawn at 24, 36 and 48 h. During the fermentation period pH and ethanol production was monitored. The results showed that fermentation at a reactor temperature of 25, 30, 35 and 40°C for a hydraulic retention time of 36 h, the ethanol production were found to be 15,, 25, 30 and 34 g/L respectively as shown in Figure 4. The fermentation broth was found to be rising from an initial pH value of 4.5 till pH 4.9 as shown in Figure 5.
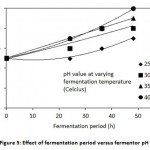 |
Figure 5: Effect of fermentation period versus fermentor pH |
Conclusion
Deriving alternate energy from solid waste helps to reduce the burden on fossil fuel dependence and environmental degradation. The major bottlenecks in gaining energy from lignocellulosic waste like bagasse are associated with breaking the recalcitrant matrix of hemicelluloses and lignin associated with the fibre. Due to complexity of the lignocellulosic substrate various pretreatment techniques have been investigated with varying degree of success depending on the extent of reduction of crystalline into amorphous cellulose. In the present study steam explosion of acid treated lignocelluloses proved to a best option towards the unlocking of lignocellulosic linkage and to reduce the crystalline nature of cellulose. The indigenous yeast isolated from bagasse yielded 32 g/l of bioethanol during a fermentation period of 36 h.
Acknowledgments
This research was supported under the in house research support 2019-2020 by Hindustan University. We also thank the Management for their constant support towards research.
Author Contributions
KV and GMA designed the study and performed the experimental investigation and evaluated the data. GMA drafted the manuscript by analyzing the results. KV re-examined and condensed the manuscript.
Conflict of Interest
No potential conflict of interest was reported by the authors.
References
- Eerhart, A. J. J. E., Faaij, A. P. C., Patel, M. K. Replacing fossil based PET with biobased PEF; process analysis energy and GHG balance. Energ. Environ. Sci. 2012; 5: 6407-6422.
- Moghaddam, L., Zhang, Z., Wellard, R. M., Bartley, J.P., O’Hara, I.M., Doherty, W.O. Characterisation of lignins isolated from sugarcane bagasse pretreated with acidified ethylene glycol and ionic liquids. Biomass Bioenerg., 2014; 70: 498-512.
- Kumar, A. K., Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feed stocks: a review. Bioproces., 2017; 4: 7. doi:10.1186/s40643-017-0137-9
- Riccio, A., Chianese, E., Tirimberio, G., Prati, M.V. Emission factors of inorganic ions from road traffic: a case study from the city of Naples (Italy). Res. Transpor. Environ., 2017; 54: 239-249.
- Isikgor, F. H., Becer, C. R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Chem., 2015; 6: 4497-4559.
- Silveira, M. H. L., Chandel, A. K., Vanelli, B. A., Sacilotto, K.S., Cardoso, E.B. Production of hemicellulosic sugars from sugarcane bagasse via steam explosion employing industrially feasible conditions: pilot scale study. Technol., Rep., 2018; 3: 138-146.
- da Costa Lopes, A. M., Brenner, M., Falé, P., Roseiro, L.B., Bogel-Łukasik, R. Extraction and purification of phenolic compounds from lignocellulosic biomass assisted by ionic liquid, polymeric resins, and supercritical CO2. ACS Sustain. Chem. Eng., 2016; 4: 3357-
- Dias, M. O., Junqueira, T. L., Cavalett, O., Cavalett, O., Pavanello, L.G., Cunha, M.P., Jesus, C.D., Maciel Filho, R., Bonomi, A. Biorefineries for the production of first and second generation ethanol and electricity from sugarcane. Energ., 2013; 109: 72-78.
- Mora-Pale, M., Meli, L., Doherty, T. V., Linhardt, R. J., Dordick, J. S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Bioeng., 2011; 108: 1229-1245.
- Chandel, A. K., Chan, E. S., Rudravaram, R., Narasu, M.L., Rao, L.V., Ravindra, P. Economics and environmental impact of bioethanol production technologies: an appraisal. Mol. Biol., 2007; 2: 014-032.
- Triwahyuni, E., Muryanto, Sudiyani, Y., Abimanyu, H. The effect of substrate loading on simultaneous saccharification and fermentation process for bioethanol production from oil palm empty fruit bunches. Energy Procedia., 2015; 68: 138-146.
- Ravindran, H., Thangalazhy-Gopakumar, S., Adhikari, S., Fasina, O., Tu, M., Via, B., Carter, E., Taylor, S. Production of bio-oil from underutilized forest biomass using an auger reactor. Energy Sources, Part A: Recovery, Utilization, and Environ. Effect., 2015; 37: 750-757.
- Nikolic, S., Mojovic´, L., Rakin, M., Pejin, D., Pejin, J. Ultrasound-assisted production of bioethanol by simultaneous saccharification and fermentation of corn meal. Food Chem., 2010; 122: 216-222.
- Jacquet, N., Maniet, G., Vanderghem, C., Delvigne, F., Richel, A. Application of steam explosion as pretreatment on lignocellulosic material. A review. Eng. Chem. Res., 2015; 54: 2593-2598.
- Robak, K., Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol.l Res., 2020; doi: https://doi.org/10.1016/j.micres.2020.126534
- Neves, P. V., Pitarelo, A. P., Ramos, L. P. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: effect of extractives content, acid catalysis and different fermentation technologies. Technol., 2016; 208: 184-194.
- Langan, P., Petridis, L., O’Neill, H. M., Pingali, S.V., Foston, M., Nishiyama, Y., Schulz, R., Lindner, B., Hanson, B.L., Harton, S., Heller, W.T., Urban, V., Evans, B.R., Gnanakaran, S., Ragauskas, A.J., Smith, J.C., Davison, B.H. Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem., 2014; 16: 63-
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D. Determination of structural carbohydrates and lignin in biomass: laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; 2008. NREL Report No.: TP-510-42618. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy.
- Miller, J. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem., 1959; 31: 426-428.
- Williams, M. B., Reese, D. R. Colorimetric determination of ethyl alcohol. Chem., 1950; 22: 1556-1561.
- Menegol, D., Scholl, A. L., Dillon, A. J. P., Camassola, M. Influence of different chemical pretreatments of elephant grass (Pennisetum purpureum, Schum.) used as a substrate for cellulase and xylanase production in submerged cultivation. Bioprocess and Biosyst. Eng., 2016; 39: 1455-1464.
- Du, B., Sharma, L. N., Becker, C., Chen, S.F., Mowery, R.A., Peter van Walsum, G., Kevin Chambliss, C. Effect of varying feedstock pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. and Bioeng., 2010; 107: 430-440.
- Kumar, S., Dheeran, P., Singh, S.P,. Mishra, I.M., Adhikari, D.K. Kinetic studies of two-stage sulphuric acid hydrolysis of sugarcane bagasse. Energ., 2015; 83: 850-858.
- Mesa, L., González, E., Cara, C., González, M., Castro, E., Mussatto, S.I. The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Eng. J., 2011; 168: 1157–1162.
- Silva Ortiz, P., de Oliveira, Jr.S. Exergy analysis of pretreatment processes of bioethanol production based on sugarcane bagasse. Energ., 2014; 76: 130–138.
- Rocha, G.J.M., Gonçalves, A.R., Oliveira, B.R., Olivares, E.G., Rossell, C.E.V. Steam explosion pretreatment reproduction and alkaline delignifi cation reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Crops Prod., 2012; 35: 274-279.
- Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., Levin, D. B. Biomass pretreatment: fundamentals toward application. Adv., 2011; 29: 675-685.
- Brodeur, G., Yau, E., Badal, K., Collier, J., Ramachandran, K.B., Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass : A Review. Res., 2011; Article ID 787532, https://doi.org/10.4061/2011/787532

This work is licensed under a Creative Commons Attribution 4.0 International License.





